Abstract
HSV-specific CD8+ T cells provide constant immunosurveillance of HSV-1 latently infected neurons in sensory ganglia, and their functional properties are influenced by the presence of latent virus. In this study, we show that ganglionic HSV-specific CD8+ T cells exhibit a higher functional avidity (ability to respond to low epitope density) than their counterparts in noninfected lungs, satisfying a need for memory effector cells that can respond to low densities of viral epitopes on latently infected neurons. We further show that lack of CD4+ T cell help during priming leads to a transient inability to control latent virus, which was associated with a PD-1/PD-L1 mediated reduced functional avidity of ganglionic HSV-specific CD8+ T cells. CD4+ T cells are not needed to maintain CD8+ T cell memory through 34 d after infection, nor do they have a direct involvement in the maintenance of HSV-1 latency.
The typical CD8+ T cell response paradigm is illustrated during acute infections. Naive T cells initially encounter Ag in draining lymph nodes (DLNs), where they expand and acquire the functional program of effector cells. Subsequently, a polyfunctional Tc1 program including expression of the cytokines IFN-γ, TNF, and IL-2, and the lytic granule (LG) components perforin and granzyme B is associated with efficient viral eradication. After elimination of the infection, the effector T cell population undergoes contraction, with retention of a long-lived memory population that is maintained through homeostatic proliferation (1, 2).
In most infectious models CD4+ T cell help during the initial differentiation of naive CD8+ T cells is required for programming of CD8+ T cells for functional memory. Nonhelped CD8+ T cells develop a memory population that exhibits functional defects and provides an inadequate recall response on subsequent exposure to Ag (3, 4). Emerging from recent studies is a scenario in which CD4+ T cells augment the programming of CD8+ T cells directly through the production of IL-2 (5, 6), and indirectly by enhancing the provision of costimulatory signals by dendritic cells (7–9).
A departure from this T cell response paradigm is observed during persistent infections with viruses such as lymphocytic choriomeningitis virus (LCMV), hepatitis C virus, and HIV (10–12). The initial programming of CD8+ T cells appears to be dysregulated, resulting in functional exhaustion of the CD4+ and CD8+ memory T cell populations. In persistent infections induced by LCMV clone 13, the exhaustion of virus-specific memory CD8+ T cells is characterized by the serial loss of production of IL-2, TNF, IFN-γ, and granzyme B (10, 13). The CD8+ T cell exhaustion appears to result in part from exposure to IL-10, and in part from their acquisition of inhibitory receptors such as PD-1, LAG-3, and CD160 (14, 15). Interestingly, mice that are deficient in CD4+ T cells at the time of clone 13 LCMV infection exhibit a more profound CD8+ T cell functional exhaustion and never clear the virus (10, 16, 17). Thus, CD4+ T cell help during the initial programming of LCMV-specific CD8+ T cells attenuates the aberrant programming that leads to functional exhaustion.
A hallmark of the herpesvirus family is their capacity to induce latent infections in which the viral genome persists for prolonged periods without production of infectious virions. Recent studies demonstrate that latency with many herpesviruses is maintained through constant immunosurveillance by CD8+ T cells (18–21). Studies in murine models of HSV-1 latency demonstrate that HSV-specific CD8+ T cells are persistently exposed to viral Ags, forming immunologic synapses and releasing LGs into the junction with latently infected neurons (22–25). Despite persistent antigenic exposure, these CD8+ T cells do not become functionally exhausted. This might reflect the fact that replicating virus is rapidly eradicated during acute infection, preventing prolonged Ag exposure during the programming of CD8+ T cells. Once programming is complete, persistent exposure to low levels of Ag does not appear to have an adverse effect on the virus-specific memory T cell population. Interestingly, CD4+ T cell deficiency during the acute phase of murine gamma herpesvirus 68 infection renders virus-specific memory CD8+ T cells incapable of maintaining viral latency, suggesting that nonhelped CD8+ T cells might be susceptible to functional exhaustion in the context of a latent viral infection (26, 27).
In this study, we show that CD4+ T cell deficiency during the programming of HSV-specific CD8+ T cells gives rise to a memory population that exhibits transient partial exhaustion characterized by elevated PD-1 levels and a reduced functional avidity that is associated with reduced ability to control HSV-1 latency in sensory ganglia.
Materials and Methods
Mice and virus
HSV-1 strain RE was grown in Vero cells, and intact virions were isolated on Optiprep gradients according to manufacturer’s instructions (Accurate Chemical and Scientific, Wesbury, NY). Six- to 8-wk-old female wild-type C57BL/6 mice were anesthetized by i.p. injection of 2.0 mg ketamine hydrochloride and 0.04 mg xylazine (Phoenix Scientific, San Marcos, CA) in 0.2 ml HBSS (BioWhittaker, Walkersville, MD). The abraded central corneas of anesthetized mice were infected by topical application of 3 μl RPMI (BioWhittaker) containing 1 × 105 PFU of HSV-1. All animal experiments were conducted in accordance with guidelines established by the University of Pittsburgh Institutional Animal Care and Use Committee.
Tissue preparation
At the indicated days postinfection (dpi), anesthetized mice were sedated and euthanized by perfusion with sterile PBS. Prior to perfusion DLNs were harvested. Trigeminal ganglions (TGs) and lungs were digested in 100 μl (TGs) or 1 ml (lungs) of DMEM (BioWhittaker) containing 10% FCS (Atlanta Biologicals, Norcross, GA) and 400 U/ml collagenase type I (Sigma-Aldrich, St. Louis, MO) for 1 h at 37°C. Tissues were then dispersed into single-cell suspensions and treated with RBC lysis buffer before staining with the designated antibodies. Data were collected on a FACSAria cytometer and analyzed by FACSDiva software (BD Biosciences, San Jose, CA).
