Abstract
Summary
Children who sustain a forearm fracture when injured have lower bone density throughout their skeleton, and have a smaller cortical area and a lower strength index in their radius. Odds ratios per SD decrease in bone characteristics measured by peripheral quantitative computed tomography (pQCT) and dual-energy X-ray absorptiometry (DXA) were similar (1.28 to 1.41).
Introduction
Forearm fractures are common in children. Bone strength is affected by bone mineral density (BMD) and bone geometry, including cross-sectional dimensions and distribution of mineral. Our objective was to identify bone characteristics that differed between children who sustained a forearm fracture compared to those who did not fracture when injured.
Methods
Children (5–16 years) with a forearm fracture (cases, n=224) and injured controls without fracture (n=200) were enrolled 28±8 days following injury. Peripheral QCT scans of the radius (4% and 20% sites) were obtained to measure volumetric BMD (vBMD) of total, trabecular and cortical bone compartments, and bone geometry (area, cortical thickness, and strength strain index [SSI]). DXA scans (forearm, spine, and hip) were obtained to measure areal BMD (aBMD) and bone area. Receiver operating characteristic (ROC) analyses were used to assess screening performance of bone measurements.
Results
At the 4% pQCT site, total vBMD, but not trabecular vBMD or bone area, was lower (−3.4%; p= 0.02) in cases than controls. At the 20% site, cases had lower cortical vBMD (−0.9%), cortical area (−2.8%), and SSI (−4.6%) (p<0.05). aBMD, but not bone area, at the 1/3 radius, spine, and hip were 2.7–3.3% lower for cases (p< 0.01). Odds ratios per 1 SD decrease in bone measures (1.28–1.41) and areas under the ROC curves (0.56–0.59) were similar for all bone measures.
Conclusions
Low vBMD, aBMD, cortical area, and SSI of the distal radius were associated with an increased fracture risk. Interventions to increase these characteristics are needed to help reduce forearm fracture occurrence.
Keywords: Bone densitometry, Epidemiology, Fracture, Orthopedics, Pediatrics, QCT
Introduction
Approximately 40% of girls and 50% of boys experience a fracture sometime during childhood and adolescence [1]. The forearm is the most common site of fracture accounting for 25–30% of all fractures in children [1]. Forearm fracture incidence peaks between ages 9–12 years in girls and 11–14 years in boys [1–4]. Of concern, the incidence of forearm fractures has increased over the last 30 years by 42% [4].
Forearm fractures among children often occur from injuries sustained during participation in sports and recreational activities [4–7], and hours spent in physical activity is a strong predictor of fracture risk [8]. However, participation in sports and recreational activities are important for child development and impart multiple health benefits. Deficits in bone strength also have been implicated in the etiology of forearm fractures, and understanding the deficits in bone strength that result in forearm fractures in the context of typical sports and recreational activities may lead to interventions to reduce their occurrence.
Bone strength is a function of the material properties of bone, often estimated by bone mineral density (BMD), and bone size or cross-sectional bone geometry. The latter can be characterized by total area, cortical area and thickness, periosteal and endosteal circumferences, and the distribution of mineral within the bone cross-section [9]. Most studies of forearm fracture in children have used dual-energy X-ray absorptiometry (DXA) to measure BMD, and have reported deficits in bone mass or density of the radius [5, 6, 10, 11] and at other skeletal sites [5, 6] among children with a forearm fracture compared to population controls. Some have found a smaller diameter of the radius in fracture cases compared to population controls [11, 12], whereas others show no difference [5]. DXA, however, is a two-dimensional imaging technique that provides a measure of areal BMD (aBMD), which is confounded by bone size, and only a limited measure of bone size, namely projected bone area.
In comparison to DXA, quantitative computed tomography (QCT) or peripheral QCT (pQCT) provides a more refined characterization of bone, including measures of volumetric BMD (vBMD), bone geometry (dimensions, area, and cortical thickness), and quantifies the distribution of mineral within the bone cross-section. Furthermore, pQCT can distinguish between cortical and trabecular bone compartments. Information on these characteristics would provide a better understanding of skeletal deficits associated with fracture risk. Few studies of children with a forearm fracture have used QCT. A cross-sectional study of girls found no difference in trabecular or cortical vBMD of the distal radius; however, girls with a fracture had a narrower diameter of the radial metaphysis than girls without a fracture [13]. A longitudinal study of pubertal girls found that those who sustained an upper limb fracture had lower integral vBMD (cortical and trabecular bone), but a greater cross-sectional area of the radial metaphysis compared to controls, and these differences persisted throughout adolescence [14]. Lastly, among males age 18 years, those with a previous forearm fracture had lower cortical and trabecular vBMD and lower cortical thickness, but no difference in periosteal circumference compared to non-fractured controls [15]. Because bone measurements were taken ≥4 years after most fractures, it is uncertain whether they accurately reflect those at the time of fracture. The discordant findings among studies make it difficult to draw conclusions about the relative importance of bone density and aspects of bone geometry for forearm fracture in children and adolescents.
Our long-term goal is to identify bone characteristics that impart strength and can be used as targets for interventions to reduce forearm fracture risk. It is important to investigate possible determinants of bone strength in the context of injuries that occur during typical childhood activities, and thus, the use of injured but non-fractured controls. The objectives of this study were to determine whether trabecular and cortical vBMD and aspects of bone geometry in the distal radius measured by pQCT differed between children who sustained a forearm fracture as a consequence of injury compared to those who sustained an injury but did not fracture. Our secondary objective was to determine how well bone characteristics measured by pQCT and by DXA predicted forearm fracture for screening purposes.
