Abstract
In recent years remarkable progress has been made towards the understanding of proposed hallmarks of cancer development and treatment. However with its increasing incidence, the clinical management of cancer continues to be a challenge for the 21st century. Treatment modalities comprise of radiation therapy, surgery, chemotherapy, immunotherapy and hormonal therapy. Radiation therapy remains an important component of cancer treatment with approximately 50% of all cancer patients receiving radiation therapy during their course of illness; it contributes towards 40% of curative treatment for cancer. The main goal of radiation therapy is to deprive cancer cells of their multiplication (cell division) potential. Celebrating a century of advances since Marie Curie won her second Nobel Prize for her research into radium, 2011 has been designated the Year of Radiation therapy in the UK. Over the last 100 years, ongoing advances in the techniques of radiation treatment and progress made in understanding the biology of cancer cell responses to radiation will endeavor to increase the survival and reduce treatment side effects for cancer patients. In this review, principles, application and advances in radiation therapy with their biological end points are discussed.
Keywords: Cancer, Radiation therapy, Linear energy transfer, Cell death.
Introduction
Cancer remains leading cause of death globally. The International Agency for Research on Cancer (IARC) recently estimated that 7.6 million deaths worldwide were due to cancer with 12.7 million new cases per year being reported worldwide. A significant proportion of this burden is borne by developing countries; 63% of cancer deaths are reported to be from developing countries 1, 2, 3. Cancer is a multigenic and multicellular disease that can arise from all cell types and organs with a multi-factorial etiology. Hanahan and Weinberg 4 have identified six cancer cell phenotypes or hallmarks of cancer: cells with unlimited proliferative potential, environmental independence for growth, evasion of apoptosis, angiogenesis, invasion and metastasis to different parts of body. If uncontrolled cell growth or metastatic spread occurs it will result in death of the individual 5. The past decade has witnessed a considerable progress towards the treatment and understanding of the earlier proposed hallmarks of cancer 6 and together with advances in early detection and in the various treatment modalities, many cancers have become curable 7.
After the discovery of X-rays in 1895, by Wilhelm Conrad Röntgen from Germany its clinical usefulness, as a means of cancer treatment was first appreciated. It is also one hundred years ago that Marie Curie won a second Nobel Prize for her research into radium, establishing her position as a pioneer in the field of radiation therapy. To mark this, 2011 has been designated the Year of Radiation therapy in the UK, celebrating a century of advances. Since that time, radiation therapy has developed into a recognized medical specialty with Radiation Oncology being a discipline in which various health and science professionals from numerous disciplines work together. Along with surgery and chemotherapy, radiation therapy or radiotherapy remains an important modality used in cancer treatment being a highly cost effective single modality treatment accounting about only 5% of the total cost of cancer care 8. Furthermore, approximately 50% of all cancer patients will receive radiation therapy during their course of illness 9, 10 with an estimation that radiation therapy contributes to around 40% towards curative treatment 11. Rapid progress in this field continues to be boosted by advances in imaging techniques, computerized treatment planning systems, radiation treatment machines (with improved X-ray production and treatment delivery) as well as improved understanding of the radiobiology of radiation therapy 12.
Principles of radiation therapy
Radiation is a physical agent, which is used to destroy cancer cells. The radiation used is called ionizing radiation because it forms ions (electrically charged particles) and deposits energy in the cells of the tissues it passes through. This deposited energy can kill cancer cells or cause genetic changes resulting in cancer cell death.
High-energy radiation damages genetic material (deoxyribonucleic acid, DNA) of cells and thus blocking their ability to divide and proliferate further 13. Although radiation damages both normal cells as well as cancer cells, the goal of radiation therapy is to maximize the radiation dose to abnormal cancer cells while minimizing exposure to normal cells, which is adjacent to cancer cells or in the path of radiation. Normal cells usually can repair themselves at a faster rate and retain its normal function status than the cancer cells. Cancer cells in general are not as efficient as normal cells in repairing the damage caused by radiation treatment resulting in differential cancer cell killing 10.
