Abstract
Purpose:
To explore the association between primary healthcare (PHC) organizational model and health-related quality of life (HRQoL) in persons with chronic disease.
Methods:
We recruited 776 patients with a primary diagnosis of one of four chronic diseases from 33 PHC clinics. Patients were interviewed at baseline, 6, 12 and 18 months. We categorized PHC model by administrative type and by a taxonomy according to organizational attributes. HRQoL was measured by disease-specific questionnaires.
Results:
Mean age was 67 years and 55.3% were female. PHC models differed with respect to case mix: community models served older patients with higher co-morbidity and lower health status. Multilevel logistic regression revealed that none of the PHC organizational models was associated with HRQoL. Having fewer co-morbidities, higher self-rated health and not using home care services were associated with higher HRQoL.
Conclusion:
Despite their having patients with more complex health problems, HRQoL in patients of community practices was equivalent to that of patients in other types of PHC organizations.
Abstract
Objet :
Explorer les liens entre le modèle d'organisation des soins de santé primaires (SSP) et la qualité de vie liée à la santé (QVS) chez les personnes atteintes d'une maladie chronique.
Méthode :
Nous avons interrogé 776 patients qui ont obtenu un diagnostic primaire pour une parmi quatre maladies chroniques dans 33 cliniques de SSP. Les patients ont été interrogés au début de l'étude, puis à 6, 12 et 18 mois. Nous avons classé les modèles de SSP selon le type d'établissement et selon une taxonomie des caractéristiques organisationnelles. Des questionnaires axés sur les maladies spécifiques ont servi à mesurer la QVS.
Résultats :
L'âge moyen des répondants était 67 ans et 55,3 % d'entre eux étaient des femmes. Les modèles de SSP différaient selon la composition des cas : les modèles communautaires offraient des services à une clientèle plus âgée présentant une plus grande comorbidité et un état de santé moindre. L'analyse de régression logistique multiniveau révèle qu'aucun des modèles d'organisation des SSP avait un lien avec la QVS. Une plus faible comorbidité, un plus haut taux d'autoévaluation en matière de santé et le non-usage des services de soins à domicile sont associés à une meilleure QVS.
Conclusion :
Malgré la présence de patients qui ont des problèmes de santé plus complexes, la QVS chez les patients des établissements communautaires est équivalente à celle des patients des autres types d'organisations de SSP.
Improving the effectiveness of healthcare in a system undergoing reform requires a greater understanding of how organizational context affects clinical outcomes (Mitchell and Shortell 1997). In particular, there has been an emphasis on effective and efficient healthcare delivery for persons with chronic health problems, especially from their primary care providers (Morgan et al. 2007; Russell et al. 2009). A main reason for this focus is that chronic conditions have significant adverse consequences on health outcomes and functioning (Yach et al. 2004), but adequate management can moderate their impact on quality of life and reduce the burden of the disease (Hung et al. 2008). Health status and health-related quality of life (HRQoL) are important outcome measures for patients with chronic conditions (Lam and Lauder 2000) and have been found to be linked with healthcare utilization and mortality (Schraeder et al. 2001).
Recent literature suggests that the way primary healthcare (PHC) is organized may influence patient health outcomes, and that structured care incorporating such elements as health system integration, enhanced clinical information systems and self-management support can optimize clinical outcomes for patients with chronic disease (Bosch et al. 2009; Hung et al. 2008; Scott et al. 2004). The Chronic Care Model, which is now widely recognized as a framework for optimizing chronic care, has incorporated these elements into its core dimensions (Wagner et al. 2001). Yet, research on organization of primary care for most chronic conditions is still limited. Furthermore, few studies have used appropriate analytical methods to explore the link between organizational factors and patient outcomes.
There are several different PHC organizational models, including solo practices, group practices, family medicine groups and community health clinics. Determining which are most beneficial for patients with chronic disease can potentially influence health policy. In addition to observed outcomes such as incidence of disease, healthcare utilization and mortality, patient-reported outcomes such as perceptions of healthcare quality, symptoms and HRQoL are increasingly becoming part of the evaluation profile in PHC settings (Maddigan et al. 2004). Patient-reported outcomes are more relevant than ever in the PHC setting given the current emphasis on providing interventions to empower patients to engage in a healthy lifestyle to prevent disease, as well as to help patients manage the symptoms, treatment, and physical and psycho-social consequences inherent in living with a chronic condition (Greenhalgh et al. 2005).
Our objective was to determine the association between PHC organizational models and quality-of-life outcomes in persons with chronic disease.