Methods
We recruited subjects for this case–control study from the patient population who underwent radiography of the forearm at Cincinnati Children’s Hospital Medical Center for a suspected forearm fracture. Both fractured and non-fractured subjects were recruited from this patient pool: all had sustained an injury to the forearm. Subjects were 5 to 16 years of age; there was no restriction regarding race/ethnicity. We excluded children with chronic diseases that affect bone, with injuries due to a motor vehicle accident, with bilateral forearm fractures or with a concurrent fracture at another skeletal site. Recruitment was stratified by sex and presence of a fracture. The goal was to have similar age distributions of forearm fracture cases and injured controls within each sex. Midway through the study we noted that fracture cases were younger than controls and had sustained injuries due to a fall rather than a collision. Thus, recruitment of controls was restricted to better match cases in terms of age and mechanism of injury (fall versus collision). The Institutional Review Board approved the study protocol, and the parent or guardian provided informed consent.
We recruited subjects as a forearm fracture case or injured control based on the initial radiography report. Subsequently, the study radiologist (TL) reread all radiographs to confirm group assignment. Criteria for classification as a forearm fracture were radiographically evident bony contour deformity of the radius or ulna on the initial imaging examination, or callus formation on a follow-up radiograph. Criteria for controls were no bony contour deformity on the initial radiograph, no callus formation on follow-up radiographs, clinical management consistent with no fracture (i.e., no cast), and no report of persistent pain at screening. All bony fractures were characterized according to bone involved (radius and/or ulna) and site (physis, metaphysis or metadiaphysis [end of the metaphysis abutting the diaphysis], or diaphysis).
PQCT scans of the non-injured forearm were obtained at enrolment with a Stratec XCT2000 6-detector scanner at sites 4% (metaphysis) and 20% (diaphysis) from the distal end of the radius. We performed a scout scan for scan positioning according to the landmarks described by Neu et al. [16]. For subjects with an open physis, the reference line was placed at the proximal edge of the physis in the center of the radius. The reference line was placed at the ulnar edge of the articular surface if the physis was closed or closing (13% of girls, 8% of boys). All scans were obtained with a voxel size of 0.2 mm and speed of 25 m/s. Scans were analyzed using the manufacturer’s software version 5.50. Scans of the 4% site were analyzed with an iterative algorithm that identifies the steepest gradient on the cortical shell to identify the periosteal bone edge (Contour mode 2). This analysis provided measures of total or integral vBMD, bone mineral content (BMC), and cross-sectional area. The interior trabecular bone region was identified using a threshold of 400 mg/cm3 (Peel mode 1) allowing measurement of trabecular vBMD.
We identified the periosteal and endosteal bone edges of scans at the 20% site using two different analysis thresholds, 710 and 280 mg/cm3 (Cort mode 1), as there has been no consensus as to a single best threshold [17, 18]. The differences between cases and controls were similar regardless of threshold used (data not shown). We presented results using thresholds that theoretically best reflect the characteristic being measured. A threshold of 710 mg/cm3 was used to identify bone edges for measurement of cortical vBMD, cortical area, and periosteal and endosteal circumferences. A threshold of 280 mg/cm3 was used to identify bone edges for BMC and the strength–strain index (SSI) in order to capture the maximum amount of bone mineral. We used the manufacturer’s software “circular ring model” to calculate periosteal and endosteal circumferences, and cortical thickness. SSI (section modulus accounting for cortical density) also was calculated by the manufacture’s software as follows: SSI = Σvoxels (A × d2 × vBMDvox/1,200 mg/cm3)/dmax, where A is the area of a voxel, d is the distance of the voxel from the center, dmax is the maximum distance of any of the voxels from the center, and vBMDvox is the vBMD for each voxel.
All subjects had DXA scans (Hologic, QDR 4500, Bedford, MA, USA) of the total body, lumbar spine and proximal femur (hip). A DXA scan of the non-injured forearm was obtained on the latter half of subjects to enable comparison with forearm pQCT measures. We performed DXA scans according to Hologic guidelines, and analyzed DXA scans by software version 12.1 using the automatic low-density option. To prevent measurement artifacts due to the cast material, we excluded the scan region containing the injured arm and replicated the information from the non-injured arm to generate all total body results. Information on BMC, aBMD, and bone area were obtained for following regions of interest (ROI) lumbar spine, total hip, femoral neck, ultradistal radius (metaphysis), and 1/3 radius (diaphysis). The total body less head (TBLH; i.e., subcranial skeleton) ROI was used instead of the total body ROI because the head contains an inconsistent proportion of total body BMC relative to body size in children [19]. Information on lean mass and fat mass was obtained from the total body scans.
Information about arm dominance and mechanism of injury was ascertained by questionnaire at enrolment. Subjects reported the type of activity in which they were engaged when injured and whether the injury was a result of a fall or a collision. We further classified mechanism of injury as low, moderate, or severe trauma according to criteria described by Landin [20] and modified by Clark et al. [21]. Low trauma: falls during informal play, ball sports, and gymnastics, from standing heights or less (<0.5 m), to a trampoline from ≤3 m; wrestling; being hit by a soccer or soft ball; and collisions with a stationary object while moving slowly. Moderate trauma: falls onto a non-resilient surface from 0.5 to 3 m, down stairs, from a moving bicycle, swing, slide, skateboard, rollerblades, or skis moving at moderate or fast speed; being hit by a bicycle; collisions between two moving objects; collision with a stationary object while moving at moderate or fast speed; and falls from standing height with another person landing on top of them. Severe trauma: falls from height >3 m, and being hit by a moving heavy object (>body weight).