Radiation can be given with the intent of cure as well as being used as a very effective modality of palliative treatment to relieve patients from symptoms caused by the cancer. Further indications of radiation therapy include combination strategies with other treatment modalities such as surgery, chemotherapy or immunotherapy. If used before surgery (neoadjuvant therapy), radiation will aim to shrink the tumor. If used after surgery (adjuvant therapy), radiation will destroy microscopic tumor cells that may have been left behind. It is well known that tumors differ in their sensitivity to radiation treatment. Table 1 shows a list of common cancers treated with radiation therapy.
Table 1.
Examples of cancers treated with radiation therapy.
| Early cancers curable with radiation therapy alone | Cancers curable with radiation therapy in combination with other modalities |
|---|---|
| Skin cancers (Squamous and Basel cell) | Breast carcinomas |
| Prostate carcinomas | Rectal and anal carcinomas |
| Lung carcinomas (non-small cell) | Local advanced cervix carcinomas |
| Cervix carcinomas | Locally advanced head and neck carcinomas |
| Lymphomas (Hodgkin's and low grade Non-Hodgkin's) | Locally advanced lung carcinomas |
| Head and neck carcinomas | Advanced lymphomas |
| Bladder carcinomas | |
| Endometrial carcinomas | |
| CNS tumors | |
| Soft tissue sarcomas | |
| Pediatric tumors |
There are two ways to deliver radiation to the location of the cancer. External beam radiation is delivered from outside the body by aiming high-energy rays (photons, protons or particle radiation) to the location of the tumor. This is the most common approach in the clinical setting. Internal radiation or brachytherapy is delivered from inside the body by radioactive sources, sealed in catheters or seeds directly into the tumor site. This is used particularly in the routine treatment of gynecological and prostate malignancies as well as in situations where retreatment is indicated, based on its short range effects.
Radiation therapy techniques
Fractionation
Radiation therapy delivered in a fractionated regime is based on the differing radiobiological properties of cancer and various normal tissues. These regimes in general amplify the survival advantage of normal tissues over cancer cells, largely based on better sublethal damage repair of radiation damage in normal cells as compared to cancer cells. Normal cells proliferate relatively more slowly compared to the rapidly proliferating cancer cells and therefore have time to repair damage before replication. Initial observations of the effects of fractionated radiation therapy in the 1920s eventually led to the development of regimes comparing different treatment schedules based on total dose, number of fractions and overall treatment time 14. Current regimes are based on the more refined linear-quadratic formula which addresses the time-dose factors for individual tumor types and normal tissues 15. A typical radiation therapy regime now consists of daily fractions of 1.5 to 3Gy given over several weeks.
Technological advances
The goal of radiotherapy is to deliver as much dose to the tumour whilst sparing normal tissue. Technological advances incorporating new imaging modalities, more powerful computers and software, and new delivery systems such as advanced linear accelerators have helped achieve this.
3D Conformal radiotherapy (3DCRT)
2D radiation therapy using rectangular fields based on plain X-ray imaging has largely been replaced by 3D radiation therapy based on CT imaging which allows accurate localization of the tumour and critical normal organ structures for optimal beam placement and shielding. The aim is to deliver radiation to the gross tumour volume (GTV), with a margin for microscopic tumour extension called the clinical target volume (CTV), and a further margin uncertainties from organ motion and setup variations called the planning target volume (PTV) 16.
Intensity modulated radiation therapy (IMRT)
IMRT allows the oncologist to create irregular-shaped radiation doses that conform to the tumour whilst simultaneously avoiding critical organs. IMRT is made possible through: a) inverse planning software and b) computer-controlled intensity-modulation of multiple radiation beams during treatment. IMRT is now available in many clinical departments and can be delivered by linear accelerators with static or dynamic multi-leaf collimators or tomotherapy machines. This has allowed improvements in the therapeutic ratio for several tumor sites, such as head and neck cancers 17, prostate cancers 18 and gynecological cancers 19.