Methods
Machro-1 is a cohort study designed to evaluate the association of PHC organizational models with various patient outcomes. We invited 90 PHC clinics – in Montreal and the Montérégie regions of the province of Quebec, Canada – that indicated (in a previous survey) that they provide services for patients with chronic disease to enrol in this phase of the study. Physicians approached their patients who had one of the required diagnoses and asked them whether they would agree to participate in the research study. Those who agreed were contacted by a trained interviewer. Patients were interviewed at baseline in a face-to-face interview and at three subsequent, six-month intervals by telephone. Recruitment and interviews were done between 2006 and 2008. Interviews consisted of a study questionnaire that collected information on sociodemographics and healthcare utilization, as well as several validated questionnaires: the Medical Outcomes Short Form-36 (SF-36) (Ware and Sherbourne 1992), which was administered at baseline and 12 months, and disease-specific HRQoL questionnaires, which were administered at all four interviews (baseline, 6, 12 and 18 months). The disease-specific questionnaires were the Health Assessment Questionnaire (HAQ), which evaluated performance in activities of daily living in patients with arthritis (Bruce and Fries 2003a,b); the Minnesota Living with Heart Failure Questionnaire (Rector and Cohn 1992), which measured the impact of chronic heart failure; the Chronic Respiratory Questionnaire (Guyatt et al. 1999), which measured the impact of chronic obstructive pulmonary disease; and the Audit of Diabetes-Dependent Quality of Life (ADDQoL) (Bradley et al. 1999), which measured diabetes-related adverse consequences on health and functioning. All questionnaires had been validated in English and Canadian French.
PHC organizations were classified using two different perspectives. First, a typology of PHC clinics based on their official administrative denomination was used. A second approach used a taxonomy of PHC organizations based on inherent attributes. The rationale for using these two approaches is that the former corresponds to what is usually described in the literature in terms of type of PHC clinic, whereas the latter classifies the PHC clinic based on organizational attributes developed according to Pineault and colleagues (2008). The taxonomic approach categorizes organizations so that it minimizes the heterogeneity within each category and maximizes the heterogeneity among categories. Administrative types included (a) solo providers (solo practitioner); (b) family medicine groups (FMGs) – a group of family physicians who work closely with nurses to offer family medicine to registered individuals; to be deemed a FMG, the organization must meet specific government standards; (c) community health centres – offering medical, home care and social services; (d) group practices – group of physicians that practise independently but share office expenses (includes walk-in clinics). Method of remuneration was fee for service for all types except community health centres, where physicians are salaried.
Five taxonomic models were identified. Four were professional models of PHC: Single-Provider Model (general practitioners who work essentially on their own), Contact Model (walk-in clinics that focus on accessibility and responding to short-term needs), Coordination Model (two to six physicians forming a medical team geared towards follow-up of regular clients with a broad range of services offered; however, there are few formal links with other care providers) and Coordination Integrated Model (such as FMGs, which are characterized by teams of caregivers composed of several physicians and nurses offering a broad range of services; the clinics usually share space and have links with specialists and other health professionals), while one was a Community-Oriented Model (teams consisting of more than six physicians as well as nurses working in a group; they often share facilities with other health professionals, offer a broad range of services and are integrated into public health structures, such as the local community health centres in Quebec). Organizational characteristics and descriptions of each taxonomic model are presented in Table 1. Some of these characteristics reflect aspects of more structured care and integration that are part of the Chronic Care Model.
TABLE 1.
Main characteristics of PHC taxonomy models
| Aspects | Professional Models | Community Model | ||||
|---|---|---|---|---|---|---|
| Single Provider | Contact | Coordination | Coordination Integrated | |||
| Vision | Responsibility | Clientele | Individuals who come to the clinic | Clientele | Clientele – Population | Population – Clientele |
| Structure | Governance | Private – Professional | Public | |||
| Integration | Low | Medium / Low | Medium / Low | High | High / Medium | |
| Resources | Quantity and variety | Low | Medium | Medium | High | High |
| Practice | Appointment – Walk-in | Mostly by appointment | Mostly walk-in | Mostly by appointment | Mixed | Mixed |
| Scope of services | Narrow | Narrow | Average | Wide | Wide | |
We first analyzed the data using unadjusted bivariate analyses to determine associations between organizational models (types and taxonomic categorizations) and health status – SF-36/HRQoL outcomes. We operationalized HRQoL in three ways in order to make the different disease-specific scales comparable. The first method was to rescale each result on a continuum from 0 (worst HRQoL) to 100 (best HRQoL). The second method was to assess change in health status as well as in HRQoL, dichotomized as deterioration (≥5% decline) versus stability or improvement. The third method consisted of dividing raw HRQoL scores into tertiles according to their respective distributions, and recoding as a binary variable (upper tertile versus lower two tertiles).