Reagents
The gB498–505 (SSIEFARL) peptide was purchased from Research Genetics (Huntsville, AL). PE-conjugated H-2Kb tetramers complexed with the gB498–505 peptide were kindly provided by the NIAID Tetramer Core Facility (Emory University Vaccine Center, Druid Hills, GA). Rat anti-mouse allophycocyanin-alexa750-conjugated and Pacific Blue-conjugated anti-CD8α (clone 53-6.7); FITC-conjugated rat anti-mouse CD107a (1d45), PE-Cy7-conjugated rat anti-mouse CD4 (RM4-5) and PD-1 (RMP1-30), and anti-mouse TNF (MP6-XT22); allophycocyanin-conjugated rat anti-mouse IFN-γ (XMG1.2), and PerCP-conjugated rat anti-mouse CD45 (30-F11) were purchased from BD Pharmingen (San Diego, CA). The appropriate isotype control Abs were purchased from BD Pharmingen or eBiosciences (San Diego, CA).
In vivo mAb treatment
Mice were treated with an i.p injection of 0.15 mg rat anti-mouse CD4 Ab clone GK1.5 (BioXcell, West Lebanon, NH) for in vivo depletion of CD4 T cells. For depletions prior to infection, injections were given 2 d before infection, followed by 1, 3, 8, 15, 22, 29, 36, 43, and 50 dpi. For depletions during contraction, treatments were given at 8, 15, 22, and 29 dpi. For PDL1 blockade, mice were treated with 0.2 mg rat anti-mouse PDL1 Ab clone 10F.9G2 (BioXcell) every 3 d for 2 wk before the desired observation point.
Phenotypic analysis of T cells
For all phenotypic analyses, cells were stained for CD45 to permit gating exclusively on bone marrow–derived cells. For analysis of CD8 and CD4 T cell populations and their phenotype, cells were stained with anti-CD8α and anti-CD4. For analysis of HSV-specific CD8+ T cells (gB-CD8s), cells were additionally stained with anti-CD8α and gB498–505 H-2Kb tetramers.
Fluorescently assisted cell sorting
At indicated time points, lungs and DLNs were dispersed into single-cell suspension and stained with anti-CD8 and gB498–505 H-2Kb tetramers. Cells were sorted at high purity (purity-32) for CD8+ gB tetramer+ cells on a FACSAria cytometer (BD Biosciences).
Intracellular cytokine staining and LG exocytosis
Dispersed TG cells or postglycoprotein B (gB) tetramer sorted cells were stimulated directly with either 10−6 M (optimal) or 10−11 M (suboptimal) gB498–505 peptide pulsed B6WT350 fibroblast targets in the presence of FITC-conjugated anti-CD107a mAb and Golgi-plug (BD Biosciences) for 6 h at 37°C/5% CO2. The optimal and suboptimal peptide concentrations are based on previous studies (28). After stimulation, cells were stained for surface expression of CD8α, followed by intracellular staining for IFN-γ and TNF-α after permeabilization and fixation via Cytofix/Cytoperm (BD Biosciences). CD107a capture on the cell surface during stimulation provides sensitive detection of LG exocytosis as previously described (29, 30).
Tetramer release assay
Tetramer release assay was performed as described (31). Single-cell TG suspensions were stained with gB498–505 tetramer for 1 h at 37°C, the cells were then washed and incubated with anti-H-2Db/Kb Ab to avoid tetramer rebinding (28-8-6; BD Pharmingen) at 37°C for the designated times. Cells were then stained with anti-CD8α and anti-CD45 mAB, and analyzed via flow cytometry to observe loss of tetramer over time. Data are CD8+ tetramer+ cells as a percentage mean fluorescence intensity (MFI) ± SEM of the maximal binding observed at time zero (n = 10 mice per group).
Detection of infectious virus on corneas
The corneal surfaces of mice were swabbed with sterile Weck-Cel surgical spears (Medtronic Solan, Jacksonville, FL) on days 2, 4, 6, and 8 after HSV-1 infection; spears were placed in 0.5 ml RPMI and frozen at −80°C until assayed. Samples were added to confluent Vero cells, incubated for 1 h at 37° C, and overlayed with 0.5% methylcellulose. The cultures were incubated for 72 h, fixed with formalin, and stained with crystal violet. Viral cytopathic effect was detected with the aid of a dissecting microscope.
Quantitative real-time PCR
Total DNA was isolated from single-cell TG suspensions using DNeasy columns according to manufacturer’s instructions (Qiagen, Valencia, CA). DNA was quantified by spectrophotometry and diluted to 1 ng/μl in nuclease-free dH2O. Twenty-five nanograms DNA or water control was mixed in duplicate with a 25-μl mixture of TaqMan Universal PCR Master Mix (Roche, Basel, Switzerland) and an HSV-1 glycoprotein H (gH)-specific primer-probe set, custom designed and synthesized by ABI Assays-by-Design service (Applied Biosystems, Foster City, CA). Samples (50 μl per well) were assayed in 96-well plates with an ABI Prism 7700 sequence detector. ABI Primer Express v1.5a software default settings were used for instrument control and data analysis (Applied Biosystems). The gH sequences were: forward primer (5′-CGACCACCAGAAAACCCTCTTT-3′), reverse primer (5′ACGCTCTCGTCTAGATCAAAGC-3′), and probe [5′-(FAM)TCCGGACCATTTTC(NFQ)-3′].
Statistics
All statistical analyses were performed with GraphPad Prism 5 (San Diego, CA) software using a two-tailed unpaired t test with 95% confidence.