Image-guided radiotherapy (IGRT)
As treatment margins become tighter and more conformal, the potential to miss tumour due to organ motion and patient setup variations become greater 20. When critical structures are close to the tumour, a slight positional error may also lead to inadvertent radiation of the normal organs. IGRT allows the detection of such errors by information acquired through pre-radiotherapy imaging which allows for correction. One such example is with daily cone-beam CT scans acquired before each treatment 21. The improved accuracy has made dose escalation feasible 22, and this has allowed an improvement in the therapeutic ratio for several tumor sites, such as head and neck cancers 23 and prostate cancers 24.
Stereotactic body radiation therapy (SBRT)
The above technological advancements have enabled SBRT, which precisely delivers very high individual doses of radiation over only a few treatment fractions to ablate small, well-defined primary and oligometastatic tumours anywhere in the body 25, 26. Due to the high radiation dose, any tissue immediately adjacent to the tumour is likely to be damaged. However as the amount of normal tissue in the high dose region is small and non-eloquent, clinically significant toxicity is low 27. SBRT has shown excellent results in the treatment of early stage non-small cell lung cancer in patients unfit for surgery. Other tumours include in the prostate, head and neck, hepatic, renal, oligometastases, spinal and pancreatic 28, 29, 30.
Types of radiation used to treat cancer: photons radiation (x-rays and gamma rays), which are widely used
Photon beams carry a low radiation charge and have a much lower mass. X-rays and gamma rays are routinely used photons in radiation therapy to treat various cancers. X- rays and gamma rays are sparsely ionizing radiations, considered low LET (linear energy transfer) electromagnetic rays and further composed of massless particles of energy are called photons. X-rays are generated by a device that excite electrons (e.g. cathode ray tubes and linear accelerators), while gamma rays originate from the decay of radioactive substances (e.g.cobalt-60, radium and cesium).
Particle radiations (electron, proton and neutron beams)
Electron beams are commonly used in everyday radiation therapy treatment and are particularly useful to treat tumours close to a body surface since they do not penetrate deeply into tissues. External beam radiation therapy is also carried out with heavier particles such as: neutrons produced by neutron generators and cyclotrons; protons produced by cyclotrons and synchrotrons; and heavy ions (helium, carbon, nitrogen, argon, neon) produced by synchrocyclotrons and synchrotrons. Proton beams are a newer form of particle beam radiation used to treat cancer. It can offer better dose distribution due to its unique absorption profile in tissues, known as the Bragg's peak, allowing deposition of maximum destructive energy at the tumor site while minimizing the damage to healthy tissues along their path. These have particular clinical use in pediatric tumors and in adults tumors located near critical structures such as spinal cord and skull base tumors, where maximal normal tissue sparing is crucial 31. Neutron beams are generated inside neutron generators after proton beams are deflected to a target. They have high LET and can cause more DNA damage than photons. The limitations have been mainly due to difficulty in generating neutron particles as well as the construction of such treatment facilities.
Particle radiation has higher LET than photons with higher biological effectiveness. Therefore, these forms of radiations may be more effective to the radioresistant cancers such as sarcomas, renal cell carcinomas, melanomas and glioblastoma 32. However, equipment for production of particle radiation therapy is considerably more expensive than for photons. The decreasing costs of cyclotrons are likely to result in a wider use of proton beam therapy in the future 33.
Biological aspects
Biological effectiveness (cell killing) of radiation depends on the linear energy transfer (LET), total dose, fractionation rate and radio-sensitivity of the targeted cells or tissues 34, 35. Low LET radiation deposits relatively a small quantity of energy whilst high LET radiation deposits higher energy on the targeted areas. Though radiation is directed to kill the tumor cell, it is inevitable that the non-cancerous normal tissues surrounding the tumour also damaged by radiation. However, the goal of radiation therapy is to maximize the dose to tumour cells while minimizing exposure to normal healthy cells 36.
Radiation therapy works through in various ways to remove the cancer cells
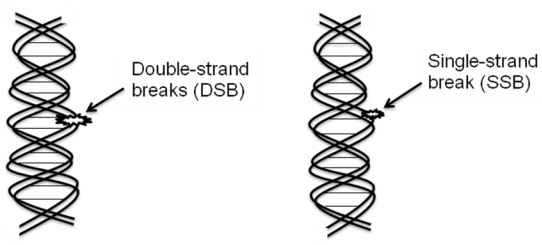
The biological target of radiation in the cell is DNA (Figure 1).