Next, we used logistic regression to model 12-month change in health status or in HRQoL (divided into a dichotomous outcome, i.e., ≥5% deterioration in health status or in HRQoL) as a function of PHC models (types and taxonomy), adjusting for covariates. Next, we conducted multi-level logistic analysis that took into consideration the longitudinal study design (repeated measures at baseline, 6, 12 and 18 months) and the structure of the data, which comprised patients nested within PHC clinics. HRQoL outcomes (upper tertile versus lower two tertiles) were entered into a three-level model (level 1 – repeated measures; level 2 – individuals; level 3 – PHC models), beginning with lower-level variables and entering variables that appeared as significant covariates in a step-forward fashion. Models were adjusted for age, sex, co-morbidity, self-rated health, socio-economic status and severity, as well as several other sociodemographic variables. Co-morbidity was characterized by the total number of conditions as reported by the participant. Health was self-rated on a scale from 1 (bad) to 5 (excellent). Socio-economic status was operationalized as a measure of patients' perceived SES (wealthy, average, poor or very poor) (Pham-Kanter 2009). Disease severity was determined by the use of home care services, a measure of utilization found to be highly correlated with severe physical and functional limitation (Stoddart et al. 2002). Other sociodemographic variables included Canadian versus immigrant (the latter defined as not being born in Canada), social support (defined as having someone to help you with your medical appointments) and being single (single, divorced or widowed, as opposed to being married or living common-law with someone). Analyses were done using SPSS 12.02 (2003) and HLM 6.03 (Raudenbush and Bryk 2002).
This project was approved by the ethics boards of the Public Health Department of Montreal and the Research Centre of the University of Montreal Hospital Centre.
Results
Among the 90 PHC clinics asked to participate in the study, 33 clinics actually provided patients for our study. From them, we recruited a sample of 776 patients with at least one of four chronic diseases diagnosed by their physician (diabetes, arthritis, heart failure and chronic obstructive pulmonary disease). At baseline, mean age of the patient sample was 67.1 years, 55.3% were female and a majority (72%) reported living with more than two health conditions. Table 2 displays sample characteristics at baseline (n=776).
TABLE 2.
Sample characteristics and PHC affiliation
| Mean age ± SD (years) | 67.13 ± 11.64 | |
| % | ||
| Female | 55.3 | |
| Education ≤ high school | 74.5 | |
| Yearly income ≤ $15,000 | 43.4 | |
| ≥ 6 co-morbidities | 25.1 | |
| Primary diagnosis | Diabetes | 33.2 |
| Heart failure | 19.3 | |
| Chronic arthritis | 27.2 | |
| COPD | 20.2 | |
| Self-rated health | Good to excellent | 66.4 |
| Fair to bad | 33.0 | |
| ≥ 4 medical consultations (12 months) | 72.7 | |
| ≥ 1 emergency department visit (12 months) | 34.8 | |
| ≥ 1 hospitalization (12 months) | 24.2 | |
| Use of home care services | 15.2 | |
| Follow-up in specialty clinic for primary diagnosis | 24.3 | |
| PHC affiliation type | Solo provider | 8.5 |
| Group practice | 34.3 | |
| Family medicine group | 21.5 | |
| Community clinic | 35.7 | |
|
PHC affiliation Taxonomic model |
Solo provider | 9.5 |
| Contact professional | 23.3 | |
| Coordination professional | 4.9 | |
| Professional integrated coordination | 28.6 | |
| Community | 33.6 | |
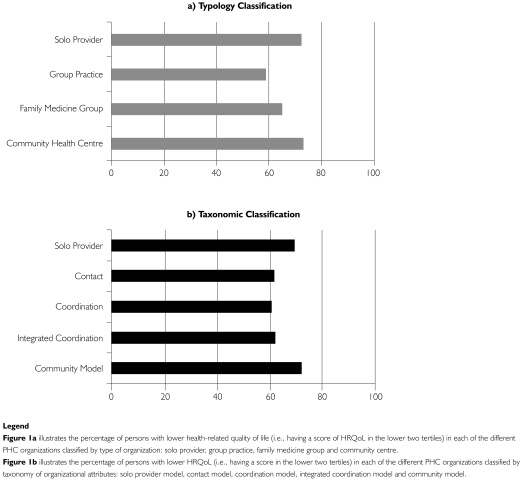
At the 18-month follow-up, 66.12% of participants had completed all of the four study interviews (513/776). Reasons for non-response included hospitalization or admission to long-term care (2.7%), death (9.4%), refusal to participate (41.6%) and loss to follow-up (46.4%). Non-respondents did not differ from respondents with respect to most sociodemographic characteristics. However, non-respondents had more co-morbidities (p=0.03), were more likely to be living with CHF (p<0.001) and were higher users of home care services (p<0.001). The distribution of PHC affiliation is also described in Table 2 for both type and taxonomic PHC organizational models. More than half of patients were followed by community health centres or FMGs. PHC organizational models differed with respect to case mix; community practices served older patients with significantly higher co-morbidity levels and lower self-rated health status. Figure 1 illustrates case mix with respect to HRQoL across PHC models, showing that group practices have the lowest proportion of patients in the lower HRQoL tertiles.