Results
CD4+ T cell ablation influences the maintenance of HSV-1 latency
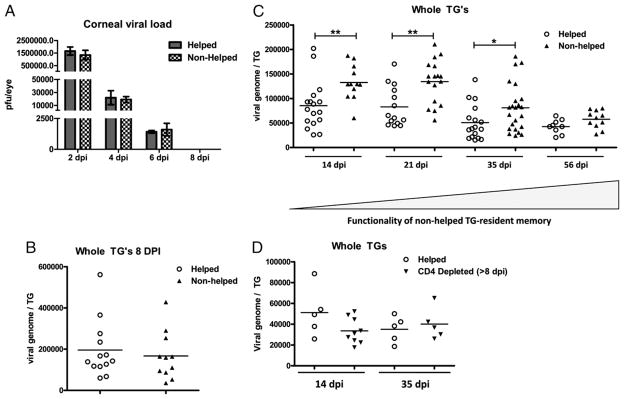
Initial studies tested the effect of CD4+ T cell ablation starting before HSV-1 corneal infection on the clearance of virus from the cornea, the establishment of viral latency in the TG, and on the maintenance of latency. Our studies used an Ab treatment protocol that effectively ablated CD4+ T cells from mice beginning 2 d before HSV-1 corneal infection and continuing through 60 dpi (Supplemental Fig. 1). In this study, we show that depletion of CD4+ T cells through 8 dpi did not influence the clearance of HSV-1 from the cornea (Fig. 1A), the kinetics of latency establishment within the TG (not shown), or the load of latent viral genome at 8 dpi (Fig. 1B). However, the CD4+ T cell ablated mice failed to maintain the virus in a latent state between 8 and 35 dpi, as indicated by a significant increase in the viral genome copy number in the TG (Fig. 1C). The latent viral load returned to normal levels by 56 dpi in CD4+ T cell ablated mice. The failure of CD4+ T cell ablated mice to control the early stages of HSV-1 latency did not reflect a direct requirement for CD4+ T cells in control of viral latency because initiating CD4 depletion at 8 dpi did not result in a similar increase in the latent viral load (Fig. 1D).
FIGURE 1.
CD4+ T cell ablation influences the maintenance of HSV-1 latency. B6 mice that received HSV-1 corneal infections were continuously depleted of CD4+ T cells beginning 2 d before (A–C) or 8 d after (D) infection. A, At the indicated dpi, eyes were swabbed with a sterile surgical spear and assayed for live virus by standard plaque assay. The data are presented as the mean ± SEM viral PFUs per cornea of nondepleted (helped) mice and those continuously CD4-depleted starting at −2 dpi (nonhelped). B–D, At the indicated dpi, DNA was extracted from TGs of and the number of copies of HSV-1 genome harbored in each TG was determined by quantitative real time PCR for the gH gene. Data from individual TGs are presented (B and C) as viral genomes per TGs of helped and nonhelped mice. D, Viral genomes per TG of nondepleted (helped) mice and those CD4-depleted starting 8 dpi (CD4-depleted > 8 dpi). *p < 0.05; **p < 0.01; n = 5–23 mice per group as indicated in scatter plots.
CD4+ T cell help does not influence the size of the HSV-specific CD8+ T cell effector or memory pool
We previously demonstrated that diminished function of memory HSV-specific CD8+ T cells within latently infected TGs was associated with failure to maintain latency, as indicated by an increase in viral genome copy number (32, 33). Therefore, we hypothesized that the absence of CD4+ T help during the first 8 d after HSV-1 infection might have resulted in an HSV-specific CD8+ effector or memory T cell population within the TG that was incapable of maintaining HSV-1 latency. We used an HSV-1 corneal infection model in C57BL/6 (B6) mice in which tetramers containing an immunodominant glycoprotein B (gB498–505) epitope were used to identify and quantify a majority of gB-CD8s.
The number of CD4+ T cell–helped (helped) and CD4+ T cell–nonhelped (nonhelped) gB-CD8s peaked at similar levels in the DLNs (data not shown) and the infected TG (Fig. 2) at 8 dpi. In the infected TG, the nonhelped gB-CD8s underwent a more rapid contraction between 8 and 14 dpi (Fig. 2A), but ultimately established a stable and numerically normal memory population (Fig. 2A). CD4+ T cell ablation starting after the peak of the effector response (8 dpi) resulted in accelerated contraction similar to that seen when CD4+ T cells were ablated from the time of infection, suggesting a direct role for CD4+ T cells in gB-CD8 T cell contraction (Fig. 2B).
FIGURE 2.
CD4+ T cell help does not influence the size of the gB-CD8+ T cell effector or memory population. B6 mice that received HSV-1 corneal infections were continuously depleted of CD4+ T cells beginning 2 d before (A) or 8 d after (B) infection. At the indicated dpi, TGs were excised and gB-CD8s were quantified by simultaneous staining with anti-CD8α mAb and tetramers containing the immunodominant HSV-1 gB498–505 epitope (gB498–505 H2-Kb). A, Mean ± SEM of the absolute number of gB-CD8s per TG in nondepleted (helped) and continuously CD4-depleted starting −2 dpi (nonhelped) mice. B, Mean ± SEM of the absolute number of gB-CD8s per TG in nondepleted (helped) and continuously CD4-depleted starting +8 dpi (CD4-depleted > 8 dpi) mice. *p < 0.05; ***p < 0.001; n = 10–15 mice per group.