Height and weight were measured in duplicate with children dressed in light clothing and without shoes. Height was measured with a stadiometer, and weight was measured with an electronic balance. Body mass index (BMI) was calculated as weight/height2 (kg/m2), and BMI Z-scores were calculated using the CDC 2000 growth reference [22]. Subjects ≥8 years were given pictures illustrating the five stages of pubertal development, breast (girls) and gonadal (boys), according to criteria of Tanner [23]. Subjects, with help of their parents, were asked to select the Tanner stage most similar to them. We assumed that subjects <8 years of age were Tanner stage 1 (pre-pubertal).
Statistical analyses were performed using JMP statistical software (version 5.1, SAS Institute, Cary, NC, USA). We conducted multiple regression analyses to determine whether bone measures differed between cases and controls. The dependent variables were the bone measures, and the primary independent variable was group (case vs. control). All bone measures, except cortical vBMD and cortical thickness, were log-transformed to remove heteroscedasticity and improve the fit of the regression models. Results were reverse transformed for presentation. To adjust for imbalances between groups and to reduce the residual error, we included the following covariates in all regression models: sex, age, black race, Tanner stage, height, and injury type (fall vs. collision) or severity. Regression models for forearm bone measures also included arm dominance and forearm length if the latter was a stronger predictor than height (based on model R2) for that bone measure. Additional variables (height2, Tanner stage-by-sex, and sex-by-fracture group interactions) were tested and kept in the model if p<0.05. Given that cortical vBMD measurements are subject to “partial volume effect” especially when the cortical shell is thin, cortical thickness was included in the regression models for cortical vBMD to statistically adjust for this potential bias [24]. We calculated the percentage difference between groups and the 95% confidence interval (95% CI) around the percentage difference [25].
We calculated Z-scores for each bone measure to enable expression of that bone measure relative to the average value given the child’s age, sex, race, height, and Tanner stage in our study sample. Z-scores were calculated as the Studentized residual (mean=0, standard deviation=1) from the multiple regression models. We then performed logistic regression analyses, where fracture group was the dependent variable, to determine the odds ratio (OR) and 95% CI associated with a change in the Z-score for each bone measure. This method enabled us to compare the relative importance of each bone measure for predicting fracture risk in a standardized way. Covariates considered in the logistic regression models were injury type or severity and arm dominance. Receiver operating characteristic (ROC) curves were generated for each bone Z-score, and the area under the curve (AUC) was calculated to reflect the potential of that bone measure for identifying individuals at risk of fracture. ROC curves are commonly used to evaluate the performance of a diagnostic test. An AUC of 1 indicates a perfect test; an AUC of 0.5 indicates that the test is equivalent to chance.
Results
We enrolled 453 participants, representing a 54% and a 49% acceptance rate for cases and controls, respectively. Enrolment and all study measurements were obtained 28± 8 days after injury. The study radiologist classified 29 subjects fracture status as “suspicious, but not definitive”, therefore they were excluded. The remaining subjects were classified as a forearm fracture case (n=224) or an injured control (n=200). Fractures occurred in the radius only n= 114, radius and ulna n=105, and ulna only n=5. Of the 219 radius fractures, 18 involved the physis (Salter-Harris II and III fractures), three distal epiphysis with extension to the articular surface, 153 distal metaphysis or metadiaphysis, 38 diaphysis, and 11 proximal radius. Of the 110 ulnar fractures, three involved the physis, 29 styloid process, 51 metaphysis or metadiaphysis, and 30 diaphysis. The numbers of fractures at each location exceed the total as some bones were fractured in multiple locations.
Among boys, fracture cases were similar to controls in height, weight, BMI, lean mass, fat mass, and whether the injured arm was the dominant vs. non-dominant arm (Table 1). Male controls were more likely to be of black race than cases (p<0.05). Among girls, fracture cases were younger than controls (p<0.05), and consequently also had lower height, weight, lean mass, and were more likely to be pre-pubertal and pre-menarchal (p<0.05). There were no differences in these measures after adjusting for age. However, girls with a fracture were more likely to have injured their non-dominant arm, whereas controls were more likely to have injured their dominant arm (p<0.05).
Table 1.
Descriptive characteristics (mean±SD) of forearm fracture cases and injured controls
| Boys
|
Girls
|
|||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| N | 106 | 103 | 118 | 97 |
| Age (year) | 11.6±2.8 | 11.5±2.3 | 10.1±2.2a | 11.0±2.6 |
| Height (cm) | 150.0±17.4 | 150.5±14.5 | 141.1±13.9b | 146.3±14.5 |
| Weight (kg) | 47.2±18.3 | 47.5±17.3 | 39.5±13.8b | 44.5±16.9 |
| Lean mass (kg) | 35.1±12.9 | 35.0±11.8 | 26.8±7.9b | 30.5±9.6 |
| Fat mass (kg) | 10.9±7.0 | 11.4±8.0 | 11.6±6.9 | 13.0±8.4 |
| Body fat (%) | 21.9±7.5 | 22.4±9.2 | 27.6±7.6 | 27.4±7.6 |
| Body mass index (kg/m2) | 20.2±4.3 | 20.4±4.6 | 19.4±4.2 | 20.2±5.2 |
| Body mass index Z-score | 0.6±1.1 | 0.6±1.2 | 0.5±1.1 | 0.5±1.1 |
| Pre-pubertalc | 43% | 42% | 44%b | 28% |
| Pre-menarcheal | – | – | 90%b | 73% |
| Black race | 13%a | 24% | 19% | 21% |
| Injured dominant arm | 38% | 47% | 42%a | 57% |
| Injury from fall (vs. collision) | 89% | 83% | 91% | 87% |
| Injury severity low (vs. moderate + severe) | 46% | 59% | 47% | 71%a |
Significantly different from controls of same sex p<0.05
Significantly different from controls of same sex, but no difference after adjusting for age p>0.05
Tanner stage I
Children reported doing a wide variety of activities when they sustained their fracture. The most common types of activities were: skating/skateboarding (13.9%), non-specific “running around” (9.4%), basketball (9.2%), bicycling (7.5%), soccer (7.1%), walking and tripped/slipped (4.0%), football (3.8%), monkey bars (3.3%), skiing/sledding (3.1%), and other (38.6%). The proportion of subjects categorized according to trauma severity was 55.1% low, 43.2% moderate, and 1.6% severe. Owing to the small number of severe trauma injuries, these were included with moderate trauma injuries for remaining analyses. Among boys, cases and controls were similar in terms of trauma severity. Among girls, fracture cases were less likely to have sustained a trauma of low severity than controls.
