Abstract
Both innate and adaptive immune cells are involved in the allograft response. But how the innate immune cells respond to allotransplants remains poorly defined. In the present study, we examined the role of NK cells and macrophages in recognizing and rejecting allogeneic cells in vivo. We found that in naïve mice NK cells are the primary effector cells in killing of allogeneic cells via “the missing self” recognition. However, in alloantigen pre-sensitized mice, NK cells are dispensable. Instead, macrophages become alloreactive and readily recognize and reject allogeneic non-self. This effect requires help from activated CD4+ T cells and involves CD40/CD40L engagement, as blocking CD40/CD40L interactions prevents macrophage mediated rejection of allogeneic cells. Conversely, actively stimulating CD40 triggers macrophage-mediated rejection in the absence of CD4+ T cells. Importantly, alloantigen primed and CD4+ T cell-helped macrophages (licensed macrophages) exhibit potent regulatory function in vivo in an acute GVHD model. Together, our data uncover an important role for macrophages in the alloimmune response and may have important clinical implications.
Keywords: Macrophages, alloimmunity, innate cells, NK cells, GVHD
INTRODUCTION
With few exceptions, a traditional view in transplantation (both bone marrow and solid organs) is that T cells are necessary and sufficient for allograft rejection. This view comes from the observation that rejection often does not occur in naïve hosts in the absence of T cells (1). However, destruction of an allograft in the effector phase of a rejection response is far more complex, involving many other cell types besides T cells. In fact, the innate immune cells such as NK cells and macrophages, are often well represented in the rejecting allografts (2, 3). As compared to T cells, much less is known about how innate immune cells influence the allograft response (graft rejection or acceptance). The common wisdom is that innate cells do not directly respond to allotransplants; but they can be brought in by activated T cells to aid the process of graft destruction. Thus, innate immune cells are thought to play a redundant or even a dispensable role in the rejection response (4).
There are several lines of evidence now that call for a reassessment of the role of innate immune cells in transplant models. For example, seminal work by Murphy et al dated back in 1987 revealed that NK cells can mediate the specificity of bone marrow graft rejection (5). We now know that NK cells are equipped with an unique receptor system that allows them to recognize and reject allogeneic cells via “missing self” or “missing ligand” recognition (6), and because of this, NK cells are in fact alloreactive capable of rejecting allotransplants (both bone marrow cells and solid grafts) (7–10). Some of the cell surface molecules on NK cells (e.g., NKG2D) have been identified as key activators of NK cells in rejection of bone marrow grafts (11). Interestingly, NK cells can also acquire additional features that are traditionally ascribed to adaptive T cells and B cells; NK cells can respond in an antigen-specific manner, undergo clonal expansion prior to becoming effector cells, and even acquire memory features capable of mediating recall responses (12). These unexpected findings drastically expand the role of NK cells in the overall immune responses. Besides NK cells, macrophages, which are thought to be non-specific inflammatory cells, can also exhibit certain degree of specificity in selected transplant models. To this end, Zecher et al recently reported that challenge of Rag−/− mice with alloantigens sensitizes host monocytes/macrophages that subsequently mediate alloantigen specific delayed type hypersensitivity response (13), suggesting that monocytes/macrophages may express a previously unknown allorecognition system that allows self non-self discrimination. In fact, in a xenogeneic islet transplant model (porcine to mouse), macrophages turn out to be the primary effector cells in destruction of islet transplants (14, 15). Once sensitized and activated by porcine xenoantigens, macrophages exhibit a high degree of antigen specificity in that they selectively and specifically destroy porcine islet transplants to which they are sensitized, but not allogeneic islets (14, 15). Similar findings were also reported in other settings (16). Considering the prevailing view of T cells in transplant models, these findings may be viewed simply as exceptions. However, these data do raise significant questions regarding the exact role of innate immune cells in response to allotransplants. The recent demonstration that kidney allograft rejection in humans following aggressive T cell depletion therapies is dominated by innate immune cells, especially monocytes/macrophages, suggests that these cells may play a much bigger role in transplant rejection than previously envisioned (17).
In the present study, we took an in vivo approach to examine the innate immune responses to allogeneic non-self cells immediately after alloantigen encounter (within the first 16 hrs) or weeks thereafter. We focused on NK cells and macrophages in this setting and found striking differences in the alloreactivity of NK cells and macrophages in naïve versus alloantigen pre-sensitized hosts. Specifically, NK cells reject allogeneic non-self cells in naïve mice, but they are dispensable in donor antigen pre-sensitized hosts. Unexpectedly, macrophages can be driven to an alloreactive mode in alloantigen pre-sensitized hosts and mediate rejection of allogeneic cells. This effect requires help from activated CD4+ T cells and involves CD40/CD40L interactions. Importantly, alloreactive macrophages display potent regulatory functions in vivo in an acute GVHD model.
MATERIALS AND METHODS
Animals
Wild type C57BL/6 mice (H-2b), perforin knockout, CD4 knockout, CD8 knockout, CD11b-GFP-DTR transgenic and Rag−/− mice on the C57BL/6 background (H-2b) were purchased from the Jackson Laboratory (Bar Harbor, ME). DBA/2 and Balb/c mice (H-2d) were also obtained from the Jackson Laboratory. Rag−/−γc−/− double mutant mice (H-2b) and C3H (H-2k) mice were obtained from the Taconic Farms (Germantown, NY). Animal care and use conformed to the guidelines established by the Animal Care Committee at Harvard Medical School in Boston, MA.