Nonhelped gB-CD8s exhibit functional alterations upon infiltrating the infected TG
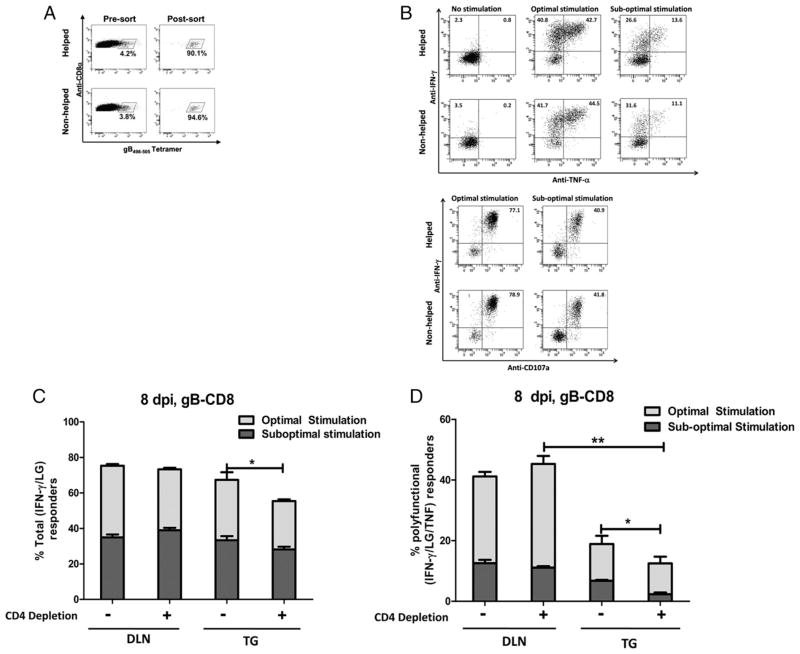
In this study we quantified four separate populations of gB-CD8s. As previously reported (28), one population of low functional avidity gB-CD8s produces effector cytokines only in response to an optimal epitope density (1 × 10−6 M gB498–505 peptide loaded fibroblasts); whereas a second high functional avidity population responds to both optimal and suboptimal (1 × 10−11 M gB498–505 peptide loaded fibroblasts) epitope densities. Both the high and low functional avidity gB-CD8 populations contain subpopulations that express different functional programs: all responding gB-CD8s produce IFN-γ and release LGs (IFN-γ/LG) when stimulated, but a subpopulation here referred to as polyfunctional also produces TNF-α (IFN-γ/TNF/LG). No gB-CD8s were single producers of TNF-α. These four subpopulations are illustrated in Fig. 3B.
FIGURE 3.
Nonhelped gB-CD8s exhibit functional alterations upon infiltrating the infected TG. B6 mice that received HSV-1 corneal infections were not depleted or continuously depleted of CD4+ T cells beginning 2 d before infection. A, DLNs were excised at 8 dpi, dispersed into single cell suspensions, and stained simultaneously with CD8α mAb and gB498–505 tetramer. Tetramer-positive CD8+ T cells were sorted via FACS with a purity of >90%, as illustrated in representative dot plots. B–D, Sorted DLN cells or dispersed TG cells were stimulated for 6 h with the B6WT350 fibroblast cell line loaded with gB498–505 peptide at optimal (1 × 10−6 M) or suboptimal (1 × 10−11 M) density, in the presence of the Golgi transport inhibitor brefeldin-A and FITC-conjugated anti-CD107a Ab, followed by intracellular staining for cytokines IFN-γ and TNF-α. B, Data are representative flow plots depicting background activation from tetramer binding, as well as IFN-γ and TNF-α production and LG release (CD107a+) following optimal and suboptimal gB peptide stimulation. C and D, Bars represent mean ± SEM total responder cells (IFN-γ/LG) or polyfunctional responder (IFN-γ/LG/TNF) cells from both the DLNs and TG at 8 dpi. The total bar represents response to optimal Ag, and the dark gray bar represents response to suboptimal Ag. *p < 0.05; **p < 0.01; n = 8–10 mice per group.
We sorted for gB-CD8s in the DLN at 8 dpi to test their functionality (Fig. 3A). Within the DLN, ~75% of both helped and nonhelped gB-CD8s responded to optimal gB peptide stimulation, and of these ~50% showed high functional avidity by responding to a suboptimal gB peptide dose (Fig. 3C). In the TG at 8 dpi, the mean frequency of helped gB-CD8s that responded to optimal gB peptide stimulation (67%) was not significantly different from their counterparts in the DLNs (Fig. 3C). The frequency of nonhelped gB-CD8s in the TG at 8 dpi that responded to optimal gB peptide stimulation (55%) was significantly reduced relative to their counterparts in the DLN (p < 0.05) and to the helped gB-CD8s in the TG (p < 0.05). The frequencies of high functional avidity helped and nonhelped gB-CD8 in the TG at 8 dpi were not significantly different from each other or from their counterparts in the DLN (Fig. 3C).
The most dramatic difference between the gB-CD8 effector cells in the DLN and TG at 8 dpi was in the low frequency of polyfunctional (IFN-γ/LG/TNF) cells in the TG (Fig. 3D). Although both the helped and nonhelped gB-CD8 in the TG showed a marked reduction in the frequency of polyfunctional cells in response to optimal gB peptide stimulation relative to their counterparts in the DLN, high functional avidity polyfunctional cells were nearly undetectable in the nonhelped gB-CD8s in the TG.