Four subjects did not get a pQCT scan, and 58 pQCT scans at the 4% site and 51 scans at the 20% site had movement, leaving 85% (n=359) and 87% (n=369) of the sample with valid scans at the 4% and 20% sites, respectively. Cases and controls had similar proportions of valid scans. Adjusted means for pQCT measures for cases and controls are given in Table 2. There was no interaction between sex and fracture group for any bone measure (p> 0.12). Fracture cases had significantly lower total vBMD (−3.2%) at the 4% site compared to controls, but there were no differences between groups in trabecular vBMD or in total bone area at the 4% site. At the 20% site, cases had lower total BMC (−3.4%), cortical vBMD (−0.9%), cortical area (−2.8%), and SSI (−4.6%) than controls, but there were no significant differences in periosteal or endosteal circumference and cortical thickness. The magnitude of difference in cortical vBMD between cases and control was smaller when including cortical thickness in the regression model.
Table 2.
Adjusted mean values of pQCT measurements for forearm fracture cases and injured controls
| Fracture Cases | Injured Controls | p value | % difference (95% CI) | |
|---|---|---|---|---|
| 4% site | (n=171) | (n=165) | ||
| Total BMC (mg) | 70.90 | 73.21 | 0.057 | −3.2 (−6.8, 0.04) |
| Total vBMD (mg/cm3) | 309.36 | 319.99 | 0.019 | −3.4 (−6.4, −0.6) |
| Total area (mm2)a | 229.2 | 228.8 | 0.91 | 0.2 (−3.0, 3.2) |
| Trabecular vBMD (mg/cm3) | 211.6 | 211.1 | 0.88 | 0.2 (−3.2, 3.6) |
| 20% site | (n=191) | (n=178) | ||
| Total BMC (mg) | 60.99 | 63.07 | 0.007 | −3.4 (−6.0, −1.0) |
| Cortical vBMD (mg/cm3)a | 1,063 | 1,073 | 0.013 | −0.9 (−1.53, −0.2) |
| Adjusted for cortical thickness | 1,064 | 1,070 | 0.059 | −0.6 (−0.2, 0.02) |
| Cortical thickness (mm) | 2.14 | 2.18 | 0.08 | −2.1 (−4.3, 0.2) |
| Cortical area (mm2) | 50.63 | 52.03 | 0.03 | −2.8 (−5.3, −0.4) |
| Periosteal circumference (mm) | 30.73 | 30.99 | 0.29 | −0.8 (−2.4, 0.7) |
| Endosteal circumference (mm) | 17.21 | 17.20 | 0.96 | 0.1 (−3.3, 3.4) |
| Strength Strain Index (mm3) | 132.5 | 138.6 | 0.025 | −4.6 (−8.8, −0.7) |
Adjusted for sex, age, black race, Tanner stage, forearm length or height, injured dominant arm, and injury severity; 95% confidence interval around the percentage difference between groups. Italicized and bolded confidence intervals exclude 1
Regression models also included a sex-by-Tanner stage interaction term
DXA scans for three subjects were lost due to a corrupt data storage device. The adjusted mean bone measures derived from the remaining DXA scans are given in Table 3. Compared to controls, fracture cases had significantly lower (−3.8% to −3.2%) BMC of the lumbar spine, total hip, femoral neck, and of the TBLH, but there were no differences in BMC at the ultradistal or 1/3 radius sites. Fracture cases also had a lower (−3.4% to −2.7%) aBMD of the lumbar spine, total hip, femoral neck, and 1/3 radius, but there were no differences in aBMD at the ultradistal radius. There was no difference in DXA measures of bone area between cases and controls except for the TBLH, which was 2.2% lower in cases than controls. Inclusion of lean mass in the regression models for the TBLH measures did not meaningfully affect the statistical significance or magnitude of difference between cases and controls.
Table 3.