Antibodies and reagents
The following anti-mouse mAbs used for cell surface staining were obtained from eBiosciences (San Diego, CA): PE-Cy7-anti-CD4 (clone GK1.5), Pacific blue-anti-CD11b (clone M1/70), PE-Cy5-anti-F4/80 (clone BM8), FITC-anti-68 (clone FA/11), PE-anti-CD40 (clone IC10), PE-anti-CD80 (clone M89-61), APC-anti-CD11c (clone N418), PE-Cy7-anti-NKG2D (clone CX5), PE-Cy7-anti-CD62L (clone MEL-14), Pacific Blue-anti-CD62L (clone MEL-14), PE-anti-CD44 (clone IM-7), and isotype control Abs. PE-anti-Ly6c (clone HK14) was purchased from Southern Biotech (Birmingham, AL). Anti-CD40L (clone MR1), anti-NK1.1 (clone PK136), and anti-CD4 mAb (clone GK1.5) were purchased from BioXcell (West Lebanon, NH) and used for in vivo experiments. An agonist anti-CD40 (clone FGK45.5) mAb was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany) and used for some in vivo experiments.
In vivo killing assay
This assay was performed as previously reported (9). Briefly, spleen cells were prepared from allogeneic donors, namely Balb/c and C3H/he mice, and syngeneic C57BL/6 mice, cells were labeled with 0.2 μM and 2.5 μM CFSE ex vivo, respectively. The CFSE-labeled allogeneic and syngeneic cells were mixed together at 1:1 ratio, and the cell mixture was injected into host mice via the tail vein. Each mouse received ~10×106 cells. The host mice were killed 16–17 h later and survival of CFSE-labeled donor cells in the host spleen was analyzed using a FACScan flow cytometer by selectively gating onto the CFSE positive cells. The ratio of CFSE labeled cells was determined by FACS and shown in relative percentage in histograms. Rejection of donor cells was calculated using the following formula: [1 − CFSElow events/CFSEhigh events] × 100%, and then compared among different groups.
Cell staining and flow cytometry
Spleen and lymph node cells were harvested and single cell suspension was prepared, cells were resuspended in PBS/0.5% BSA and stained with fluorochrome-conjugated Abs on ice for 30 minutes. The cells were washed twice in PBS/BSA and fixed in 1% paraformaldehyde prior to FACS analysis. All samples were acquired using the FACScan or LSRII (BD Biosciences, Mountain View, CA). Data analysis was performed using the FlowJo software (Treestar, Ashland, OR).
Quantitative Real-time PCR
Total cellular RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed into cDNA with ABI Prism TaqMan reverse transcription method (9). Expression of genes of interest and of GAPDH control was assessed in simplex RT-PCR with FAM and VIC probes (Applied BioSystems, Foster City, CA). All the TaqMan primers and probe sets were purchased from Applied BioSystems. Transcript levels of target genes were calculated according to the 2−ΔCt formulas as provided by the manufacturer (ABI PRISM 7700 user bulletin, Applied BioSystems) and expressed as arbitrary units for comparison.
Determination of NO production
A fluorogenic assay was used to quantitate nitric oxide (NO) producing cells (18). Briefly, macrophages (2×105 cells/200 μl) were incubated in the presence of 1 μM of 4-amino-5-methylamino-2′, 7′-difluorescein (DAF-FM) diacetate, a green-fluorescent probe for detecting NO producing cells (Invitrogen, Calsbad, CA), at 37°C in 5% CO2 incubator for 40 min. After the incubation, cells were washed with fresh RPMI 1640 medium and further incubated for an additional 20 minutes. After that, cells were incubated with an Fc-blocker, followed by staining with anti-CD11b and F4/80 Abs. NO production by macrophages was assessed by the intensity of green fluorescence (excitation/emission at ~490/515 nm) in the F4/80+ population and data were presented in histograms.
Reactive oxygen species (ROS) production
ROS production was assessed with a fluorogenic assay (19). Briefly, macrophages (2×105 cells/200 μl) were incubated with 1 μM APF, a green-fluorescent probe for detecting ROS (Invitrogen), at 37°C for 40 min in an incubator with 5% CO2. After the incubation, cells were washed to remove excessive probe, followed by blocking with an Fc-blocker. Cells were further stained with anti-CD11b and anti-F4/80 Abs. ROS production by macrophages was assessed by FACS by assessing the intensity of green fluorescence (excitation/emission at ~490/515 nm) in the F4/80+ population.
Isolation of macrophages
Mice were anesthetized and a ventral midline incision exposed the peritoneal cavity, and a sterile 24-gauge cannula was inserted into the vena porta. The liver was preperfused in situ with collagenase A solution (0.05% in RPMI 1640) at a flow rate of 6 ml/min for 30 s at 35°C. After perfusion the liver were excised, sliced, and further incubated for 20 minutes, followed by gently pushing the digested tissue through a nylon filter (88 um). The cell suspension was collected and cells pelleted through centrifugation, and then washed twice at 500g for 8 minutes. Cells were resuspended in PBS and gently layered on a double Percoll gradient (30% and 70%), followed by centrifugation at 800g for 15 minutes. Cells between the 30% to 70% gradient interface were collected and macrophages (CD11b+F4/80+) were further sorted using MoFlo high speed cell sorter.
Cell depletion in vivo
Depletion of NK cells in vivo was accomplished using a depleting anti-NK1.1 mAb (clone PK136); the mAb was given at 0.25mg i.p. for two consecutive days. CD4+ T cells were depleted with a depleting anti-CD4 mAb (clone GK1.5); mice were injected with the anti-CD4 mAb i.p. at 0.25mg for 2 consecutive days. Cell depletion with these protocols has been always >90%, as assessed by FACS (20). Gadolinium chloride (GdCl3) (Sigma Aldrich, St. Louis, MO) was used to inhibit macrophages in vivo (21). Mice were given gadolinium chloride at 20mg/kg/day for 3 consecutive days before performing the in vivo killing assays.