Direct effects of radiation: Radiation can directly interact with cellular DNA and cause damage (Figure 2A).
Indirect effects of radiation: The indirect DNA damage caused by the free radicals is derived from the ionization or excitation of the water component of the cells (Figure 2B).
Figure 1.
The biological target of radiation in the cell is DNA. Extensive damage to cancer cells DNA can lead to cell death. DNA double-strand breaks (DSBs) are more responsible for most cells killing, even a single DSB is sufficient to kill a cell or disturb its genomic integrity by the radiation treatment.
Figure 2.
Radiation act directly or indirectly on the cellular DNA.
Double strand DNA breaks are irreparable and more responsible than the single strand DNA breaks for most of cell killing in cancer as well as surrounding normal cells.
Radiation therapy and cell death
Radiation therapy can kill cancer cells by a variety of mechanisms. The main goal of radiation therapy is to deprive cancer cells of their multiplication potential and eventually kill the cancer cells. Cancer cells whose DNA is damaged beyond repair stop dividing and die.
However, the mechanism of cell death response to irradiation is complex. Thus, identifying the importance of radiation induced cell death and further mechanisms involved has potential clinical implications for improving outcomes with radiation therapy.
Types and characteristics of cell death
Radiation therapy, like most anticancer treatments, achieves its therapeutic effect by inducing different types of cell death 37 (Figure 3). Radiation therapy does not kill cancer cells right away. It takes hours, days or weeks of treatment before cancer cells start to die after which cancer cells continue dying for weeks to months after radiation therapy ends.
Figure 3.
Types of cell death induced by radiation. Radiation mainly kills the cells either by apoptosis or mitotic catastrophe.
Apoptosis: Programmed cell death or apoptosis is a major cell death mechanisms involved in cancer therapy and in radiation therapy in particular 38, 39, 40. Apoptosis is characterized by cell shrinkage and formation of apoptotic bodies. Mitochondria play a major role for the apoptotic cell death 41. Blebbing of cell membrane is often seen with condensed chromatin with nuclear margination and with DNA fragmentation. In general, the cellular membrane of apoptotic cells remains intact. Induction of apoptosis in cancer cells plays an important role in the efficacy of radiation therapy 37, 42.
Mitotic cell death or Mitotic catastrophe: This type cell death occurs during or after aberrant mitosis (cell division) and is caused by mis-segregation of chromosomes leading to formation of giant cells with aberrant nuclear morphology, multiple nuclei. Cells often have one or more micronuclei and with centrosome over duplication 43, 44. After irradiation, most of solid tumor cell death occurs predominantly as a result of aberrant mitotic events 45.
The above two types of cell death account for the majority of ionizing radiation induced cell death.
Necrosis: Cells visibly swell with breakdown of cell membrane. Cells have an atypical nuclear shape with vacuolization, non-condensed chromatin and disintegrated cellular organelles along with mitochondrial swelling and plasma membrane rupture followed by subsequent loss of intracellular contents 46.
Following radiation, necrosis is seen less frequently but does occur in cancer cell lines or tissues.
Senescence: Senescence refers to a state of permanent loss of cell proliferative capacity. Senescent cells are viable but non-dividing, stop to synthesize DNA, become enlarged and flattened with an increased granularity. Senescence has been reported to occur in cancer cells following extensive cellular stress in the form of DNA damage induced by radiation treatment 47, 48 and later die mainly by the process of apoptosis.
Autophagy: Autophagy is a more recent phenomenon described. It is a form of cancer cell death in response to radiation. Autophagy is a genetically regulated form of programmed cell death in which the cell digests itself that involves autophagic/lysosomal compartment. It is characterized by the formation of double-membrane vacuoles in the cytoplasm, which sequester organelles such as condensed nuclear chromatin and ribosomes 49, 50.