FIGURE 1.
Case mix at baseline as described by percentage of persons in the lower two tertiles of HRQoL across types of PHC organizations: (a) typology classification; (b) taxonomic classification
Unadjusted bivariate analyses using an aggregated HRQoL measure (0 to 100 scale) indicated modest but significant differences across PHC models. Patients followed in group practices reported higher HRQoL (67.3/100), while those affiliated with community health centres presented with lower HRQoL (61.5/100) (p<0.001). From a taxonomic perspective, contact as well as coordination models (integrated and non-integrated) were both associated with higher patient-reported HRQoL, with respective means of 65.1/100 and 67.5/100 (p=0.004) compared to community-oriented models (61.2/100, p<0.005).
No deterioration in health status (<5% decline in SF-36 score over the first 12 months) was calculated across organizational models. In our sample, 58.3% of participants experienced no deterioration in physical health, while 72.5% of them reported no deterioration in mental health. For HRQoL, 73.1% of patients reported no deterioration in their quality of life (≥5% decline) (Table 3).
TABLE 3.
Change in health status and HRQoL over 12 months (n=613)
| Mean Score at Baseline | Mean Score (12 Months) | “No Deterioration” (<5% Decline) Over 12 Months | |
|---|---|---|---|
| Health Status | |||
| FS-36 Physical (SD) | 52.97 (14.33) | 54.93 (21.82) | 58.3 |
| SF-36 Mental (SD) | 58.05 (9.25) | 64.97 (17.07) | 72.5 |
| HRQoL (0–100) | |||
| (SD) | 64.92 (21.67) | 64.83 (20.25) | 73.1 |
Table 4 displays results from a logistic regression analysis, modelling no deterioration (<5% deterioration vs. ≥5% deterioration) as a function of PHC organization, type of chronic disease, co-morbidity and sociodemographic variables. Patients with a primary diagnosis of diabetes and those who were older were less likely to have deteriorated in physical health. Persons with a primary diagnosis of heart failure and those born in Canada were less likely to decline in their mental health. No deterioration in HRQoL was associated with a primary diagnosis of diabetes. Neither type (Table 4) nor taxonomic model (data not shown) of PHC organization showed significant associations with change in physical or mental health status during the first year of follow-up. Factors associated with deterioration in health status were increased co-morbidity (for both physical and mental health) and lower perceived socio-economic status (mental health). None of the variables under study was significantly associated with deterioration in HRQoL.
TABLE 4.
Factors associated with no deterioration in health status or HRQoL over 12 months (logistic regression)
| Physical Health OR (95% CI) |
Mental Health OR (95% CI) |
HRQoL OR (95% CI) |
|
|---|---|---|---|
| Organization | |||
| Family medicine group | 0.97 (0.57,1.65) | 1.14 (0.64,2.04) | 0.94 (0.53,1.67) |
| Solo practitioner | 1.29 (0.59,2.83) | 0.85 (0.38,1.90) | 0.87 (0.38,1.99) |
| Group practice | 1.13 (0.66,1.93) | 1.59 (0.87,2.91) | 1.03 (0.58,1.83) |
| Community health clinic (REF) | 1 | 1 | 1 |
| Chronic Disease | |||
| Arthritis | 0.95 (0.54,1.67) | 1.10 (0.61,1.99) | 1.17 (0.64,2.15) |
| Heart failure | 1.74 (0.82,3.28) | 2.13 (1.16,3.91) | 0.68 (0.36,1.29) |
| Diabetes | 2.03 (1.16,3.55) | 1.69 (0.85,3.34) | 1.94 (1.05,3.59) |
| COPD (REF) | 1 | 1 | 1 |
| Co-morbidity | |||
| Number of co-morbid conditions | 0.78 (0.70,0.86) | 0.88 (0.78,0.98) | 0.92 (0.82,1.02) |
| Sociodemographics | |||
| Age | 1.02 (1.01,1.04) | 1.00 (0.98,1.02) | 1.01 (0.98,1.03) |
| Female sex | 0.94 (0.63,1.42) | 1.13 (0.72,1.78) | 1.25 (0.81,1.93) |
| College education | 1.15 (0.73,1.83) | 1.23 (0.73,2.08) | 1.06 (0.65,1.74) |
| Low self-perceived SES | 0.81 (0.49,1.33) | 0.45 (0.27,0.75) | 1.18 (0.68,2.03) |
| Canadian vs. immigrant | 1.54 (0.86,2.76) | 2.04 (1.12,3.73) | 1.35 (0.73,2.50) |
| Social support | 1.29 (0.26,6.39) | 2.28 (0.46,11.41) | 0.94 (0.17,5.31) |
| Single | 0.90 (0.59,1.38) | 0.78 (0.49,1.24) | 0.93 (0.59,1.47) |
Notes: REF = reference category.