Adoptive cell transfer
In some experiments where immunodeficient Rag−/− or Rag−/−γc−/− mice were used as hosts, CD4+ T cells from C57BL/6 mice were prepared by MACS assisted cell sorting and 20×106 cells were injected into each host via the tail vein.
Acute graft versus host disease in vivo
Male Rag−/−γc−/− mice (H-2b) were used as hosts, and each host was given 40×106 allogeneic Balb/c T cells via the tail vein to induce the graft versus host response. The severity of the response was assessed by weight loss and tissue pathology. Groups of host mice were also given CD11b+F4/80+ macrophages (2×106 per host) prepared from naïve B6 mice or B6 mice that were pre-sensitized with Balb/c cells. In some experiments, the host mice received 40×106 T cells from Balb/c mice and 4×106 CD4+ T cells from syngeneic B6 mice injection to prime the host macrophages. The severity of GVHD, as assessed by weight loss, was monitored and shown.
Statistics
Mann-Whitney nonparametric statistics were used to determine the level of significance among groups, and a P value less than 0.05 was considered significant.
RESULTS
NK cells in naïve hosts reject allogeneic non-self via “missing self” recognition
We labeled allogeneic Balb/c (H-2d) and syngeneic B6 (H-2b) spleen cells with different molar concentrations of CFSE, mixed them at 1:1 ratio, and injected them into naïve B6 hosts (H-2b). Survival of CFSE-labeled cells was examined 6 to 16 hrs later by FACS. As shown in Fig 1A, the allogeneic Balb/c cells were mostly rejected in B6 hosts, while survival of syngeneic B6 cells was not affected. Depletion of NK cells from the B6 hosts prevented rejection of Balb/c cells, and both the labeled Balb/c cells and B6 cells survived equally well in the NK depleted hosts, confirming that NK cells are the effector cells mediating the killing of allogeneic Balb/c cells (9). This effect requires perforin and is associated with NK cell degranulation, as revealed by staining for CD107a expression (Fig 1B). In this in vivo cytotoxic assay, killing of allogeneic cells by host NK cells was ~50% within 16 hrs of alloantigen exposure (Fig 1C).
Figure 1. NK cells promptly kill allogeneic non-self in naïve B6 hosts.
(A) CFSE-labeled Balb/c (0.2 μM) and B6 (2.5 μM) spleen cells were mixed at a 1:1 ratio and injected into naïve C57BL/6 mice with or without NK depletion, and a cohort of perforin knockout mice (Pfr KO) were used as hosts in some experiments. Survival of CFSE-labeled cells in the host mice was determined 16 hrs later by flow cytometry and shown. Data presented are one of three independent experiments. (B) Analysis of CD107a expression by NK cells, a marker of degranulation, with or without challenging with allogeneic Balb/c cells. Data shown are selectively gated on NK1.1+ cells 5h after injection of allogeneic Balb/c cells in vivo. One of three independent experiments is shown. (C) Rejection of allogeneic Balb/c cells in vivo by naïve B6, NK depleted B6 mice, and perforin knockout mice 16 hrs after injection of CFSE-labeled cells. The bars represent SD of three individual samples and the results shown are representative of three independent experiments.
Rejection of allogeneic cells in pre-sensitized hosts is NK-independent
In this strain combination, donor Balb/c cells express H-2Dd, which is the ligand for the NK activating receptor Ly49D in B6 hosts (22). To examine whether NK cells can be sensitized by donor antigens in vivo, a phenomenon that has been described in other models (12, 23), we challenged B6 mice with Balb/c spleen cells first (priming phase), and 14 days later we performed the same in vivo killing assay as described above (assay phase) to assess whether NK cells exhibit features of memory against the same donor antigens. As compared to that in naïve hosts (~50% killing), a much greater killing of donor Balb/c cells (but not syngeneic B6 cells), was indeed observed in the pre-sensitization hosts (Fig 2), and virtually all donor cells were killed 16 hrs later in the sensitized hosts (>95%). Surprisingly, depletion of NK cells from the pre-sensitized hosts was no longer effective in preventing rejection of donor Balb/c cells, and the donor cells were completely destroyed regardless of NK depletion (Fig 2). NK cell depletion in the primed hosts was confirmed by flow cytometry (data not shown), excluding the possibility of residual NK cells in killing of donor cells.
Figure 2. Rejection of allogeneic non-self in donor alloantigen pre-sensitized mice.
(A) Wt C57BL/6, CD8KO, CD4KO mice were pre-sensitized with allogeneic Balb/c spleen cells (10×106 per mouse). Two weeks later, NK cells were depleted from these mice (PK136, 0.25 mg/day for 2 days), followed by transferring CFSE labeled syngeneic and allogeneic cells into them for performing the in vivo killing assay. Wt C57BL/6 mice pre-sensitized with Balb/c cells without NK depletion were used as controls. Survival of CFSE-labeled cells was assessed by flow cytometry 16 hrs after cell transfer. The plots shown are representative data of 1 of 4 experiments. (B) Rejection of allogeneic Balb/c cells by donor antigen pre-sensitized hosts was calculated and shown. The histograms are mean ± SD of 4 individual experiments.
One possibility is that in the primed hosts, T cells especially CD8+ T cells, may become cytotolytic, which then reject the allogeneic Balb/c cells in spite of NK depletion. To test this possibility, we pre-sensitized CD4KO and CD8KO mice with donor Balb/c cells. NK cells were depleted 14 days later and then performed the in vivo killing assay thereafter. Interestingly, we observed that CD8KO mice that were depleted of NK cells still rejected donor Balb/c cells in a similar fashion as wt B6 mice. However, rejection of donor Balb/c cells was prevented in NK depleted CD4KO mice (Fig 2). These findings suggest that in donor antigen primed hosts, NK cells and CD8+ T cells are both dispensable for rejection of donor cells upon re-challenge, but CD4+ T cells are somehow required.