Various genes and intracellular pathways have been reported to be involved in the different types of radiation induced cell death. Apoptosis has been associated with the ATM-p53-Bax-Cytochrome c-Caspases pathway 51, whilst mitotic catastrophe involves the p53-Caspases-Cytochrome c cascade 52. In the necrosis, TNF (alpha) -PARP-JNK-Caspases pathway 53 is involved and the MYC-INK4A-ARF-p53-p21 pathway has been implicated in senescence 54. With autophagy, the PI3K-Akt-mTOR cascade is thought to be important 55. Though most of these pathways are interrelated for radiation induced cancer cell death, much remains to be understood in the cell death pathways that generate cancer cell tumorigenesis and radiation treatment resistance. However, the precise mechanism(s) responsible for radiation induced different mode of cancer cell death have not been fully elucidated. In recent years, knowledge is rapidly increasing regarding the various molecular pathways involved in determining cell death after exposure to radiation. Areas of interest include studying the mechanisms of DNA damage response and repair, intracellular signaling in response to single or fractionated radiation as well as the effects of radiation on the tumor microenvironment. With advances in genome sequencing, tumor profiling should allow more individualized treatment with risk stratification approaches 56 and allow for a more accurate molecular targeted anticancer approach of radiation therapy 10 in the next decade.
Conclusions
Radiation remains an important modality for cancer treatment with ongoing efforts towards designing new radiation treatment modalities and techniques which continue to improve the survival and quality of life of cancer patients. With the improved clinical outcomes of cancer treatment, minimizing radiation therapy related toxicities has also become a priority. The emergence of mechanistic biological studies together with improvements in radiation technology has improved the sparing of normal cells/tissues through dose fractionation and conformal radiation techniques. Radiation is also being delivered in combination with molecular targeted therapy with the aim of further improving the therapeutic ratio of the radiation treatment 10, 57, 58.
Though ionizing radiation remains one of the most effective tools in the therapy of cancer cure, answers to a number of questions remain: 1. What criteria drive the cancer cells in the selection of a particular type of cell death pathway? 2. How does a cancer cell switch from a recovery (repair) program to destructive cell death? 3. Ways to optimize the effectiveness of radiation therapy in combination with other modalities of treatment? 4. Would it be possible to lower radiation therapy effects to normal tissues? Answers to these and other questions together with ongoing advancements in radiation therapy technology and techniques will ultimately lead to continued improvement in cancer treatment.
Authors' contributions
RB and KWY wrote the first draft of the manuscript. All authors contributed to the development of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This work was supported to RB by National Cancer Center (Award number: NRFRS10122) Singapore.
References
- 1.International Agency for Research on Cancer (IARC) GLOBOCAN 2008, Cancer incidence and mortality worldwide. Lyon, France: IARC; 2010. [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg R. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;44:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Pollack LA, Rowland JH, Crammer C, Stefanek M. Introduction: charting the landscape of cancer survivors' health-related outcomes and care. Cancer. 2009;115:4265–4269. doi: 10.1002/cncr.24579. [DOI] [PubMed] [Google Scholar]
- 8.Ringborg U, Bergqvist D, Brorsson B, Cavallin-Ståhl E, Ceberg J, Einhorn N, Frödin JE, Järhult J, Lamnevik G, Lindholm C, Littbrand B, Norlund A, Nylén U, Rosén M, Svensson H, Möller TR. The Swedish Council on Technology Assessment in Health Care: systematic overview of radiotherapy for cancer including a prospective survey of radiotherapy practice in Sweden 2001-summary and conclusions. Acta Oncol. 2003;42:357–365. doi: 10.1080/02841860310010826. [DOI] [PubMed] [Google Scholar]