Adjusted mean values of DXA measurements for forearm fracture cases and injured controls
| Fracture Cases | Injured Controls | p value | % difference (95% CIa) | |
|---|---|---|---|---|
| Lumbar spine | (n=223) | (n=200) | ||
| BMC (g) | 32.04 | 33.13 | 0.02 | −3.4 (−6.4, −0.6) |
| aBMD (g/cm2) | 0.668 | 0.691 | 0.005 | −3.3 (−5.8, −1.1) |
| Bone area (cm2) | 48.16 | 48.41 | 0.49 | −0.5 (−2.0, 0.9) |
| Total hip | (n=223) | (n=200) | ||
| BMC (g) | 20.83 | 21.62 | 0.015 | −3.8 (−7.0, −0.8) |
| aBMD (g/cm2) | 0.781 | 0.805 | 0.005 | −3.1 (−5.3, −1.0) |
| Bone area (cm2) | 26.85 | 27.18 | 0.20 | −1.2 (−3.0, 0.6) |
| Femoral neck | (n=223) | (n=200) | ||
| BMC (g) | 3.55 | 3.68 | 0.007 | −3.6 (−6.4, −1.1) |
| BMD (g/cm2) | 0.751 | 0.777 | 0.002 | −3.4 (−5.7, −1.3) |
| Bone area (cm2) | 4.73 | 4.74 | 0.78 | −0.2 (−1.5, 1.1) |
| 1/3 radius | (n=107) | (n=128) | ||
| BMC (g) | 1.47 | 1.50 | 0.15 | −2.3 (−5.6, 0.8) |
| aBMD (g/cm2) | 0.588 | 0.604 | 0.009 | −2.7 (−4.9, −0.8) |
| Bone area (cm2) | 3.01 | 3.04 | 0.46 | −0.9 (−3.5, 1.5) |
| Ultradistal radius | (n=107) | (n=128) | ||
| BMC (g) | 1.04 | 1.08 | 0.09 | −3.8 (−8.6, 0.6) |
| aBMD (g/cm2) | 0.346 | 0.355 | 0.11 | −2.9 (−6.5, 0.6) |
| Bone area (cm2) | 2.96 | 2.98 | 0.57 | −0.6 (−2.9, 1.6) |
| Total body less head | (n=221) | (n=200) | ||
| BMC (g) | 1,052 | 1,086 | 0.004 | −3.2 (−5.5, 1.1) |
| BMC adj lean mass (g)b | 1,072 | 1,102 | 0.002 | −2.7 (−4.4, −0.9) |
| Bone area (cm2) | 1,326 | 1,355 | 0.0014 | −2.2 (−3.6, −0.9) |
| BA adj lean mass (g)b | 1,355 | 1,378 | 0.003 | −1.8 (−2.9, −0.7) |
Covariates included in all regression models were injury type (fall vs. collision), age, black race, Tanner stage, height, and injured dominant arm (arm analyses only). Height2 was included in regression models for lumbar spine BMC and aBMD, total hip bone area, and total body bone area. A sex-by-Tanner stage interaction term was included in models for lumbar spine BMC and aBMD, total hip BA, and 1/3 radius BMC
95% confidence interval around the percentage difference between groups
Adjusted for lean mass
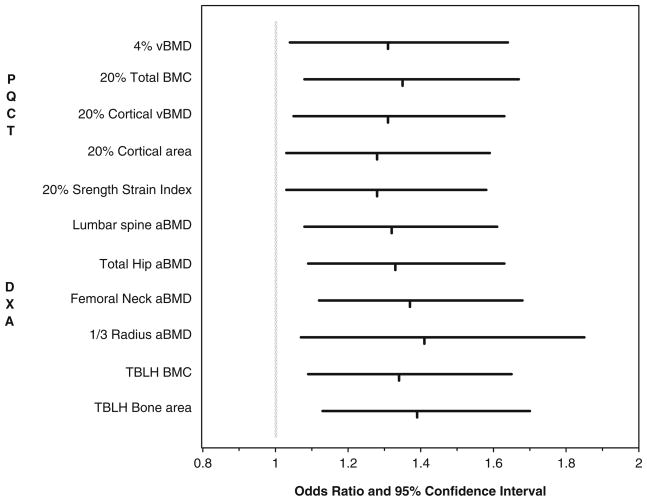
The OR, 95% CI, and area under the ROC curves for each bone measure by pQCT and DXA that significantly differed between cases and controls are given in Fig. 1. The OR associated with a 1 SD decrease in bone measures ranged from 1.28 (95% CI 1.03, 1.59) to 1.41 (95% CI 1.07, 1.85). The AUC ranged from 0.56 to 0.59 when just considering bone measures, and ranged from 0.63 to 0.65 when considering bone measures and injury severity. Despite the fact that bone Z-scores for pQCT measures were only weakly to moderately correlated with those from DXA (r=0.11 to 0.60), inclusion of any one pQCT measure with any DXA measure in ROC analyses had a negligible effect on the AUC (e.g., 0.65 increased to 0.66).
Fig. 1.
Risk of forearm fracture associated with a 1 standard deviation decrease in Z-scores for bone measures derived from pQCT and DXA scans. The vertical dash is the odds ratio and the horizontal line is the 95% confidence interval
Discussion
We investigated characteristics of bone that were associated with forearm fractures in healthy children who sustained injuries during routine informal play, sports, and activities of daily living. We found that children with a forearm fracture had lower cortical, total vBMD, and aBMD and smaller cortical area in the distal radius, which contribute to bone strength, compared to children who were injured but did not fracture. By comparing bone characteristics of children with a forearm fracture to those who were injured but did not fracture, we were better able to isolate the impact of bone characteristics associated with bone strength (accounting for age, maturation, sex, and race) rather than injury risk. The use of pQCT provided specific measures of vBMD and cross-sectional bone geometry in the distal radial metaphysis and diaphysis, which have distinct morphologic characteristics, providing an advantage over prior studies using DXA. Notably, however, pQCT was not more robust than DXA in identifying bone deficits that were predictive of fracture.
Characteristics of the radial metaphysis and trabecular bone
The distal radial metaphysis is largely comprised of trabecular bone surrounded by a thin cortical shell. Children with a forearm fracture had lower total vBMD in the metaphysis than injured controls, but there was no difference in trabecular vBMD. This difference points to the cortical shell as being important for bone strength at this site, as the cortical shell is included in the total, but not the trabecular vBMD measurement. We could not measure the vBMD or thickness of the cortical shell of the metaphysis with our pQCT as it is too thin. Cortical thickness in the metaphysis also was found to be an important predictor of bone strength in cadaveric radius specimens from elderly persons [26]. Cheng et al. found that pubertal girls with an arm fracture had lower vBMD on the periosteal edge of the metaphysis, which may reflect a thinner cortical shell as well [14]. In contrast to our findings in children, young women and men with a prior arm fracture and older women with a Colles’ fracture have reductions in trabecular vBMD in the metaphysis. The discrepancy in findings may reflect age-specific changes in bone characteristics that affect fracture risk [14, 15, 27].