Macrophages act as effector cells in rejection of allogeneic cells in pre-sensitized hosts
To dissect whether CD4+ T cells directly reject donor Balb/c cells or indirectly via the activation of other cell types in pre-sensitized hosts, we performed a series of adoptive transfer experiments using Rag−/−γc−/− mice as host mice (B6 background, H-2b); these mice have ample myeloid cells but are deficient for T, B, and NK cells (24). As shown in Fig 3 and consistent with our published data (9), both Balb/c and B6 cells survived equally well in the Rag−/−γc−/− hosts, as assessed by the in vivo killing assay. Next, we adoptively transferred syngeneic B6 CD4+ T cells into Rag−/−γc−/− mice first, and then performed the in vivo killing assay14 days later. As shown in Fig 3A, no killing of donor cells was observed under this condition, suggesting that CD4+ T cells alone do not directly kill donor Balb/c cells. In a different setting in which syngeneic B6 CD4+ T cells and donor Balb/c cells were transferred at the same time into Rag−/−γc−/− hosts (priming phase), and 14 days latter we performed the in vivo killing assay to assess rejection of donor Balb/c cells. As shown in Fig 3A (upper panel), donor Balb/c cells were completely rejected by the Rag−/−γc−/− hosts whereas syngeneic B6 cells survived. Depletion of CD4+ T cells from the T cell-transferred Rag−/−γc−/− hosts at the time of performing the in vivo killing assay did not affect the rejection of donor Balb/c cells (Fig 3A, lower panel), supporting the notion that CD4+ T cells don’t directly reject the donor cells. Interestingly, reconstituting Rag−/−γc−/− hosts with syngeneic B6 CD4+ T cells and donor Balb/c cells did not result in the rejection of third party C3H cells (H-2k), demonstrating a degree of donor antigen specificity in this model.
Figure 3. Rejection of allogeneic non-self in Rag−/−γc−/− hosts passively transferred with different syngeneic and allogeneic cell types.
(A) Rag−/−γc−/− mice were left unmanipulated or transferred with allogeneic Balb/c cells (10×106 per mouse), syngeneic CD4+ T cells (20×106 per mouse), or both, and the in vivo killing assay was performed two weeks later using either donor Balb/c cells or the third party C3H cells. Groups of host mice were also treated with a depleting anti-CD4) mAb (clone GK1.5, 0.25mg/day for 2 days) or with GdCl3 (20 mg/kg/day for 3 days) before performing the in vivo killing assay. Survival of CFSE-labeled syngeneic and allogeneic cells in the treated hosts was determined by FACS and shown. The plots shown are representative of 1 of 4 independent experiments. (B). Rag−/−γc−/− mice were used as host mice for adoptive cell transfer. Licensed macrophages were prepared from B6.CD11b-DTR transgenic mice pre-sensitized with Balb/c allogeneic cells for 2 wks, and each host was given 2×106 macrophages via tail vein injection. A cohort of host mice transferred with licensed CD11b-DTR macrophages was also treated with DT (25ng/day for 2 days), and the in vivo killing assay was performed thereafter. Data shown are 1 of 3 independent experiments.
As Rag−/−γc−/− mice are deficient for T, B, and NK cells (24), and depletion of transferred CD4+ T cells at the assay phase did not affect rejection of donor Balb/c cells. This prompted us to examine whether macrophages (the only major immune cells in these mice) play a significant role in this model. As shown in Fig 3A (lower panel), in Rag−/−γc−/− hosts that were transferred with B6 CD4+ T cells and Balb/c spleen cells, treatment with gadolinium to inhibit macrophages before the killing assay indeed markedly inhibited the rejection of donor Balb/c cells, and when compared to the untreated controls, a significant fraction of donor Balb/c cells survived in gadolinium treated Rag−/−γc−/− hosts. These findings suggest a key role for macrophages in rejection of allogeneic Balb/c cells in vivo.
To further ascertain this notion, we repeated the same killing assay in Rag−/−γc−/− hosts in which macrophages from donor antigen pre-sensitized CD11b-DTR transgenic mice were adoptively transferred (licensed macrophages). As shown in Fig 3B, both syngeneic B6 and allogeneic Balb/c cells survived equally well in the Rag−/−γc−/− hosts, and transferring the licensed CD11b-DTR macrophages resulted in the rejection of allogeneic Balb/c cells. Treatment of the host mice with diphtheria toxin (DT) at the assay phase to eliminate macrophages rescued the allogeneic Balb/c cells from being rejected in vivo, providing definitive proof that macrophages can recognize and reject allogeneic cells upon donor antigen pre-sensitization.