- 9.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 10.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 11.Barnett GC, West CM, Dunning AM, Elliott RM, Coles CE, Pharoah PD, Burnet NG. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9:134–142. doi: 10.1038/nrc2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nature. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 13.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nature. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 15.Ellis F. Dose, time and fractionation: a clinical hypothesis. Clin Radiol. 1969;20:1–7. doi: 10.1016/s0009-9260(69)80043-7. [DOI] [PubMed] [Google Scholar]
- 16.International Commission on Radiation Units. Prescribing, recording and reporting photon beam therapy. Supplement to ICRU Report 50. Bethesda: International Commission on Radiation Units and Measurement. MD: ICRU; 1999. [Google Scholar]
- 17.Feng FY, Kim HM, Lyden TH, Haxer MJ, Feng M, Worden FP, Chepeha DB, Eisbruch A. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 18.Wang-Chesebro A, Xia P, Coleman J, Akazawa C, Roach M 3rd. Intensity-modulated radiotherapy improves lymph node coverage and dose to critical structures compared to with three-dimensional conformal radiation therapy in clinically localized prostate cancer. Int J Radiat Oncol Phys. 2006;66:654–662. doi: 10.1016/j.ijrobp.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Mundt AJ, Lujan AE, Rotmensch J, Waggoner SE, Yamada SD, Fleming G, Roeske JC. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Phys. 2002;52:1330–1337. doi: 10.1016/s0360-3016(01)02785-7. [DOI] [PubMed] [Google Scholar]
- 20.Langen KM, Jones DT. Organ motion and its management. Int J Radiat Oncol Biol Phys. 2001;50:265–278. doi: 10.1016/s0360-3016(01)01453-5. [DOI] [PubMed] [Google Scholar]
- 21.Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2002;53:1337–1349. doi: 10.1016/s0360-3016(02)02884-5. [DOI] [PubMed] [Google Scholar]
- 22.Gill S, Thomas J, Fox C, Kron T, Rolfo A, Leahy M, Chander S, Williams S, Tai KH, Duchesne GM, Foroudi F. Acute toxicity in prostate cancer patients treated with and without image-guided radiotherapy. Radiat Oncol. 2011;6:145. doi: 10.1186/1748-717X-6-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duma MN, Kampfer S, Wilkens JJ, Schuster T, Molls M, Geinitz H. Comparative analysis of an image-guided versus a non-image-guided setup approach in terms of delivered dose to the parotid glands in head-and-neck cancer IMRT. Int J Radiat Oncol Biol Phys. 2010;77:1266–1273. doi: 10.1016/j.ijrobp.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 24.Barney BM, Lee RJ, Handrahan D, Welsh KT, Cook JT, Sause WT. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT) Int J Radiat Oncol Biol Phys. 2011;80:301–305. doi: 10.1016/j.ijrobp.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, Teh BS, McGarry RC, Cardenes HR, Timmerman RD. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 26.Tipton K, Launders JH, Inamdar R, Miyamoto C, Schoelles K. Stereotactic body radiation therapy: scope of the literature. Ann Intern Med. 2011;154:737–745. doi: 10.7326/0003-4819-154-11-201106070-00343. [DOI] [PubMed] [Google Scholar]
- 27.Lo SS, Moffatt-Bruce SD, Dawson LA, Schwarz RE, Teh BS, Mayr NA, Lu JJ, Grecula JC, Olencki TE, Timmerman RD. The role of local therapy in the management of lung and liver oligometastases. Nat Rev Clin Oncol. 2011;8:405–416. doi: 10.1038/nrclinonc.2011.75. [DOI] [PubMed] [Google Scholar]
- 28.Wu QJ, Wang Z, Yin FF. The impact of respiratory motion and treatment technique on stereotactic body radiation therapy for liver cancer. Med Phys. 2008;35:1440–1451. doi: 10.1118/1.2839095. [DOI] [PubMed] [Google Scholar]
- 29.National radiotherapy implementation group report. Stereotactic body radiotherapy; Clinical review of the evidence for SBRT. UK: NRIG; 2010. [Google Scholar]
- 30.Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat Oncol. 2011;6:3. doi: 10.1186/1748-717X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laramore GE. Role of particle radiotherapy in the management of head and neck cancer. Current Opin Oncol. 2009;21:224–231. doi: 10.1097/cco.0b013e328329b716. [DOI] [PubMed] [Google Scholar]