The cortical shell in the metaphysis may be particularly important for bone strength during growth. About 90% of radial bone growth occurs distally [28]. As the radius elongates, bone on the periosteal edge of the metaphysis is resorbed enabling maintenance of the overall shape of the metaphysis. This process results in a very thin cortical shell in the metaphysis that persists until the cessation of growth and maturation [29, 30], and helps to explain why the metaphysis and the metadiaphysis are common sites of forearm fracture in children. With the exception of growth and maturation, there is a paucity of information on factors that influence cortical thickness and density in the metaphysis.
The distal metaphysis of the radius may experience a significant amount of compressive force during a fall. Biomechanically, cross-sectional area as well as vBMD is important for resistance to fracture in the presence of compressive forces. However, we did not detect differences in cross-sectional bone area at the 4% radius site. Because the diameter rapidly narrows along the length of the metaphysis it is possible that the area and/or diameter at other sites are more important. In contrast to our findings and those of others [14, 15], Skaggs et al. found that girls with a forearm fracture had an 8.4% narrower radius measured at the site of fracture, an average of 10% of the radial length near the end of the metaphysis, compared to matched controls without a fracture [13]. However, there was no difference in either total or trabecular vBMD in the metaphysis [13]. The discrepancy in findings among studies may be due to different sites in the metaphysis being measured.
Characteristics of the radial diaphysis and cortical bone
The radial diaphysis is comprised of cortical bone surrounding the medullary cavity. The cortex is thicker than in the metaphysis enabling measurement of cortical bone characteristics. Children with a forearm fracture had a lower cortical vBMD at the 20% site compared to injured controls. Importantly, this effect was still evident when we statistically adjusted for cortical thickness to prevent a spurious association due to “partial volume effect” [29]. Darelid et al. [15] also found a lower cortical vBMD in 18-year-old males ≥4 years after fracture compared to controls, whereas others found no difference [13, 14]. Reduced cortical vBMD may reflect increased intra-cortical remodeling and cortical porosity. Intra-cortical remodeling and porosity are known to be greater in children due to their rapid growth [30, 31] and fracture risk is known to increase around peak growth velocity [15]. We still observed differences in cortical density between groups when statistically accounting for age, attained growth and maturation. Lower vBMD among fracture cases might reflect differences in calcium intake or vitamin D status affecting the availability of mineral to fill bone remodeling spaces or under-mineralized osteoid. Consistent with this speculation, Goulding et al. found that children with repeated forearm fractures were more likely to be milk avoiders [12].
The smaller cortical area at the 20% radius site in children with a forearm fracture compared to injured controls likely reflects both a slightly smaller periosteal circumference and a slightly larger endosteal circumference, as neither alone significantly differed between groups. Cortical dimensions of the radius increase in response to mechanical loading [32]. Thus, investigation of arm loading histories of children with and without forearm fractures is warranted. The combined effects of lower cortical vBMD and smaller cortical area resulted in a lower SSI in fracture cases compared to controls. The SSI expresses strength to withstand bending and torsion, the types of forces that commonly affect this site. Interestingly, SSI did not discriminate between cases and controls better than measures of cortical vBMD or cortical area alone, despite being a function of both bone characteristics. Fracture studies involving cadaveric radii found that fracture load was most strongly related to measures that incorporated both vBMD and dimensions, such as SSI or total BMC [33].
Bone characteristics measured by DXA
DXA measures of aBMD at the distal 1/3 radius site (diaphysis) were lower in children with a fracture compared to controls, which is consistent with other studies using DXA [5, 6, 10]. Overall, odds ratios in our study were smaller than in other studies, which may reflect residual confounding in other studies by not using an injured control group. That we did not find a difference by DXA in aBMD at the ultradistal site (metaphysis) whereas we did by pQCT illustrates a technical advantage of pQCT. Furthermore, we did not detect differences in projected bone area from DXA scans of the forearm, although results from other studies are inconsistent with this finding [5, 11, 12].
Interestingly, in our sample of children and adolescents, we found that measures of aBMD throughout the skeleton predicted forearm fracture similarly. Jones et al. also found this lack of site specificity for predicting upper limb fractures in children and adolescents [34], whereas among adults, site-specific measures of aBMD are stronger predictors of fracture at that site [35]. These generalized skeletal deficits may help explain why children who have experienced a forearm fracture are at greater risk of experiencing a fracture elsewhere in their skeleton as well as another forearm fracture [36]. Overall, DXA was as good as pQCT for identifying bone deficits.
Implications for screening and intervention
Bone densitometry, by pQCT or DXA, alone is likely to be inefficient for screening or identifying healthy children at risk of a forearm fracture as the areas under the ROC curve for all of our bone measures were low. These findings are consistent with others that have evaluated the ability of DXA to identify children with a forearm fracture [10]. Further research is needed to identify other risk factors to help in developing screening and prevention strategies. We were not able to determine the relative importance of behavioral factors that may affect risk of injury, as we sought to match cases and controls on presence of injury to minimize potential confounding. Some studies have found obesity to be associated with an increased forearm fracture risk [5, 6, 13]. We, however, did not find this association. One potential reason is that we used injured controls instead of non-injured population controls, and it is possible that obesity may be related to risk of overall injury [37].