Macrophage-mediated rejection of allogeneic non-self requires CD4+ T cell help
Clearly, macrophages are capable of rejecting allogeneic non-self, but this response requires donor antigen priming and host CD4+ T cells. To determine the role of CD4+ T cells in this response, we challenged the Rag−/−γc−/− hosts with allogeneic Balb/c cells with or without syngeneic CD4+ T cells, andphenotyped macrophages two weeks later. Macrophages were identified by the expression of F4/80. As shown in Fig 4, amongst the cell surface markers examined, CD40 expression was noticeably upregulated following donor antigen priming, while other markers did not show marked differences. As activated CD4+ T cells express CD40L (CD154) (25), one possibility is that activated CD4+ T cells may engage CD40 on macrophages to trigger macrophage activation, which allows macrophages to be alloreactive. To test this possibility, we again transferred allogeneic Balb/c cells and syngeneic CD4+ T cells together into Rag−/−γc−/− hosts, the host mice were then treated with anti-CD154 mAb to block CD40/CD40L interactions. Two weeks later, we performed the in vivo killing assay to assess rejection of CFSE labeled allogeneic Balb/c cells. As shown in Fig 5A, the CFSE-labeled Balb/c cells survived in unmanipulated Rag−/−γc−/− hosts, but completely rejected in Rag−/−γc−/− hosts previously transferred with Balb/c cells and B6 CD4+ T cells. Interestingly, rejection of Balb/c cells was completely inhibited by blocking the CD40/CD40L pathway (Fig 5A). Similarly, transferring CD4+ T cells from CD40L knockout mice (CD40L deficient T cells) to Rag−/−γc−/− mice failed to trigger rejection of allogeneic Balb/c cells upon donor antigen priming (Fig 5A), demonstrating a critical role for CD40/CD40L interactions in rejection of allogeneic cells by macrophages. Importantly, treatment of Rag−/−γc−/− hosts (transferred with CD4+ T cells and primed with allogeneic Balb/c cells) with anti-CD154 at the time of performing the killing assay did not prevent rejection of allogeneic Balb/c cells (Fig 5B). Thus, CD40/CD40L interactions at the priming phase are required to drive macrophages to an alloreactive mode.
Figure 4. The phenotype of macrophages challenged with or without allogeneic Balb/c cells and syngeneic CD4+ T cells in vivo.
Rag−/−γc−/− mice were transferred with 20×106 syngeneic B6 CD4+ T cells with or without 10×106 allogeneic Balb/c cells via the tail vein. Two weeks later, mice were sacrificed; spleen cells were prepared and stained with mAbs directed against F4/80 and various other cell surface molecules, and expression of such molecules was analyzed by flow cytometry by gating onto the F4/80+ macrophages. Cells from naïve Rag−/−γc−/− mice were processed in an identical way and used as controls. The plots shown are representative of 1 of 3 independent experiments.
Figure 5. Role of the CD40/CD40L pathway in macrophage mediated rejection of allogeneic non-self.
(A) Rag−/−γc−/− mice were transferred with syngeneic B6 CD4+ T cells and allogeneic Balb/c cells and treated with anti-CD154 (clone MR1) at 0.25 mg for 2 days or a control IgG. Groups of Rag−/−γc−/− mice were transferred with syngeneic CD4+ T cells deficient for CD40L plus allogeneic Balb/c cells. The in vivo killing assay using CFSE-labeled indicator cells was performed 2 weeks later, and killing of CFSE-labeled syngenic and allogeneic indicator cells was shown. (B) The Rag−/−γc−/− mice were challenged with allogeneic Balb/c cells and syngeneic CD4+ T cells, as described above, MR1 was given at the time of performing the killing assay 2 weeks after the priming phase, and survival of CFSE-labeled indicator cells was assessed by FACS and shown. (C) The Rag−/−γc−/− mice were transferred with allogeneic Balb/c cells only. The host mice were treated with an agonist anti-CD40 (clone FGK45.5) or a control IgG (0.1mg i.p.) at the time of donor antigen priming. Some hosts received the agonist anti-CD40 only without allogeneic Balb/c cells. Two weeks later, the in vivo killing assay was performed and survival of CFSE-labeled indicator cells was assessed by FACS and shown. All results shown in this Figure are representative data of three independent experiments.
To further examine the role of CD40 in macrophage activation, we primed the Rag−/−γc−/− mice with allogeneic Balb/c cells, and instead of transferring syngeneic CD4+ T cells at the time of priming, we injected the hosts with an agonist anti-CD40 mAb (FGK45.5) to specifically engage CD40 on macrophages. Rag−/−γc−/− mice given the agonist anti-CD40 mAb (FGK45.5) without allogeneic Balb/c cells were included as controls. Two weeks later, the in vivo killing assay was performed to assess the rejection of allogeneic Balb/c cells. As shown in Fig 5C, stimulation of the CD40 receptor at the time of alloantigen priming triggered rejection of allogeneic Balb/c cells, whereas CD40 engagement alone failed to do so. Additionally, treatment host mice with the agonist anti-CD40 mAb alone did not induce rejection of Balb/c cells in donor antigen primed Rag−/−γc−/− mice (Fig 5C). Collectively, these data show that both donor antigen encounter and CD40 engagement in the priming phase are required to allow macrophages to reject allogeneic cells in vivo.
Macrophages reject allogeneic non-self cells via phagocytosis
To explore the mechanisms by which macrophages reject allogeneic cells, we again transferred Rag−/−γc−/− mice with Balb/c cells with or without syngeneic B6 CD4+ T cells, and then examined several pathways known to induce target cell death. We observed that expression of NO and ROS by macrophages, molecules that are implicated in macrophage-induced cell death (18), was not markedly different regardless the presence or absence of donor alloantigens and syngeneic CD4+ T cells. Additionally, resting or licensed macrophages did not express detectable levels of Perforin and Granzyme B (data not shown), suggesting that macrophages unlikely use such molecules to mediate rejection of allogeneic cells.