- 32.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25:953–964. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 33.Ma CM, Maughan RL. Within the next decade conventional cyclotrons for proton radiotherapy will become obsolete and replaced by far less expensive machines using compact laser systems for the acceleration of the protons. Med Phys. 2006;33:571–573. doi: 10.1118/1.2150220. [DOI] [PubMed] [Google Scholar]
- 34.Hall EJ. Cancer caused by x-rays-a random event? Lancet Oncol. 2007;8:369–370. doi: 10.1016/S1470-2045(07)70113-4. [DOI] [PubMed] [Google Scholar]
- 35.Baskar R. Emerging role of radiation induced bystander effects: Cell communications and carcinogenesis. Genome Integr. 2010;1:13. doi: 10.1186/2041-9414-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 37.Verheij M. Clinical biomarkers and imaging for radiotherapy-induced cell death. Cancer Metastasis Rev. 2008;27:471–480. doi: 10.1007/s10555-008-9131-1. [DOI] [PubMed] [Google Scholar]
- 38.Dewey WC, Ling CC, Meyn RE. Radiation-induced apoptosis: relevance to radiotherapy. Int J Radiat Oncol Biol Phys. 1995;33:781–796. doi: 10.1016/0360-3016(95)00214-8. [DOI] [PubMed] [Google Scholar]
- 39.Rupnow BA, Knox SJ. The role of radiation-induced apoptosis as a determinant of tumor responses to radiation therapy. Apoptosis. 1999;4:115–143. doi: 10.1023/a:1009675028784. [DOI] [PubMed] [Google Scholar]
- 40.Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer. 2009;9:321–326. doi: 10.1038/nrc2615. [DOI] [PubMed] [Google Scholar]
- 41.Fogg VC, Lanning NJ, MacKeigan JP. Mitochondria in cancer: at the crossroads of life and death. Chin J Cancer. 2011;30:526–539. doi: 10.5732/cjc.011.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31:363–372. doi: 10.1007/s13277-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 43.Sato N, Mizumoto K, Nakamura M, Ueno H, Minamishima YA, Farber JL, Tanaka M. A possible role for centrosome overduplication in radiation-induced cell death. Oncogene. 2000;19:5281–5290. doi: 10.1038/sj.onc.1203902. [DOI] [PubMed] [Google Scholar]
- 44.Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 45.Jonathan EC, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol. 1999;3:77–83. doi: 10.1016/s1367-5931(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 46.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roninson I. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- 48.Schmitt CA. Cellular senescence and cancer treatment. Biochim Biophys Acta. 2007;1775:5–20. doi: 10.1016/j.bbcan.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Ito H, Daido S, Kanzawa T, Kondo S, Kondo Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol. 2005;26:1401–1410. [PubMed] [Google Scholar]
- 50.Kuwahara Y, Oikawa T, Ochiai Y, Roudkenar MH, Fukumoto M, Shimura T, Ohtake Y, Ohkubo Y, Mori S, Uchiyama Y, Fukumoto M. Enhancement of autophagy is a potential modality for tumors refractory to radiotherapy. Cell Death Dis. 2011;2:e177.. doi: 10.1038/cddis.2011.56. doi: 10.1038/cddis.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitt CA. Senescence, apoptosis and therapy-cutting the lifelines of cancer. Nat Rev Cancer. 2003;3:286–295. doi: 10.1038/nrc1044. [DOI] [PubMed] [Google Scholar]
- 52.Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 53.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, Lowe SW. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 55.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 56.Starmans MH, Zips D, Wouters BG, Baumann M, Lambin P. The use of a comprehensive tumour xenograft dataset to validate gene signatures relevant for radiation response. Radiother Oncol. 2009;92:417–422. doi: 10.1016/j.radonc.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Brown JM. Therapeutic targets in radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:319–326. doi: 10.1016/s0360-3016(00)01482-6. [DOI] [PubMed] [Google Scholar]
- 58.Tofilon PJ, Saxman S, Coleman CN. Molecular targets for radiation therapy: bringing preclinical data into clinical trials. Clin Cancer Res. 2003;9:3518–3520. [PubMed] [Google Scholar]