Early CD4+ T cell help is required to avert gB-CD8 functional compromise
The frequency of helped gB-CD8s in the TG that responded to an optimal gB peptide stimulation remained constant during 8–56 dpi (Fig. 4A). Interestingly, the frequency of those cells that exhibited a high functional avidity by responding to a suboptimal gB peptide dose increased dramatically over the same period (Fig. 4A). In contrast, the nonhelped gB-CD8s showed a sharp decline in functionality at 8–14 dpi, when only 14% exhibited a high functional avidity. At 14–35 dpi, the frequency of nonhelped gB-CD8s that responded to an optimal dose of gB peptide declined further, but the frequency of high functional avidity cells increased. The frequency of both populations remained significantly reduced (p < 0.05), relative to the helped gB-CD8s at 35 dpi. By 56 dpi, the nonhelped and the helped gB-CD8s showed the same frequency of cells capable of responding to an optimal and suboptimal dose of gB peptide.
FIGURE 4.
Early CD4+ T cell help is required to avert gB-CD8 functional compromise. B6 mice that received HSV-1 corneal infections were not depleted or continuously depleted of CD4+ T cells beginning 2 d before infection (nonhelped, A and B), or beginning at 8 dpi (C). At the indicated dpi, dispersed TG cells were stimulated with an optimal or suboptimal dose of gB peptide and IFN-γ, and TNF-α production and LG release were quantified as in Fig. 2. Data are presented as (A) mean percent ± SEM of gB-tetramer+ CD8+ T cells (gB-CD8) from CD4-depleted and nondepleted mice that responded (IFN-γ/LG) to optimal or suboptimal gB peptide stimulation, (B) mean percent ± SEM of polyfunctional (IFN-γ/LG/TNF) gB-CD8 in response to optimal or suboptimal gB peptide stimulation, and (C) mean percent ± SEM of total (IFN-γ/LG) or polyfunctional (IFN-γ/LG/TNF) gB-CD8 in response to optimal or suboptimal gB peptide stimulation at 35 dpi. *p < 0.05; **p < 0.01; ***p < 0.001; n = 8–10 mice per group.
It is noteworthy that in addition to an increased frequency of high functional avidity cells within the overall gB-CD8 responding population in the TG at 8–56 dpi (IFN-γ/LG; Fig. 4A), the helped gB-CD8s also exhibited a gradual increase in the polyfunctional (IFN-γ/LG/TNF) subpopulation (Fig. 4B), and most of these cells exhibited high functional avidity by 56 dpi. Thus, in the helped gB-CD8 population in the TG, there was a marked enrichment of polyfunctional and high functional avidity cells over time within the latently infected TG. In contrast, at 8–14 dpi the nonhelped gB-CD8 exhibited a further decline in polyfunctional cells, which was particularly prominent among the high functional avidity cells that represented only 4% of the nonhelped gB-CD8s at 14 dpi (Fig. 4B). This reduced functionality combines with a reduced number of nonhelped gB-CD8s in the TG at 14 dpi (Fig. 2A) to define a highly compromised gB-CD8 population. The frequency of polyfunctional cells within the nonhelped gB-CD8 population increased at 14–35 dpi, but remained significantly reduced (p < 0.01) relative to their helped counterparts after optimal and suboptimal gB peptide stimulation. By 56 dpi, the nonhelped gB-CD8s regained full functionality. The augmentation of gB-CD8 functionality was imprinted by CD4+ T cells during the first 8 d after infection, because depletion of CD4+ T cells beginning at 8 dpi had no effect on gB-CD8 function at 35 dpi (Fig. 4C).
Latently infected TGs selectively promote the enrichment of high functional avidity gB-CD8 during establishment of memory
We have previously demonstrated that the microenvironment of the latently infected TG can influence functional properties of the resident gB-CD8 memory population, presumably owing to persistent low-level exposure to viral Ags (28). To determine whether the increased frequency of polyfunctional and high functional avidity gB-CD8s was unique to latently infected TGs, we analyzed the functionality of gB-CD8s in the noninfected lungs of the same mice at 35 and 56 dpi (Fig. 5A). As shown in Fig. 5B and 5C, the gB-CD8 population in the lungs already showed a high frequency of polyfunctional cells by 35 dpi, and these remained high at 56 dpi. Thus, the acquisition of polyfunctional cells over time appeared to be inherent to the transition of gB-CD8s into memory. In contrast, the frequency of high functional avidity cells within both the overall responding gB-CD8 population (IFN-γ/LG) and within the polyfunctional (IFN-γ/LG/TNF) population declined significantly (p < 0.01) between 35 and 56 dpi, when they constituted only 22% of the gB-CD8 population. The selective increase (total response p < 0.01; polyfunctional p < 0.001) in high functional avidity cells in the TG relative to the lungs is illustrated in Fig. 5D and 5E. Although acquisition of polyfunctionality appears to occur normally in gB-CD8s during the transition from an effector to memory population, latently infected tissue appears to provide a selective pressure for accumulation of high functional avidity gB-CD8.
FIGURE 5.
Latent virus influences some of the functional changes that occur in gB-CD8 during the establishment of memory. A, Lungs were harvested at 35 or 56 dpi, dispersed cells were stained with gB498–505 tetramers, and sorted gB-CD8 were ≥ 90% gB-tetramer+ as illustrated in a representative dot plot. Sorted gB-CD8s were stimulated with an optimal or suboptimal dose of gB peptide and IFN-γ and TNF-α production and LG release were quantified as in Fig. 2. B, Representative flow plots depicting background activation from tetramer binding, as well IFN-γ, TNF-α, and LG responses to optimal and suboptimal gB peptide stimulation. C, Bars represent mean ± SEM of total (IFN-γ/LG) responders or polyfunctional (IFN-γ/LG/TNF) responders. The total bar represents the frequency of cells responding to optimal gB peptide stimulation, and the dark gray bar represents the frequency of high functional avidity cells responding to a suboptimal gB peptide dose. Bars represent the frequency of high functional avidity total (D) (IFN-γ/LG) and polyfunctional (E) (IFN-γ/LG/TNF) gB-CD8 responders in the TG and lungs. *p < 0.05; **p < 0.01; ***p < 0.001; n = 8–10 mice/group.