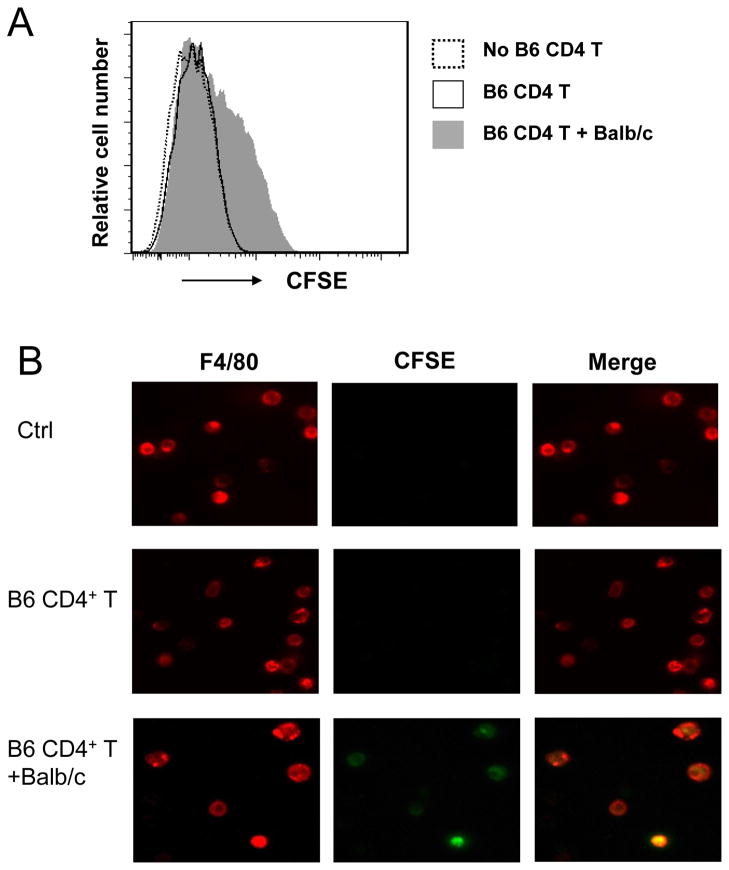
We then determined whether licensed macrophages reject allogeneic cells by phagocytosis. To this end, Rag−/−γc−/− mice were pre-sensitized with Balb/c alloantigens with or without B6 CD4+ T cells. Naïve Rag−/−γc−/− mice were used as control. Two weeks later, all these mice were injected i.v. CFSE-labeled allogeneic Balb/c spleen cells. Macrophages were isolated 5 hrs later, further purified by MACS, and then labeled with PE-Cy5-anti-F4/80. Phagocytosis of CFSE labeled cells was determined by both flow cytometry and con-focal microscopy. In this assay, ingestion of CFSE+ cells by macrophages can be detected by a shift in fluorescence intensity (Fig 6A). As shown in Fig 6B, only donor antigen primed and CD4+ T cell activated macrophages (licensed macrophages) phagocytosed allogeneic Balb/c cells. These data suggest that rejection of allogeneic cells by macrophages is by phagocytosis rather than by direct killing.
Figure 6. Licensed macrophages reject allogeneic cells via phagocytosis.
Groups of Rag−/−γc−/− mice were transferred with 20×106 syngeneic C57BL/6 CD4+ T cells with or without 10×106 allogeneic Balb/c cells. Naïve Rag−/−γc−/− mice were as control. Two weeks later, all these mice were injected i.v. with 5×106 CFSE-labeled Balb/c spleen cells, and 5 hrs later macrophages were isolated from the host liver, enriched by MACS assisted cell sorting, and further stained with PE-Cy5-anti-F4/80. The cell preparation was analyzed by flow cytometry (A) and by fluorescence microscopy (B). Phagocytosis was identified by a shift in fluorescence intensity in the FACS plots or the appearance of yellow cells under the fluorescence microscope. The results shown are representative of three independent experiments.
Licensed macrophages exhibit potent regulatory functions in vivo
We used an acute GVHD model to determine whether licensed macrophages exhibit immunoregulatory role in vivo by eliminating donor T cells. We injected Balb/c T cells to Rag−/−γc−/− mice to induce acute GVHD responses, and groups of recipient mice also received licensed macrophages from donor antigen primed B6 mice or macrophages from naïve B6 mice. Development of GVHD responses was monitored by changes in bodyweight and by tissue histology. As shown in Fig 7, injection of allogeneic Balb/c T cells to Rag−/−γc−/− mice induced a significant weight loss, and this is associated with extensive cellular infiltration in the intestine (data not shown). This effect was significantly ameliorated in recipient mice transferred with licensed macrophages, while macrophages from naïve B6 mice did not show any protective effects.
Figure 7. Licensed macrophages exhibit potent regulatory function in vivo in an acute GVHD model.
Groups of male Rag−/−γc−/− mice were given 40 × 106 Balb/c T cells via tail vein injection, and mice were then followed for signs of GVHD. The severity of GVHD was assessed by weight loss over a time period of 3 weeks. Some mice received additional CD11b+F4/80+ macrophages (2×106 per mouse) purified from naïve B6 mice or B6 mice pre-sensitized with Balb/c cells for 2 wks, or syngeneic B6 CD4+ T cells. Changes in body weights among different groups were monitored and shown. Values are mean ±SD of 5 mice at individual time points, and one of two sets of experiments was shown. (★ P value < 0.05, ★★ P value < 0.01).
DISCUSSION
In this report, we provide further evidence supporting the presence of an allorecognition system on macrophages, as recently brought up by Zecher et al (13). We extended those initial observations and further demonstrated that macrophages can recognize and reject allogeneic cells and this response requires donor antigen priming and help from activated T cells. The activated and T cell-helped macrophages (licensed macrophages) are surprisingly potent in rejection of allogeneic non-self in vitro and in protection against T cell-mediated acute GVHD in vivo. We also showed that rejection of allogeneic non-self by licensed macrophages is mediated primarily by phagocytosis and help delivered by activated T cells is mainly through the CD40/CD40L pathway. These findings, together with findings from other groups (14–16), highlight the importance of macrophages in alloimmunity, a feature that is not formally attributed to them, and the complexity of interactions with other cell types in the alloimmune response.
