Abstract
Case histories of two unrelated patients suffering from sensory ataxic neuropathy, dysarthria/ dysphagia and external ophthalmoplegia (SANDO) are reported. Both patients showed compound heterozygosity for POLG1 gene mutations, and presented with symptom of the clinical characteristics of SANDO. A patient with a p.A467T and p.W748S, well-known mutations showed a progressive course with early onset and multisystem involvement, including symptoms characteristics for mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). The second patient showed a less well-known p.T251I and p.G848S mutations with late onset and dysphagia/dysarthria dominated, moderate symptoms. This later is the second published case history, when these POLG1 gene mutations are the possible background of late onset SANDO, dominantly presenting with bulbar symptoms.
Key words: SANDO, heterozygote POLG1 mutations
Introduction
Several case histories with a remarkably uniform clinical picture, consisting of sensory ataxic neuropathy, dysarthria and ophthalmoparesis (SANDO) have been published during the last twenty years. Both, dominant and recessive missense mutations have been reported in the gene encoding the mitochondrial DNA polymerase gamma enzyme (POLG1) in patients with this syndrome (1-3). Multi-organ involvement affecting central nervous system, liver, stomach and intestine has been documented, and the possibility of sudden cardiac arrest also has been emphasized (4, 5). Here we report case histories of two patients, with different compound heterozygous POLG1 mutations, demonstrating the characteristics of SANDO, but with different clinical pictures and different disease courses.
Case histories
Patient 1
A 48 year-old female patient has been followed at our department since the age of 35. Her symptoms started when she was about 25 years old with paresthesia and sensory loss in the extremities. She had two pregnancies in her early twenties, but she had to receive fertility stimulation. Her menopause occurred at her age of 35. At first admission, physical neurological investigation revealed limited eye movements into every direction, without double vision, absent deep tendon reflexes, moderate sensory loss of superficial sensation in all extremities, and severe sensory loss of deep sensations. Neither definite paresis, nor ataxia or gait disturbances were found. During the period of follow up, the patient experienced a continuous deterioration. The sensory loss increased, followed by gait disturbances. Since her early forties, she has been suffering from repeated cramps and twitching in her muscles. She also noticed difficulties in swallowing of fluids, and has been suffering from continuous constipation. She has also been suffering from anxiety and depression for 5-6 years. During the last 2 years she has become wheelchair-bound. Now, at 48 years of age, physical investigation showed total ophthalmoplegia, mild dysarthria and dysphagia, moderate sensory loss of the superficial sensations in the upper extremities, and moderate superficial -, but severe deep sensory loss in the lower extremities. Severe gait ataxia was seen.
Chemical laboratory investigation showed normal parameters, without muscle or liver enzyme increase. Molecular genetic investigation with DNA sequencing of the POLG1 gene discovered two heterozygote mutations, a c.1399G > A giving the amino acid substitutions alanin to threonine (p.A467T) and a c.2243G > C, resulting in a trytophane to serine exchange (p.W748S). Neurophysiology demonstrated axonal sensory and motor neuropathy. EEG recordings, including sleep deprivation demonstrated generalized slow-wave abnormities, with sharp waves in the rear temporal regions, without epileptic discharges. Brain MRI did not show any pathology. Constipation has been accounted to hypomotility of the intestines by repeated gastroenterological investigations. The patient received high dose multivitamin and Q10 treatment, without obvious effect furthermore, she has been receiving antidepressive and anxiolytic treatment.
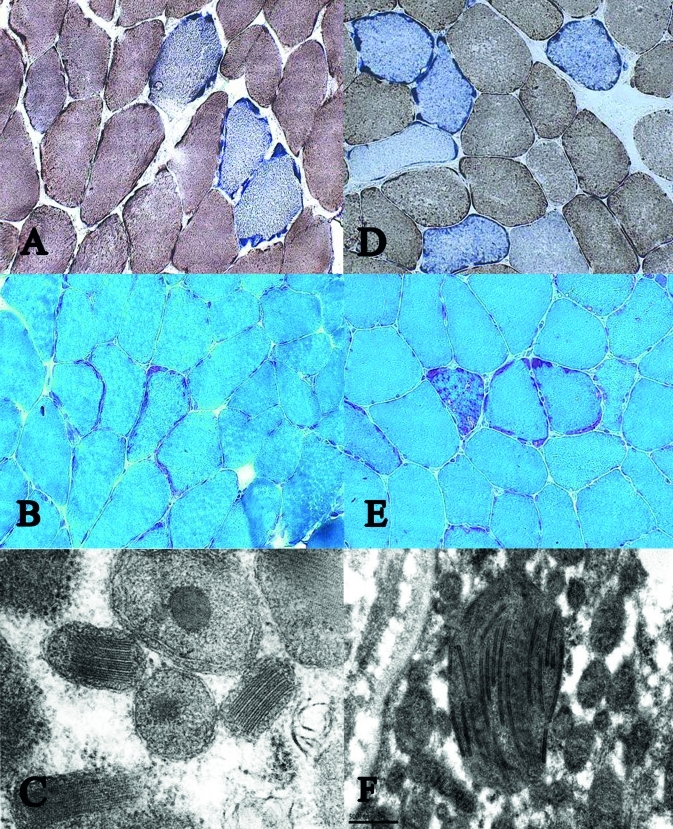
Muscle biopsy from m tibialis anterior demonstrated several ragged-red -, and COX-negative fibres. Electron microscopy showed increased amount of glycogen and neutral fat in the intermyofibrillar and subsarcolemmal area, mitochondrial proliferation, with bizarre mitochondrial morphology, crystalloid mitochondrial inclusions, and mitochondria without internal structures (Fig. 1 a, b, c).