Numbers in bold indicate statistically significant associations.
The multilevel logistic regression adjusted for patient covariates and clustering effects. In the model, the largest portion of the variance in HRQoL was attributed to individual factors, whereas only 4.3% was accounted for by organizational factors. None of the PHC organizational models were significantly associated with HRQoL. Nevertheless, lower severity in terms of having fewer co-morbid conditions, higher self-rated health and not using home care services were all associated with higher HRQoL (Table 5).
TABLE 5.
Factors associated with higher HRQoL* (multilevel logistic regression models)
| OR (95% CI) | ||
|---|---|---|
| Intercept | 0.59 (0.38; 0.96) | |
| PHC taxonomic model | Community-Oriented Model | Reference Category |
| Single Provider Model | 1.19 (0.56; 2.56) | |
| Contact Model | 1.42 (0.75; 2.71) | |
| Coordination Model | 1.48 (0.73; 3.00) | |
| Coordination Integrated Model | 0.83 (0.57; 1.23) | |
| Covariates | Followed by a specialist | 0.51 (0.33; 0.78) |
| Home care services user | 0.28 (0.17; 0.47) | |
| Number of co-morbidities | ||
| <3 | Reference Category | |
| 3–5 | 0.14 (0.02; 0.29) | |
| ≥6 | 0.44 (0.29; 0.68) | |
| Perceived health status good/excellent | 4.50 (2.95; 6.88) | |
| Age (years) | 0.98 (0.96; 0.99) | |
| Female sex | 1.25 (0.86; 1.82) | |
| Perceived SES | ||
| Rich | Reference Category | |
| Moderate | 0.69 (0.38; 1.29) | |
| Poor or very poor | 1.03 (0.59; 1.81) | |
| Time (repeated measures) | 0.98 (0.91; 1.06) | |
Upper tertile of HRQoL.
Numbers in bold indicate statistically significant associations.
Discussion
We found that PHC organizational models were not associated with HRQoL outcomes in patients with chronic disease over an 18-month follow-up. However, individual-level factors – such as older age, higher co-morbidity, worse perception of overall health, being followed in a specialty clinic and using home care services – were linked with lower HRQoL.
Differences in case mix may explain why we did not find differences in HRQoL outcomes among the various PHC organizational models. Despite the fact that community practices have patients with more complex health problems, their patients' HRQoL was as good as in any of the other models. We did attempt to adjust for case mix by including perceived health status, co-morbidity and use of home care services. Adjusting for case mix is tricky and may be prone to bias, because risks do not remain constant over time (Nicholl 2007). Further, persons with chronic disease who are in need of more comprehensive care may seek this care from certain PHC organizations precisely because of the ability of these organizations to respond to their needs.
Although we found that there is no difference between models in terms of HRQoL, there may be some reason to suspect that community models are preferable for managing patients with chronic disease. Community models of PHC organization may have greater facility for developing and implementing strategies that adequately compensate for higher levels of morbidity among their clientele. Physicians working in these community models were salaried rather than remunerated by fee for service, unlike those working in the other models. A salaried physician may benefit patients who have more complex needs (including those with more multi-morbidity) because physicians may spend a longer time with patients and be more apt to implement certain interventions that are not traditionally covered under the fee-for-service structure. Russell and colleagues (2009) reported that community health clinics in Ontario performed higher in chronic disease management process measures (especially with respect to evidence-based practices in diabetic care) than other PHC models. They also determined that the presence of a nurse practitioner was associated with higher disease management scores, whereas larger practices and higher patient load per physician were linked to lower scores. There was little effect on clinical outcomes, and HRQoL was not measured in their study.
A few studies have shown that specific organized programs may be effective in improving HRQoL outcomes in patients with chronic disease (Counsell et al. 2007; Scott et al. 2004). One intervention targeted older persons with chronic disease and consisted of a “cooperative healthcare clinic group model” in which patients met with their primary care physician, nurse and if needed, other health professionals on a monthly basis (Scott et al. 2004). A second intervention was aimed at low-income seniors and consisted of home-based care managed by a nurse and social worker in collaboration with the primary care physician and a geriatrics interdisciplinary team (Counsell et al. 2007). Both these interventions consisted of substantial comprehensive management programs, which may involve considerable expense. Neither type of program has been implemented in our context.