The lower BMD and cortical area in children with a forearm fracture provide support for broad-based interventions to increase these bone characteristics. Although the odds ratios associated with a 1 SD decrease in these bone measures were low, bone measures are potentially modifiable. This is important in light of evidence indicating that a fracture during childhood may be a marker of future bone fragility during growth; the risk of fracture is twofold higher among children who already have had a fracture [38].
Limitations
The study was limited by its retrospective design. We minimized potential bias by obtaining measurements shortly after fracture; it is unlikely that bone measures changed significantly in this time interval. Despite rigorous criteria to ensure that controls did not have a fracture, it is possible that some occult fractures were missed, which would have caused us to underestimate the magnitude of differences between groups. Female cases were slightly younger than female controls and were more likely to have injured their non-dominant arm. Therefore, we statistically adjusted for age, pubertal maturation, and dominance of the injured arm in our analyses. Although unlikely, it is possible that this was not sufficient to adequately account for differences between groups. We also had limited ability to quantify the exact type and magnitude of forces on bone that occurred during the injury. Children have difficulty in providing precise descriptions of the heights, speeds, and arm positions while injured. Nonetheless, it was recently shown that among older men and women, and among children, bone density was lower among those who fractured in high trauma as well as low trauma situations [21, 39]. Our study was limited by the resolution of our pQCT scanner, but it is unlikely to have resulted in spurious findings between groups. A higher resolution scanner would have allowed us to directly investigate the narrow cortex of the metaphysis and would have provided more precise and accurate measures of the cortex in the diaphysis. Also, correct positioning of the reference line is difficult when the physis is fusing and this potentially could have added error to our measurements. Lastly, we studied healthy children with a forearm fracture, and we cannot extrapolate our findings about the utility of densitometry to predict fractures in children with chronic medical conditions or fractures at other skeletal sites.
In summary, children with a forearm fracture have lower vBMD of the total bone in the radial metaphysis, likely reflecting a thinner and less dense cortex, and lower vBMD of cortical bone and cortical area in the radial diaphysis compared to children who are injured but did not fracture. These findings provide support for research to identify interventions to increase these bone characteristics, and thereby bone strength, in order to ultimately reduce fracture occurrence. The diagnostic efficiency of BMD and geometric measures by pQCT and DXA was low. Additional research is needed to identify other factors that influence fracture risk so that better screening measures may be developed.
Acknowledgments
We would like to thank the subjects and their families who generously donated their time to participate in this study. We also would like to thank the staff responsible for data collection Donna Bianchi, Ashwini-Roy Chaudhury, Gemma Uetrecht and Caroline Wood.
Funding This project was supported by NIH grants AR47242 and RR08084, from the General Clinical Research Centers Program
Footnotes
Conflicts of interest None.
Contributor Information
H. J. Kalkwarf, Email: Heidi.Kalkwarf@cchmc.org, Division of General and Community Pediatrics, Cincinnati Children’s Hospital Medical Center, ML-7035, 3333 Burnet Avenue, Cincinnati, OH 45229, USA
T. Laor, Email: Tal.Laor@cchmc.org, Department of Radiology, Cincinnati Children’s Hospital Medical Center, ML-5031, 3333 Burnet Avenue, Cincinnati, OH 45229, USA
J. A. Bean, Email: Judy.Bean@cchmc.org, Epidemiology and Biostatistics, Cincinnati Children’s Hospital Medical Center, ML-5041, 3333 Burnet Avenue, Cincinnati, OH 45229, USA
References
- 1.Jones IE, Williams SM, Dow N, Goulding A. How many children remain fracture-free during growth? A longitudinal study of children and adolescents participating in the Dunedin Multidisciplinary Health and Development Study. Osteoporos Int. 2002;13:990–995. doi: 10.1007/s001980200137. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res. 2004;19:1976–1981. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 3.Jones IE, Cannan R, Goulding A. Distal forearm fractures in New Zealand children: annual rates in a geographically defined area. N Z Med J. 2000;113:443–445. [PubMed] [Google Scholar]
- 4.Khosla S, Melton LJ, 3rd, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA. 2003;290:1479–1485. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 5.Goulding A, Cannan R, Williams SM, Gold EJ, Taylor RW, Lewis-Barned NJ. Bone mineral density in girls with forearm fractures. J Bone Miner Res. 1998;13:143–148. doi: 10.1359/jbmr.1998.13.1.143. [DOI] [PubMed] [Google Scholar]
- 6.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy X-ray absorptiometry study. J Pediatr. 2001;139:509–515. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 7.Manias K, McCabe D, Bishop N. Fractures and recurrent fractures in children; varying effects of environmental factors as well as bone size and mass. Bone. 2006;39:652–657. doi: 10.1016/j.bone.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Clark EM, Ness AR, Tobias JH. Vigorous physical activity increases fracture risk in children irrespective of bone mass: a prospective study of the independent risk factors for fractures in healthy children. J Bone Miner Res. 2008;23:1012–1022. doi: 10.1359/jbmr.080303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouxsein ML, Karasik D. Bone geometry and skeletal fragility. Curr Osteoporos Rep. 2006;4:49–56. doi: 10.1007/s11914-006-0002-9. [DOI] [PubMed] [Google Scholar]