TCR affinity does not correlate with functional avidity in nonhelped gB-CD8
The reduced functionality of nonhelped gB-CD8s in the TG at 35 dpi could be due to low TCR affinity or exposure to a variety of immunomodulatory signals in vivo (31, 34, 35). To differentiate these two types of influences, we used a tetramer release assay to compare the TCR affinity of helped and nonhelped gB-CD8s obtained from TGs at 35 dpi. As illustrated in Fig. 6, the non-helped gB-CD8s exhibited an average TCR affinity that was essentially identical to that of the helped gB-CD8s, suggesting that the reduced functionality of the nonhelped gB-CD8s detected directly ex vivo resulted from in vivo exposure to immunoregulatory influences.
FIGURE 6.

TCR affinity does not correlate with functional compromise in nonhelped memory. TGs were harvested and dispersed at 35 dpi. TG suspensions were stained with gB498–505 tetramer and incubated with anti-H-2Db/Kb Ab at 37°C for the designated times to observe tetramer dissociation. Cells were then stained with anti-CD8α and anti-CD45 mAb, and analyzed via flow cytometry. Data represent the MFI of CD8+ tetramer+ cells as a percentage ± SEM of the maximum MFI observed at time zero. n = 10 mice per group.
The reduced functionality of nonhelped gB-CD8 is associated with increased PD-1 expression
We next determined whether the functional compromise observed in nonhelped gB-CD8s in TGs at 8–35 dpi was associated with enhanced expression of the inhibitory receptor PD-1. At 8 dpi, PD-1+ expression was significantly elevated on nonhelped gB-CD8s in both the DLN (Fig. 7A) and TG (Fig. 7B, 7C). Increased PD-1 expression was manifest as both an increased frequency and increased level (MFI) of PD-1 expression. The frequency of PD-1+ nonhelped gB-CD8s gradually declined in the TG, but remained significantly elevated through 35 dpi. The increased PD-1 expression during this time was associated with reduced functionality (Fig. 5A, 5B). By 56 dpi, PD-1 expression on nonhelped gB-CD8s in the TG was further reduced and no longer significantly different from that on helped gB-CD8s; this was concurrent with complete functional recovery.
FIGURE 7.
The dysregulated function of nonhelped gB-CD8s is associated with increased PD-1 expression. B6 mice that received HSV-1 corneal infections were not depleted or continuously depleted of CD4+ T cells beginning 2 d before infection. DLNs were excised at 8 dpi, TGs were excised at the designated dpi, and dispersed cells were stained simultaneously with anti-CD8α, anti-CD45, anti-PD1, and gB498–505 tetramer and analyzed via flow cytometry. Representative dot plots illustrate PD1 expression (based on isotype control) by (A) non–CD4-depleted (helped) gB-CD8 in DLN at 8 dpi and (B) helped and CD4-depleted (nonhelped) gB-CD8 in the TG at 8, 21, and 56 dpi. The MFI and frequency of the PD1+ cells is indicated in each plot (C). Symbols represent the mean ± SEM frequency of PD1+ helped and nonhelped gB-CD8 in the TG at the designated dpi. The wedge under the graph represents the corresponding change in functionality. *p < 0.05; **p < 0.01; ***p < 0.001; n = 8–10 mice per group.
Blockade of PD-L1 restores function to nonhelped gB-CD8 in the TG
Because transient functional dysregulation of nonhelped gB-CD8s in the TG was associated with elevated PD-1 expression, we predicted that blocking PD-1/PD-L1 interaction would restore normal function to these cells. TGs were obtained from mice at 35 or 56 dpi following 2 wk of systemic treatment with anti-PD-L1 or control mAb. At 35 dpi the nonhelped gB-CD8s in the TGs of mice that were treated with control mAb exhibited the anticipated reduction in the frequency of total gB-CD8s (Fig. 8A) and poly-functional (Fig. 8C) capable of responding to gB peptide-pulsed targets. However, functionality was completely restored to the nonhelped gB-CD8s following 2 wk of anti-PD-L1 mAb treatment (Fig. 8A, 8C). In contrast, anti-PD-L1 treatment did not influence the function of helped gB-CD8 in TG at 35 dpi or the function of helped or nonhelped gB-CD8s in the TGs at 56 dpi (Fig. 8B, 8D).
FIGURE 8.
Blockade of PD-1L restores function to nonhelped gB-CD8 in the TG. Untreated and CD4-ablated mice were treated via i.p. injection of 200 μg anti-PDL1 blocking or isotype control mAb for 2 wk prior to 35 dpi (A and C) and 56 dpi (B and D). Dispersed TGs were stimulated with an optimal or suboptimal dose of gB peptide, and IFN-γ and TNF-α production and LG release were quantified as in Fig. 2. Bars represent the mean ± SEM frequency of total (IFN-γ/LG) or polyfunctional (IFN-γ/LG/TNF) helped (from nondepleted mice) and nonhelped (from CD4-depleted mice) gB-CD8s in control (isotype) and anti-PD-L1 (αPDL1)-treated mice. The total bar represents response to optimal Ag, and the dark gray bar represents response to suboptimal Ag. *p < 0.05; **p < 0.01; n = 10 mice per group.