An observational study (Hung et al. 2006) also supports the notion that organizational attributes are linked to better patient outcomes. Hung and associates analyzed data on 4,735 patients in 57 primary care practice-based research networks and found that persons seen in hospital/university health system–owned practices and multi-specialty practices tended to have lower health status. Practices that used patient registries, had health promotion champions onsite and supported behavioural change had patients with better health status. However, unlike our study, patients included in their study did not necessarily have a chronic disease. Furthermore, the study was cross-sectional: it is unclear whether practices with more comprehensive care are geared more towards sicker patients, while those that champion prevention and promotion deal with healthier persons. In contrast to the studies mentioned above, Bosch and colleagues (2009) reported that PHC organizational factors (structured chronic care features) were not associated with HRQoL in patients with chronic heart failure.
We found that HRQoL was associated with individual patient-level factors. This finding is supported in the literature, as the following factors have been linked to lower HRQoL: lower education, lower income, female sex and younger age in an American nationwide cohort of patients followed in primary care (Hung et al. 2006); lower function and older age for persons with chronic heart failure followed in primary care (Bosch et al. 2009). Higher co-morbidity, disease burden, financial constraints and less social activity were associated with lower health status and physical function scores (Bayliss et al. 2003).
In our study, change in HRQoL differed among the four primary diagnostic categories; persons with diabetes were much less likely to have deteriorated over time. Results from an international study indicated that arthritis, chronic lung disease and congestive heart failure had the highest impact on SF-36 physical summary score (Alonso et al. 2004). Lam and Lauder (2000) reported that knee osteoarthritis is more disabling than hypertension and diabetes from the patient's point of view; however, they used a generic measure of function (the Dartmouth COOP Functional Health Assessment) and not a specific HRQoL questionnaire. Different measures of HRQoL may emphasize different conditions (Saarni et al. 2006). A common finding across all studies, including ours, is that co-morbidities reduce HRQoL in persons with chronic disease (Alonso et al. 2004; Keles et al. 2007; Lam and Lauder 2000).
There are several strengths and limitations to our study. A main strength is the longitudinal study design, enabling us to follow changes in patient outcomes. Our study had doctor-confirmed diagnoses as opposed to self-reported diagnoses, an approach that ensures the presence of specific chronic diseases. Also, we used validated, condition-specific questionnaires for HRQoL and a generic measure of health status (SF-36). The use of advanced analytical strategies, such as multilevel modelling, further contributes to the growing body of literature that seeks to link organizational or system-level data with patient outcomes.
In terms of limitations, the relatively small number of clinics that could be assessed through this study, as well as the uneven number of patients recruited in various clinics, may have limited our capacity to detect associations between organizational models and HRQoL measures. In addition, the follow-up was limited to 18 months, and there was not much change in health status or HRQoL over that period. This finding may be a result of the slow evolution of many chronic diseases, particularly in ambulatory patients, and the loss to follow-up of more severe cases. Another explanation may be the phenomenon of “response shift.” Persons may learn to adapt and cope with existing limitations, and thus their perspectives change. When individuals experience a change in their state of health, they may also change their internal standards (recalibration), their values (reprioritization) or the meaning (reconceptualization) of the target construct one is asking them to self-report, in this case HRQoL (Schwartz et al. 2004; Sprangers and Schwartz 1999). We did not look at individual organizational attributes and their associations with patient outcomes. Instead, we chose to view the organization as a whole; we analyzed organization according to the administrative type and also the taxonomic organizational model that uses organizational attributes in its classification. Finally, the 33 clinics that participated in the study, although similar to the 57 that did not participate (in terms of providing services to persons with chronic disease), differed from the entire population of clinics (n=473), the majority of which were not inclined to follow patients with chronic disease. Thus, our results may not be generalizable to all PHC clinics. Nevertheless, the results may be applicable to clinics that provide chronic disease services.
Conclusion
Our results provide useful insights that may help in guiding the development of PHC practice models to adequately meet the needs of patients with chronic conditions and improve health outcomes. It appears that organizations exhibiting more structured and comprehensive care processes cater to patients with more complex health needs. Our study highlights the challenge of linking organizational data to patient outcomes in view of differences in case mix, as well as the need to develop methodological solutions for this important area of research.
Acknowledgements
This study was funded by the Canadian Institutes of Health Research. We also acknowledge the support of the Institut national de santé publique du Québec, the Direction de santé publique de l'Agence de la santé et des services sociaux de Montréal and the Agence de la santé et des services sociaux de la Montérégie. We wish to thank Jean-Louis Larochelle, Dr. Raynald Pineault and Marjolaine Hamel for their valuable contribution to this project. Drs. Feldman and Lévesque both hold career awards from the Fonds de la recherche en santé du Québec.
Contributor Information
Debbie Ehrmann Feldman, Institut national de santé publique du Québec and Direction de santé publique de Montréal, Professor, Université de Montréal, Montreal, QC.
Jean-Frédéric Lévesque, Institut national de santé publique du Québec and Direction de santé publique de Montréal, Centre de recherche du Centre hospitalier de l'Université de Montréal, Montreal, QC.