- 10.Jones G, Boon P. Which bone mass measures discriminate adolescents who have fractured from those who have not? Osteoporos Int. 2008;19:251–255. doi: 10.1007/s00198-007-0458-1. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R. Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistent bone fragility? J Bone Miner Res. 2006;21:501–507. doi: 10.1359/jbmr.051215. [DOI] [PubMed] [Google Scholar]
- 12.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20:2090–2096. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- 13.Skaggs DL, Loro ML, Pitukcheewanont P, Tolo V, Gilsanz V. Increased body weight and decreased radial cross-sectional dimensions in girls with forearm fractures. J Bone Miner Res. 2001;16:1337–1342. doi: 10.1359/jbmr.2001.16.7.1337. [DOI] [PubMed] [Google Scholar]
- 14.Cheng S, Xu L, Nicholson PHF, Tylavsky FA, Lyytikainen A, Wang Q, Suominen H, Kujala UM, Kroger H, Alen M. Low volumetric BMD is linked to upper-limb fracture in pubertal girls and persists into adulthood: a seven-year cohort study. Bone. 2009;45:480–486. doi: 10.1016/j.bone.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Darelid A, Ohlsson C, Rudang R, Kindblom JM, Mellstrom D, Lorentzon M. Trabecular volumetric bone mineral density is associated with previous fracture during childhood and adolescence in males—the GOOD study. J Bone Miner Res. 2009;25:537–544. doi: 10.1359/jbmr.090824. [DOI] [PubMed] [Google Scholar]
- 16.Neu CM, Manz F, Rauch R, Merkel A, Schoenau E. Bone densities and bone size at the distal radius in healthy children and adolescents: a study using peripheral quantitative computed tomography. Bone. 2001;28:227–232. doi: 10.1016/s8756-3282(00)00429-4. [DOI] [PubMed] [Google Scholar]
- 17.Kontulainen S, Liu D, Manske S, Jamieson M, Sievanen H, McKay H. Analyzing cortical bone cross-sectional geometry by peripheral QCT: comparison with bone histomorphometry. J Clin Densitom. 2007;10:86–92. doi: 10.1016/j.jocd.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Ward KA, Adams JE, Hangartner TN. Recommendations for thresholds for cortical bone geometry and density measurement by peripheral quantitative computed tomography. Calcif Tissue Int. 2005;77:275–280. doi: 10.1007/s00223-005-0031-x. [DOI] [PubMed] [Google Scholar]
- 19.Taylor A, Konrad PT, Norman ME, Karcke HT. Total body bone mineral density in young children: influence of head bone mineral density. J Bone Miner Res. 1997;12:652–655. doi: 10.1359/jbmr.1997.12.4.652. [DOI] [PubMed] [Google Scholar]
- 20.Landin LA. Fracture patterns in children. Acta Orthop Scand Suppl. 1983;54:1–109. [PubMed] [Google Scholar]
- 21.Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res. 2008;23:173–179. doi: 10.1359/jbmr.071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Tanner JM. Growth at adolescence. Blackwell Scientific; Oxford: 1962. [Google Scholar]
- 24.Schoenau E, Neu CM, Rauch R, Manz F. Gender-specific pubertal changes in volumetric cortical bone mineral density at the proximal radius. Bone. 2002;31:110–113. doi: 10.1016/s8756-3282(02)00802-5. [DOI] [PubMed] [Google Scholar]
- 25.Cole TJ. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med. 2000;19:3109–3125. doi: 10.1002/1097-0258(20001130)19:22<3109::aid-sim558>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Spadaro JA, Werner FW, Brenner RA, Fortino MD, Fay LA, Edwards WT. Cortical and trabecular bone contribute strength to the osteopenic distal radius. J Orthop Res. 1994;12:211–218. doi: 10.1002/jor.1100120210. [DOI] [PubMed] [Google Scholar]
- 27.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22:425–433. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 28.Pritchett J. Growth plate activity in the upper extremity. Clin Orthop. 1991;268:235–242. [PubMed] [Google Scholar]
- 29.Rauch F, Neu C, Manz F, Schoenau E. The development of metaphyseal cortex—implications for distal radius fractures during growth. J Bone Miner Res. 2001;16:1547–1555. doi: 10.1359/jbmr.2001.16.8.1547. [DOI] [PubMed] [Google Scholar]
- 30.Kirmani S, Christen D, vanLenthe GH, Fischer PR, Bouxsein ML, McCready LK, Melton LJ, 3rd, Riggs BL, Amin S, Muller R, Khosla S. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24:1033–1042. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos Int. 1994;4:382–398. doi: 10.1007/BF01622201. [DOI] [PubMed] [Google Scholar]
- 32.Ducher G, Courteix D, Meme S, Magni C, Viala JF, Benhamou SL. Bone geometry in response to long-term tennis playing and its relationship with muscle volume: a quantitative magnetic resonance imaging study in tennis players. Bone. 2005;37:457–466. doi: 10.1016/j.bone.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Muller ME, Webber CE, Bouxsein ML. Predicting the failure load of the distal radius. Osteoporos Int. 2003;14:345–352. doi: 10.1007/s00198-003-1380-9. [DOI] [PubMed] [Google Scholar]
- 34.Jones G, Ma D, Cameron F. Bone density interpretation and relevance in Caucasian children aged 9–17 years of age: insights from a population-based fracture study. J Clin Densitom. 2006;9:202–209. doi: 10.1016/j.jocd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ, 3rd, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 36.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15:2011–2018. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- 37.Goulding A, Jones IE, Taylor RW, Piggot JM, Taylor D. Dynamic and static tests of balance and postural sway in boys: effects of previous wrist bone fractures and high adiposity. Gait Posture. 2003;17:136–141. doi: 10.1016/s0966-6362(02)00161-3. [DOI] [PubMed] [Google Scholar]
- 38.Goulding A, Rockell JE, Black RE, Grant AM, Jones IE, Williams SM. Children who avoid drinking cow’s milk are at increased risk for prepubertal bone fractures. J Am Diet Assoc. 2004;104:250–253. doi: 10.1016/j.jada.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]