Figure 1.
Gomori trichrom (a), COX/SDH (b) and ultra sturcture (c) of patient 1, and Gomori trichrom (d), COX/ SDH (e) and ultra sturcture (f) of patient 2.
Patient 2
A 68 year-old male patient has been followed at our department for 3 years. His complains started in his fifties with moderate sensory loss in both legs, and mild gait disturbances. Dysphagia, dysarthria occurred when he was about 60 years old. At admission, at his age of 65, the physical investigation revealed bilateral moderate ptosis, moderately limited, conjugated eye movements into every direction. Weak soft palatal and swallowing reflexes were seen. He had difficulties to swallow fluids and had nasal dysarthria. There were neither paresis in the muscles of trunk and extremities, nor physical signs of myasthenia were found. Deep tendon reflexes were weak in the upper extremities and were absent in the lower extremities. He had impaired touch, cold, pinprick sensation and a profound loss of position and vibration senses, in the lower extremities. Moderate lower limb- and gait ataxia were seen. Autonomic functions were intact. During the last 3 years of follow up, a mild progression was seen.
The red- and white blood cell counts, serum electrolyte-, serum lipid- and liver enzyme levels, including creatine kinase were within the normal range. Acethylcholin receptor antibodies were not found. Two mutations of the POLG1 gen were found by genetic investigations; mutation, a c.752C > T with an aminoacid exchange from threonine to isoleucine (p.T251I), and a mutation c.2542G > A, with exchange from glycine to serine (p.G848S). Neurophysiology demonstrated axonal sensory neuropathy. Myography did not find any pathology. Brain MRI investigation described lacunar ischemia.
Muscle biopsy from m tibialis anterior showed the same pathology as that found in case 1, but more raggedred fibres were seen, and perhaps less ultra structural abnormities (Fig. 1 d, e, f).
Discussion
Sensory ataxic neuropathy, dysarthria and ophthalmoparesis (SANDO) is a characteristic clinical triad, which has been attributed to mutations of the gene encoding the mitochondrial DNA polymerase gamma enzyme (POLG1). Most cases present with an initial stage of sensory neuropathy, a second stage of progressive external ophahlmoplegia and dysarthria, which is then followed by other symptoms, often with epilepsia or myoclonus (2). Different dominant and recessive missence mutations of this gene have been described with various clinical phenotypes (3-5). Our patients had a characteristic clinical triad of SANDO, and both with dysphagia/dysarthria.
Patient 1 presented with a p.A467T and p.W748S compound heterozygous mutations. Both mutations are known, and several case histories with these compound mutations have been published (6, 7). Central nervous system involvement is characteristic of these mutations, as epilepsy, myoclonus, headache and bilateral high signal lesions of the posterior horn. Our patient also presented with central nervous system symptoms, as she had myoclonus, EEG abnormalities, and presumably her anxiety and depression could also – at least partly - be attributed to metabolic brain disturbances. Vissing and co-workers published case histories of two sisters with features of neurogastrointestinal encephalomyopathy (MNGIE), without signs of leukoencephalopathy (8). They demonstrated multiple mtDNA deletions, but could not find any thymidine phophorylase (TP) gene mutation, the usual genetic background of MNGIE. Further molecular genetic investigation found three missense mutations in POLG1 gene, of which two (N846S and P587L) mutations were not described before, and the third one was a known T251I mutation (9). Besides dysphagia, our patient was also suffering from continuous constipation, mimicking the symptoms of MNGIE. Gonadal hypofunction and premature menopause have been described as a sign of multi-organ involvement in POLG1 mutations (4). These symptoms also complicated the clinical picture of our case. Multi-organ involvement and progressive course of our patient support the finding of Tzoulis and colleagues, that compound heterozygous p.A467T and p.W748S POLG1 mutations present a severe clinical phenotype of SANDO (7).
The second patient had p.T251I, and p.G848S compound heterozygous mutations. These mutations have previously been published, only in two publications. Lamantea and co-workers reported a member of a family, with ophthalmoplegia with proximal muscle weakness, but no detailed clinical picture and disease course were described (3). These authors also presented several individuals with an isolated p.T251I heterozygous mutation without any clinical symptoms. Weiss and co-workers published a case with late onset, when these two mutations were compound, and combined with a third, p.P587L heterozygous mutation (10). The leading symptoms of their patient were ophthalmoplegia, severe nasal flaccid dysarthria and mild tongue weakness. Besides vibration and joint position sensation loss, moderate gait ataxia and mild proximal muscle weakness were found. In our patient, the symptoms also developed relatively late, in his fifties, with slowly progressive sensory loss and moderate ataxia, which later on complicated with expressed dysarthria/ dysphagia and moderate ophthalmoplegia.