A significant finding of our study is that macrophages can be driven to an alloreactive mode in which they recognize and reject allogeneic non-self in an antigen specific manner. Once in this mode, macrophages are surprisingly potent in rejection of allogeneic non-self cells in vivo. This process requires two signals. Specifically, engagement of alloantigens by macrophages is required, but alone is insufficient to mediate the alloreactivity of macrophages. T cell help is also required, and we demonstrate that T cell help is mediated primarily through the CD40/CD40L pathway. Both signals need to be delivered simultaneously to macrophages in order to license macrophages to respond to allogeneic non-self. Another unexpected feature is that licensed macrophages exhibit donor antigen specificity in that macrophages sensitized against allogeneic Balb/c cells do not show reactivity to third party C3H cells upon re-challenge. It is unlikely that this type of specificity is provided by T cells despite the fact that T cell help is required, as an agonist anti-CD40 mAb can replace activated T cells in driving activated macrophages to the alloreactive mode. While exciting, the molecular nature of such allorecognition remains unknown and is not addressed in the present study. But our findings are reminiscent of those reported by others in a xenogenic islet transplant model (14). In the xenogenic model, mouse macrophages are remarkably potent in rejecting porcine islet transplants; this effect also requires priming of mouse macrophages by porcine islets and the presence of activated CD4+ T cells at the time of priming (14). Importantly, porcine antigen primed and CD4+ T cell activated macrophages, upon transferring into immunodeficient NOD/SCID hosts, induce prompt rejection of porcine islet xenografts, whereas unprimed macrophages or those primed without CD4+ T cells fail to do so. Additionally, macrophages primed with porcine xenoantigens (along with activated CD4+ T cells) fail to reject rat islet xenografts or mouse islet allografts (14). Collectively, these data provide compelling evidence that macrophages are capable of recognizing allotransplants in an antigen specific manner.
The exact molecular nature of this macrophage allorecognition system remains to be defined. But there are several possibilities that may explain this phenomenon. Macrophages are known to express the inhibitory receptor Sirpα, which binds to its ubiquitously express ligand CD47 (26). Thus, the CD47/Sirpα is an important inhibitory pathway in suppressing macrophage activation, and species incompatibility between CD47 and Sirpα often triggers rejection of xenogenic cells by macrophages (27). But the role of this pathway in the allogeneic setting is less clear. CD47 is ubiquitously expressed by all tissues and cells, and it is not shown to have allospecificity in the mouse (26). Also, macrophages express an extensive array of pattern recognitions receptors including TLRs that recognize conserved structures from bacteria and viruses (28); these receptors may also recognize conserved residues in molecules in the mouse. Some of these receptors are known to respond to endogenous danger signals or inflammatory ligands such as HMGB1 and uric acid (29). However, such ligands are highly conserved and should be shared between self and non-self tissues and cells. Alternatively, the specificity of licensed macrophages may have been imprinted at the time of alloantigen priming (like homing property of certain cell types), but this possibility remains to be tested. Considering the inducible nature of this allorecognition system as shown in our study, this allorecognition system may be quite different from that described in other settings (14). This is a significant issue that warrants further investigation.
There are several mechanisms by which macrophages damage or kill target cells, and such mechanisms include the production of cytotoxic or cytostatic cytokines, reactive oxygen species, and rejection of target cells by phagocytosis (27). It is not known how licensed macrophages chose one over another, but different effector mechanisms are likely activated by different pathways or under different context. In our study, phagocytosis seems to be a primary effector mechanism in vivo in rejection of allogeneic cells, as licensed macrophages in our study do not produce reactive oxygen species, nor do they express typical cytolytic molecules. However, the in vivo situation is far more complex, the possibility of a multiplicity of mechanisms involved in the protection against GVHD can not be excluded.
Our data expand the traditional role of macrophages as inflammatory cells or antigen-presenting cells and further suggest that macrophages can be directly alloreactive in transplant settings. Clearly, elimination of allogeneic T cells by licensed macrophages in a GVHD setting is desirable and beneficial to the hosts. However, in solid organ transplantation, graft-infiltrating macrophages, once becoming alloreactive, may contribute significantly to graft damage and poor transplant outcomes. Considering the importance of CD40/CD40L interactions in licensing macrophages, cell types that can express CD40L may have the potential to drive macrophages to an alloreactive mode, and in addition to activated T effector cells, memory T cells, activated endothelial cells, or even platelets all express CD40L (30–32), and therefore may act as key partners in macrophage-mediated rejection. This may help explain why allografts with ample infiltrating macrophages tend to have poor outcomes in response to conventional immunosuppression therapies (17). Our data also suggest that the tolerogenic effects of anti-CD154 mAb in transplant survival may extend to the inhibition of macrophage licensing, in addition to blocking T cell costimulation. In conclusion, our data suggest that macrophages can be driven to an alloreactive mode, and this process requires donor antigen priming and help from activated T cells. Importantly, our data support the notion that macrophages express an allorecognition system that allows macrophages to respond to allotransplants under certain conditions (13, 14). A better appreciation of this system may have important clinical implications.
Acknowledgments
This work was supported by the National Institutes of Health (R01 AI080779 and R56 AI080779 to XCL). XX was supported by a T32 award (T32AI070085) from the National Institutes of Health.
We thank members in the Li lab for countless discussions and Alice E. Li at ASAP Editing for help with language editing.