Discussion
The involvement of CD4+ T cell help in CD8+ T cell responses is complex and likely influenced by factors such as the nature of the immunogen, the activation status of APCs, and the microenvironment in which the effector and memory CD8+ T cells reside. In this study, we use a unique model system in which CD8+ T cell priming occurs in the context of an acute HSV-1 infection, but the functional properties of the resulting HSV-specific CD8+ T cell memory population can then be examined within disparate tissue microenvironments with or without persistent antigenic exposure (28). Moreover, in this model, persistent low-dose antigenic exposure in latently infected ganglia does not lead to CD8+ T cell functional exhaustion, as seen with chronic viral infections that are characterized by extended exposure to high levels of viral Ags after initial infection. In this model, long-term functionality of the ganglionic gB-CD8+ T cell population is required to maintain the virus in a latent state (24, 32, 33). We use this model system to explore the influence of CD4+ T cells on the gB-CD8 response.
We demonstrate that CD4+ T cells influence the gB-CD8 response in two ways. First, the gB-CD8 effector population in the TG undergoes a more rapid contraction in the absence of CD4+ T cells. This effect is exerted at the time of contraction, versus during the initial programming of the naive gB-CD8, because contraction is accelerated when CD4+ T cell ablation is initiated before infection or after the peak accumulation of effector gB-CD8 in the TG at 8 dpi. The mechanism by which CD4+ T cells influence CD8+ T cell contraction is currently unclear.
Accelerated contraction alone did not influence the functional characteristics, immunosurveillance capability, or size of the gB-CD8 memory pool established in the TG. Mice that were depleted of CD4+ T cells beginning at 8 dpi showed an ~50% reduction in gB-CD8 at 14 dpi, but the capacity of the remaining CD8+ T cells to maintain HSV-1 latency was not compromised, as indicated by a similar latent HSV-1 genome copy number in TGs. This finding is probably a reflection of the fact that the remaining gB-CD8 were functionally normal, and at any given time only a small number of latently infected neurons appear to require CD8+ T cell protection from reactivation (36). These findings establish that CD4+ T cells are not required for either the establishment or maintenance of HSV-1 latency in the TG following corneal infection.
Of greater consequence was the influence of CD4+ T cells on the initial programming of gB-CD8. Depriving mice of CD4+ T cell help during the initial programming and expansion phase of the CD8+ T cell response to HSV-1 had no effect on the size of the gB-CD8 effector population in the TG at 8 dpi. This contrasts with findings from a flank HSV-1 infection model, in which CD4 T cell deprivation resulted in reduced gB-CD8 effector expansion (37). Important differences in the two studies include the strain of HSV-1 used and the route of infection, both of which might influence the amount of CD4+ T cell help required for the expansion of HSV-specific CD8 effector cells. We also saw no functional differences between the helped and nonhelped gB-CD8 effector cells in the DLNs at 8 dpi. However, the nonhelped gB-CD8 did exhibit a progressive functional compromise following infiltration of the TG at 8–35 dpi. The compromise was initially most pronounced among the high functional avidity polyfunctional (IFN-γ/LG/TNF) cells at 8 dpi, but progressive loss of the capacity to produce IFN-γ and release LGs was observed at 14 and 35 dpi. The concurrent nature of the functional compromise in the nonhelped gB-CD8 population in the TG and escape of HSV-1 from latency, as indicated by elevated HSV-1 genome copy number, underlines the importance of gB-CD8 functionality in maintaining HSV-1 in a latent state.
We previously demonstrated that gB-CD8s are maintained in the latently infected TG of IL-15 knockout mice, but lost in their noninfected lungs (28). Those findings were consistent with the notion that exposure to viral Ags within the TG supplants the need for homeostatic signals in maintaining proliferation and survival of memory gB-CD8. Latently infected neurons express low levels of both MHC class I and viral proteins (23, 24). Based on these observations, one might predict that high functional avidity gB-CD8s that are capable of detecting the low epitope densities likely present on latently infected neurons would be best suited to provide immunosurveillance and survive within latently infected ganglia. Our findings are consistent with this prediction, because high functional avidity gB-CD8s are highly enriched in the latently infected TG, but not in the noninfected lungs, and transient compromise in the nonhelped high functional avidity gB-CD8s was associated with failure to maintain HSV-1 latency. These findings are also consistent with the recent demonstration that CD8+ T cells in latently infected TGs represent a tissue-resident subpopulation of memory CD8+ T cells that possess unique properties (38).
In a chronic LCMV infection model, intermediate levels of PD-1 expression on LCMV-specific CD8+ T cells was associated with partial exhaustion that could be reversed by in vivo PD-L1 blockade (10). In our model, intermediate PD-1 expression (based on both frequency of positive cells and level of expression per cell) on nonhelped gB-CD8s at 8–35 dpi was associated with partial functional exhaustion. This exhaustion was similar to that seen in the LCMV infection model, with TNF-α being more severely affected before other effector mechanisms as exhaustion progressed (39). Moreover, in vivo blockade of PD-1/PD-L1 interaction effectively reversed the exhaustion in nonhelped gB-CD8s. This finding is in agreement with a recent study that observed cells that were PD-1int but not PD-1hi were capable of functional recovery (40).
The source of PD-L1 that engages PD-1 on gB-CD8s in latently infected ganglia is not clear, but several observations point to latently infected neurons as likely candidates. First, it appears that PD-1 inhibits TCR signaling when the TCR and PD-1 ligands are expressed on the same cell (41). Second, extended PD-1/PD-L1 exposure is required for CD8+ T cell functional exhaustion and gB-CD8s closely associate and form immunologic synapses with latently infected neurons (10, 23, 24). Finally, we have observed PD-L1 expression on neurons in latently infected TGs (R. L. Hendricks, B. S. Sheridan, and T. L. Cherpes, unpublished observations).