Our patients had no definite paresis in the skeletal muscles of the extremities, but the muscle biopsies taken from m. tibialis anterior demonstrated identical pathology, with ragged-red, COX negative fibres and mitochondrial ultra structural abnormities. The degree of the pathological changes did not correlate with the severity of the clinical symptoms.
Both patients demonstrated characteristics of SANDO, with axonal neuropathy, ataxia, dysarthria and ophthalmoplegia. Dysphagia was also present in both cases. The symptoms of the p.A467T and p.W748S compound heterozygous patient 1 started in early adulthood and showed severe neuromuscular symptoms with intestinal and multi-organ involvement, and this case history gave a further evidence of POLG mutation, as a genetic background of MNGIE-like syndrome. The patient 2 with p.T251I, and p.G848S compound heterozygous mutations presented with late onset, dominantly with bulbar symptoms. This case history shows that dysarthria/dysphagia could occur late in the life, as a sign of a genetically determined mitochondrial metabolic disorder.
Acknowledgements
We thank Dr. Anna Gencik, dr. Heinz D. Gabriel and dr. Melanie Kuhn, Center for Medical Genetic, Osnabrueck D-49076, Germany, and dr. Jan Vissing and Marianne Schwartz, Juliane Marie Center, Clinical Genetic, University of Copenhagen, Rigsh, hospitalet, Copenhagen DK- 2100, Denmark, for the molecular genetic investigations. We thank Gunnvor Sjöö, Bo Häggqvist, Bengt-Arne Fredriksson and Liv Gröntoft for excellent technical assistance.
References
- 1.Zeviani M, Bresolin N, Gellera C, et al. Nucleus-driven multiple large-scale deletions of the human mitochondrial genome: a new autosomal dominant disease. Am J Hum Genet. 1990;47:904–914. [PMC free article] [PubMed] [Google Scholar]
- 2.Domburg PH, Gabreëls-Festen AA, Gabreëls FJ, et al. Mitochondrial cytopathy presenting as hereditary sensory neuropathy with progressive external ophthalmoplegia, ataxia and fatal myoclonic epileptic status. Brain. 1996;119:997–1010. doi: 10.1093/brain/119.3.997. [DOI] [PubMed] [Google Scholar]
- 3.Lamantea E, Tiranti V, Bordoni A, et al. Mutations of mitochondrial DNA polymerase gammaA are a frequent cause of autosomal dominant or recessive progressive external ophthalmoplegia. Ann Neurol. 2002;52:211–219. doi: 10.1002/ana.10278. [DOI] [PubMed] [Google Scholar]
- 4.Luoma P, Melberg A, Rinne JO, et al. Parkinsonism, premature menopause, and mitochondrial DNA polymerase-gamma mutations: clinical and molecular genetic study. Lancet. 2004;364:875–882. doi: 10.1016/S0140-6736(04)16983-3. [DOI] [PubMed] [Google Scholar]
- 5.Lewis W, Day BJ, Kohler JJ, et al. Decreased mtDNA, oxidative stress, cardiomyopathy, and death from transgenic cardiac targeted human mutant polymerase gamma. Lab Invest. 2007;87:326–335. doi: 10.1038/labinvest.3700523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakonen AH, Heiskanen S, Juvonen V, et al. Mitochondrial DNA polymerase W748S mutation: a common cause of autosomal recessive ataxia with ancient European origin. Am J Hum Genet. 2005;77:430–441. doi: 10.1086/444548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzoulis C, Engelsen BA, Telstad W, et al. The spectrum of clinical disease caused by the A467T and W748S POLG mutations: a study of 26 cases. Brain. 2006;129:1685–1692. doi: 10.1093/brain/awl097. [DOI] [PubMed] [Google Scholar]
- 8.Vissing J, Ravn K, Danielsen ER, et al. Multiple mtDNA deletions with features of MNGIE. Neurology. 2002;59:926–929. doi: 10.1212/wnl.59.6.926. [DOI] [PubMed] [Google Scholar]
- 9.Goethem G, Schwartz M, Löfgren A, et al. Novel POLG mutations in progressive external ophthalmoplegia mimicking mitochondrial neurogastrointestinal encephalomyopathy. Eur J Hum Genet. 2003;11:547–549. doi: 10.1038/sj.ejhg.5201002. [DOI] [PubMed] [Google Scholar]
- 10.Weiss MD, Santo RP. Sensory ataxic neuropathy with dysarthria and ophthalmoparesis (SANDO) in late life due to compound heterozygous POLG mutations. Muscle Nerve. 2010;41:882–885. doi: 10.1002/mus.21636. [DOI] [PubMed] [Google Scholar]