References
- 1.Hall BM, Dorsch SE. Cells mediating allograft rejection. Immunol Rev. 1984;77:31–59. doi: 10.1111/j.1600-065x.1984.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 2.Li XC. The significance of non-T cell pathways in graft rejection: Implications for transplant tolerance. Transplantation. 2010;90:1043–1047. doi: 10.1097/TP.0b013e3181efcfe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alegre ML, Florquin S, Goldman M. Cellular mechanisms underlying acute graft rejection: time for reassessment. Curr Opin Immunol. 2007;19:563–568. doi: 10.1016/j.coi.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Nankivell BJ, Alexander SI. Rejection of kidney allograft. New England Journal of Medicine. 2010;363:1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 5.Murphy WJ, Kumar V, Bennett M. Rejection of bone marrow allografts by mice with severe combined immune deficiency (SCID): evidence that natural killer cells can mediate the specificity of marrow graft rejection. J Exp Med. 1987;165:1212–1217. doi: 10.1084/jem.165.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 7.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of Donor Natural Killer Cell Alloreactivity in Mismatched Hematopoietic Transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 8.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. Journal of Experimental Medicine. 2006;203:1851–1858. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemer A, Xiao X, Degauque N, Edtinger K, Wei H, Demirci G, Li XC. The Innate NK Cells, Allograft Rejection, and a Key Role for IL-15. J Immunol. 2008;180:7818–7826. doi: 10.4049/jimmunol.180.12.7818. [DOI] [PubMed] [Google Scholar]
- 10.Kroemer A, Edtinger K, Li XC. The innate NK cells in transplant rejection and tolerance induction. Curr Opin Organ Transplantation. 2008;13:339–343. doi: 10.1097/MOT.0b013e3283061115. [DOI] [PubMed] [Google Scholar]
- 11.Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nature Immunology. 2005;6:938–945. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zecher D, van Rooijen N, Rothstein DM, Shlomchik WD, Lakkis FG. An Innate Response to Allogeneic Nonself Mediated by Monocytes. The Journal of Immunology. 2009;183:7810–7816. doi: 10.4049/jimmunol.0902194. [DOI] [PubMed] [Google Scholar]
- 14.Yi S, Hawthorne WJ, Lehnert AM, Ha H, Wong JKW, van Rooijen N, Davey K, Patel AT, Walters SN, Chandra A, O’Connell PJ. T Cell-Activated Macrophages Are Capable of Both Recognition and Rejection of Pancreatic Islet Xenografts. The Journal of Immunology. 2003;170:2750–2758. doi: 10.4049/jimmunol.170.5.2750. [DOI] [PubMed] [Google Scholar]
- 15.Fox A, Mountford J, Braakhuis A, Harrison LC. Innate and Adaptive Immune Responses to Nonvascular Xenografts: Evidence That Macrophages Are Direct Effectors of Xenograft Rejection. The Journal of Immunology. 2001;166:2133–2140. doi: 10.4049/jimmunol.166.3.2133. [DOI] [PubMed] [Google Scholar]
- 16.Zhan Y, Brady JL, Irawaty W, Thomas HE, Kay TW, Lew AM. Activated macrophages require T cells for xenograft rejection under the kidney capsule. Immunol Cell Biol. 2003;81:451–458. doi: 10.1046/j.1440-1711.2003..x. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Dhanireddy KK, Kirk AD. Human Monocytes as Intermediaries between Allogeneic Endothelial Cells and Allospecific T Cells: A Role for Direct Scavenger Receptor-Mediated Endothelial Membrane Uptake in the Initiation of Alloimmunity. The Journal of Immunology. 2006;176:750–761. doi: 10.4049/jimmunol.176.2.750. [DOI] [PubMed] [Google Scholar]
- 18.Ghosn EEB, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, Bortoluci KR, Almeida SR, Herzenberg LA, Herzenberg LA. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proceedings of the National Academy of Sciences. 2010;107:2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinoshita M, Uchida T, Sato A, Nakashima M, Nakashima H, Shono S, Habu Y, Miyazaki H, Hiroi S, Seki S. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. Journal of Hepatology. 2010;53:903–910. doi: 10.1016/j.jhep.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 20.Vu MD, Amanullah F, Li Y, Demirci G, Sayegh MH, Li XC. Different costimulatory and growth factor requirements for CD4+ and CD8+ T cell mediated rejection. Journal of Immunology. 2004;173:214–221. doi: 10.4049/jimmunol.173.1.214. [DOI] [PubMed] [Google Scholar]
- 21.Hardonk MJ, Dijkhuis FW, Hulstaert CE, Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol. 1992;52:296–302. doi: 10.1002/jlb.52.3.296. [DOI] [PubMed] [Google Scholar]
- 22.Johansson S, Johansson M, Rosmaraki E, Vahlne G, Mehr R, Salmon-Divon M, Lemonnier F, Karre K, Hoglund P. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med. 2005;201:1145–1155. doi: 10.1084/jem.20050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proceedings of the National Academy of Sciences. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 25.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 26.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 27.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, Sykes M, Yang YG, Ohdan H. Role for CD47-SIRP ± signaling in xenograft rejection by macrophages. Proceedings of the National Academy of Sciences. 2007;104:5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunological Reviews. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 29.Matsuoka N, Itoh T, Watarai H, Sekine-Kondo E, Nagata N, Okamoto K, Mera T, Yamamoto H, Yamada S, Maruyama I, Taniguchi M, Yasunami Y. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. The Journal of Clinical Investigation. 2010;120:735–743. doi: 10.1172/JCI41360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiao SL, McNiff JM, Pober JS. Memory T Cells and Their Costimulators in Human Allograft Injury. The Journal of Immunology. 2005;175:4886–4896. doi: 10.4049/jimmunol.175.8.4886. [DOI] [PubMed] [Google Scholar]
- 31.Vu MD, Clarkson MR, Yagita H, Turka LA, Sayegh MH, Li XC. Critical, but Conditional, Role of OX40 in Memory T Cell-Mediated Rejection. J Immunol. 2006;176:1394–1401. doi: 10.4049/jimmunol.176.3.1394. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Zhang X, Mannon RB, Kirk AD. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. Journal of Clinical Investigation. 2006;116:769–774. doi: 10.1172/JCI27155. [DOI] [PMC free article] [PubMed] [Google Scholar]