Valérie Lemieux, Agente de planification, programmation et recherche, Direction de santé publique de Montréal, Montreal, QC.
André Tourigny, Institut national de santé publique du Québec, Scientific Director, Centre d'excellence sur le vieillissement de Québec, St-Sacrement Hospital, Montreal, QC.
Jean-Pierre Lavoie, Researcher, Centre de santé et de services sociaux Cavendish, Adjunct Professor, School of Social Work, McGill University, Montreal, QC.
Pierre Tousignant, Institut national de santé publique du Québec and Direction de santé publique de Montréal, Centre de recherche du Centre hospitalier de l'Université de Montréal, Associate Professor, McGill University, Montreal, QC.
REFERENCES
- Alonso J., Ferrer M., Gandek B., Ware J.E., Jr., Aaronson N.K., Mosconi P., Rasmussen N.K., Bullinger M., Fukuhara S., Kaasa S., Leplege A. 2004. “Health-Related Quality of Life Associated with Chronic Conditions in Eight Countries: Results from the International Quality of Life Assessment (IQOLA) Project.” Quality of Life Research 13: 283–98 [DOI] [PubMed] [Google Scholar]
- Bayliss E.A., Steiner J.F., Fernald D.H., Crane L.A., Main D.S. 2003. “Descriptions of Barriers to Self-Care by Persons with Comorbid Chronic Diseases.” Annals of Family Medicine 1: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M., van der Weijden T., Grol R., Schers H., Akkermans R., Niessen L., Wensing M. 2009. “Structured Chronic Primary Care and Health-Related Quality of Life in Chronic Heart Failure.” BMC Health Services Research 9: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley C., Todd C., Gorton T., Symonds E., Martin A., Plowright R. 1999. “The Development of an Individualized Questionnaire Measure of Perceived Impact of Diabetes on Quality of Life: The ADDQoL.” Quality of Life Research 8: 79–91 [DOI] [PubMed] [Google Scholar]
- Bruce B., Fries J.F. 2003a. “The Stanford Health Assessment Questionnaire: A Review of Its History, Issues, Progress and Documentation.” Journal of Rheumatology 30: 167–78 [PubMed] [Google Scholar]
- Bruce B., Fries J.F. 2003b. “The Stanford Health Assessment Questionnaire: Dimensions and Practical Applications.” Health and Quality of Life Outcomes 1: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell S.R., Callahan C.M., Clark D.O., Tu W., Buttar A.B., Stump T.E., Ricketts G.D. 2007. “Geriatric Care Management for Low-Income Seniors: A Randomized Controlled Trial.” Journal of the American Medical Association 298: 2623–33 [DOI] [PubMed] [Google Scholar]
- Greenhalgh J., Long A.F., Flynn R. 2005. “The Use of Patient Reported Outcome Measures in Routine Clinical Practice: Lack of Impact or Lack of Theory?” Social Science and Medicine 60: 833–43 [DOI] [PubMed] [Google Scholar]
- Guyatt G.H., King D.R., Feeny D.H., Stubbing D., Goldstein R.S. 1999. “Generic and Specific Measurement of Health-Related Quality of Life in a Clinical Trial of Respiratory Rehabilitation.” Journal of Clinical Epidemiology 52: 187–92 [DOI] [PubMed] [Google Scholar]
- Hung D.Y., Glasgow R.E., Dickinson L.M., Froshaug D.B., Fernald D.H., Balasubramanian B.A., Green L.A. 2008. “The Chronic Care Model and Relationships to Patient Health Status and Health-Related Quality of Life.” American Journal of Preventive Medicine 35: S398–S406 [DOI] [PubMed] [Google Scholar]
- Hung D.Y., Rundall T.G., Crabtree B.F., Tallia A.F., Cohen D.J., Halpin H.A. 2006. “Influence of Primary Care Practice and Provider Attributes on Preventive Service Delivery.” American Journal of Preventive Medicine 30: 413–22 [DOI] [PubMed] [Google Scholar]
- Keles H., Ekici A., Ekici M., Bulcun E., Altinkaya V. 2007. “Effect of Chronic Diseases and Associated Psychological Distress on Health-Related Quality of Life.” Internal Medicine Journal 37: 6–11 [DOI] [PubMed] [Google Scholar]
- Lam C.L., Lauder I.J. 2000. “The Impact of Chronic Diseases on the Health-Related Quality of Life (HRQoL) of Chinese Patients in Primary Care.” Family Practice 17: 159–66 [DOI] [PubMed] [Google Scholar]
- Maddigan S.L., Majumdar S.R., Guirguis L.M., Lewanczuk R.Z., Lee T.K., Toth E.L., Johnson J.A. 2004. “Improvements in Patient-Reported Outcomes Associated with an Intervention to Enhance Quality of Care for Rural Patients with Type 2 Diabetes: Results of a Controlled Trial.” Diabetes Care 27: 1306–12 [DOI] [PubMed] [Google Scholar]