This of course, begs the question of why nonhelped gB-CD8 express higher levels of PD-1 than their CD4-helped counterparts. We cannot formally rule out the possibility that nonhelped gB-CD8s upregulate PD-1 expression because of encountering higher levels of viral Ag in the DLNs or TG. However, we consider this unlikely because CD4-sufficient and CD4-deficient mice eliminate replicating virus from the cornea and TG with similar kinetics and establish latency with a similar load of viral DNA at 8 dpi. Instead, we favor the hypothesis that the threshold level of TCR signaling required for PD-1 expression is set at a lower level when gB-CD8s are programmed in the absence of CD4+ T cell help. The threshold level of TCR signaling required to induce PD-1 expression might then be met by nonhelped, but not helped, gB-CD8s through an encounter with Ag. Of interest is how the nonhelped gB-CD8 pool still retained functionality despite elevated PD-1 expression within the DLN at 8 dpi. We believe concordant expression of costimulatory molecules with PD-L1 on APCs within the DLN may slow or prevent the onset of exhaustion. As these cells enter the TG and undergo prolonged interactions with neurons, they undergo progressive functional exhaustion. Elucidating the mechanisms leading to reduced PD-1 expression and functional recovery of nonhelped gB-CD8 in HSV-1 latently infected TGs at 35–56 dpi will require further study.
Several recent reports emphasize the importance of CD8+ T cells in maintaining HSV-1 in a latent state within the TG. Effective immunosurveillance of latently infected neurons requires CD8+ T cells with the capacity to respond to low levels of viral epitopes that likely exist on infected neurons. Our current findings demonstrate that CD4+ T cell help during initial programming is important in generating a memory CD8+ T cell population with sufficient sensitivity to provide this surveillance. Because HSV-1 reactivation from latency is the primary cause of recurrent herpetic disease, any vaccine designed to prevent reactivation will need to incorporate both CD4 and CD8 epitopes.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01-EY05945 (to R.L.H.), P30-EY08098 (to R.L.H.), and K23-AI064396 (to T.L.C.); an unrestricted grant from Research to Prevent Blindness (New York, NY); and the Eye and Ear Foundation of Pittsburgh.
We thank Dawn Maker and Jessica Spehar for technical assistance, Nancy Zurowski for flow cytometry acquisition, and the National Institute of Allergy and Infectious Diseases Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA) for supplying tetramers.
Abbreviations used in this paper
- DLN
draining lymph node
- dpi
days postinfection
- gB
glycoprotein B
- gB-CD8
HSV-specific CD8+ T cell
- LCMV
lymphocytic choriomeningitis virus
- LG
lytic granule
- MFI
mean fluorescence intensity
- TG
trigeminal ganglion
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no conflicting financial interests.
References
- 1.Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 2.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 3.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 4.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181:7445–7448. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 7.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. [see comments] Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 8.Bullock TNJ, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 9.Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci USA. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 11.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, Ahmed R, Amara RR. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. [see comments] J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Lint AL, Kleinert L, Clarke SR, Stock A, Heath WR, Carbone FR. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. J Virol. 2005;79:14843–14851. doi: 10.1128/JVI.79.23.14843-14851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibbetts SA, van Dyk LF, Speck SH, Virgin HW., 4th Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J Virol. 2002;76:7125–7132. doi: 10.1128/JVI.76.14.7125-7132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon CO, Holtappels R, Tervo HM, Böhm V, Däubner T, Oehrlein-Karpi SA, Kühnapfel B, Renzaho A, Strand D, Podlech J, et al. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J Virol. 2006;80:10436–10456. doi: 10.1128/JVI.01248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decman V, Kinchington PR, Harvey SA, Hendricks RL. γ interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J Virol. 2005;79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias P, Shea AL, Inglis C, Giannoni F, Lee LN, Sarawar SR. Primary clearance of murine gammaherpesvirus 68 by PKCtheta−/− CD8 T cells is compromised in the absence of help from CD4 T cells. J Virol. 2008;82:11970–11975. doi: 10.1128/JVI.01053-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. Latent virus influences the generation and maintenance of CD8+ T cell memory. J Immunol. 2006;177:8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8 + T cells. J Exp Med. 2004;199:925–936. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betts MR, Price DA, Brenchley JM, Loré K, Guenaga FJ, Smed-Sorensen A, Ambrozak DR, Migueles SA, Connors M, Roederer M, et al. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol. 2004;172:6407–6417. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- 31.La Gruta NL, Doherty PC, Turner SJ. A correlation between function and selected measures of T cell avidity in influenza virus-specific CD8+ T cell responses. Eur J Immunol. 2006;36:2951–2959. doi: 10.1002/eji.200636390. [DOI] [PubMed] [Google Scholar]
- 32.Cherpes TL, Busch JL, Sheridan BS, Harvey SA, Hendricks RL. Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation. J Immunol. 2008;181:969–975. doi: 10.4049/jimmunol.181.2.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J Immunol. 2007;179:322–328. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.al-Ramadi BK, Jelonek MT, Boyd LF, Margulies DH, Bothwell AL. Lack of strict correlation of functional sensitization with the apparent affinity of MHC/peptide complexes for the TCR. J Immunol. 1995;155:662–673. [PubMed] [Google Scholar]
- 35.Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 36.Sawtell NM, Thompson RL. Comparison of herpes simplex virus reactivation in ganglia in vivo and in explants demonstrates quantitative and qualitative differences. J Virol. 2004;78:7784–7794. doi: 10.1128/JVI.78.14.7784-7794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajasagi NK, Kassim SH, Kollias CM, Zhao X, Chervenak R, Jennings SR. CD4+ T cells are required for the priming of CD8+ T cells following infection with herpes simplex virus type 1. J Virol. 2009;83:5256–5268. doi: 10.1128/JVI.01997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 39.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci USA. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.