- Mitchell P.H., Shortell S.M. 1997. “Adverse Outcomes and Variations in Organization of Care Delivery.” Medical Care 35: NS19–NS32 [DOI] [PubMed] [Google Scholar]
- Morgan M.W., Zamora N.E., Hindmarsh M.F. 2007. “An Inconvenient Truth: A Sustainable Healthcare System Requires Chronic Disease Prevention and Management Transformation.” Healthcare Papers 7: 6–23 [DOI] [PubMed] [Google Scholar]
- Nicholl J. 2007. “Case-Mix Adjustment in Non-Randomised Observational Evaluations: The Constant Risk Fallacy.” Journal of Epidemiology and Community Health 61: 1010–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham-Kanter G. 2009. “Social Comparisons and Health: Can Having Richer Friends and Neighbors Make You Sick?” Social Science and Medicine 69: 335–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineault R., Lévesque J.F., Roberge D., Hamel M., Lamarche P., Haggerty J. 2008. L' Accessibilité et la continuité des services de santé : une étude sur la première ligne au Québec. Rapport de recherche. Montréal: : Direction de santé publique de l'Agence de la santé et des services sociaux de Montréal, Institut national de santé publique, Centre de recherche de l'Hôpital Charles LeMoyne [Google Scholar]
- Raudenbush S.W., Bryk A.S. 2002. Hierarchical Linear Models: Applications and Data Analysis Methods (Advanced Quantitative Techniques in the Social Sciences). Thousand Oaks, CA: Sage Publications [Google Scholar]
- Rector T.S., Cohn J.N. 1992. “Assessment of Patient Outcome with the Minnesota Living with Heart Failure Questionnaire: Reliability and Validity During a Randomized, Double-Blind, Placebo-Controlled Trial of Pimobendan. Pimobendan Multicenter Research Group.” American Heart Journal 124: 1017–25 [DOI] [PubMed] [Google Scholar]
- Russell G.M., Dahrouge S., Hogg W., Geneau R., Muldoon L., Tuna M. 2009. “Managing Chronic Disease in Ontario Primary Care: The Impact of Organizational Factors.” Annals of Family Medicine 7: 309–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarni S.I., Harkanen T., Sintonen H., Suvisaari J., Koskinen S., Aromaa A., Lonnqvist J. 2006. “The Impact of 29 Chronic Conditions on Health-Related Quality of Life: A General Population Survey in Finland Using 15D and EQ-5D.” Quality of Life Research 15: 1403–14 [DOI] [PubMed] [Google Scholar]
- Schraeder C., Shelton P., Sager M. 2001. “The Effects of a Collaborative Model of Primary Care on the Mortality and Hospital Use of Community-Dwelling Older Adults.” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 56: M106–M112 [DOI] [PubMed] [Google Scholar]
- Schwartz C.E., Sprangers M.A., Carey A., Reed G. 2004. “Exploring Response Shift in Longitudinal Data.” Psychology and Health 19: 51–69 [Google Scholar]
- Scott J.C., Conner D.A., Venohr I., Gade G., McKenzie M., Kramer A.M., Bryant L., Beck A. 2004. “Effectiveness of a Group Outpatient Visit Model for Chronically Ill Older Health Maintenance Organization Members: A 2-Year Randomized Trial of the Cooperative Health Care Clinic.” Journal of the American Geriatrics Society 52: 1463–70 [DOI] [PubMed] [Google Scholar]
- Sprangers M.A., Schwartz C.E. 1999. “Integrating Response Shift into Health-Related Quality of Life Research: A Theoretical Model.” Social Science and Medicine 48: 1507–15 [DOI] [PubMed] [Google Scholar]
- SPSS Base 12.0 for Windows. 2003. Chicago: SPSS [Google Scholar]
- Stoddart H., Whitley E., Harvey I., Sharp D. 2002. “What Determines the Use of Home Care Services by Elderly People?” Health and Social Care in the Community 10: 348–60 [DOI] [PubMed] [Google Scholar]
- Wagner E.H., Austin B.T., Davis C., Hindmarsh M., Schaefer J., Bonomi A. 2001. “Improving Chronic Illness Care: Translating Evidence into Action.” Health Affairs 20: 64–78 [DOI] [PubMed] [Google Scholar]
- Ware J.E., Jr., Sherbourne C.D. 1992. “The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual Framework and Item Selection.” Medical Care 30: 473–83 [PubMed] [Google Scholar]
- Yach D., Hawkes C., Gould C.L., Hofman K.J. 2004. “The Global Burden of Chronic Diseases: Overcoming Impediments to Prevention and Control.” Journal of the American Medical Association 291: 2616–22 [DOI] [PubMed] [Google Scholar]