Abstract
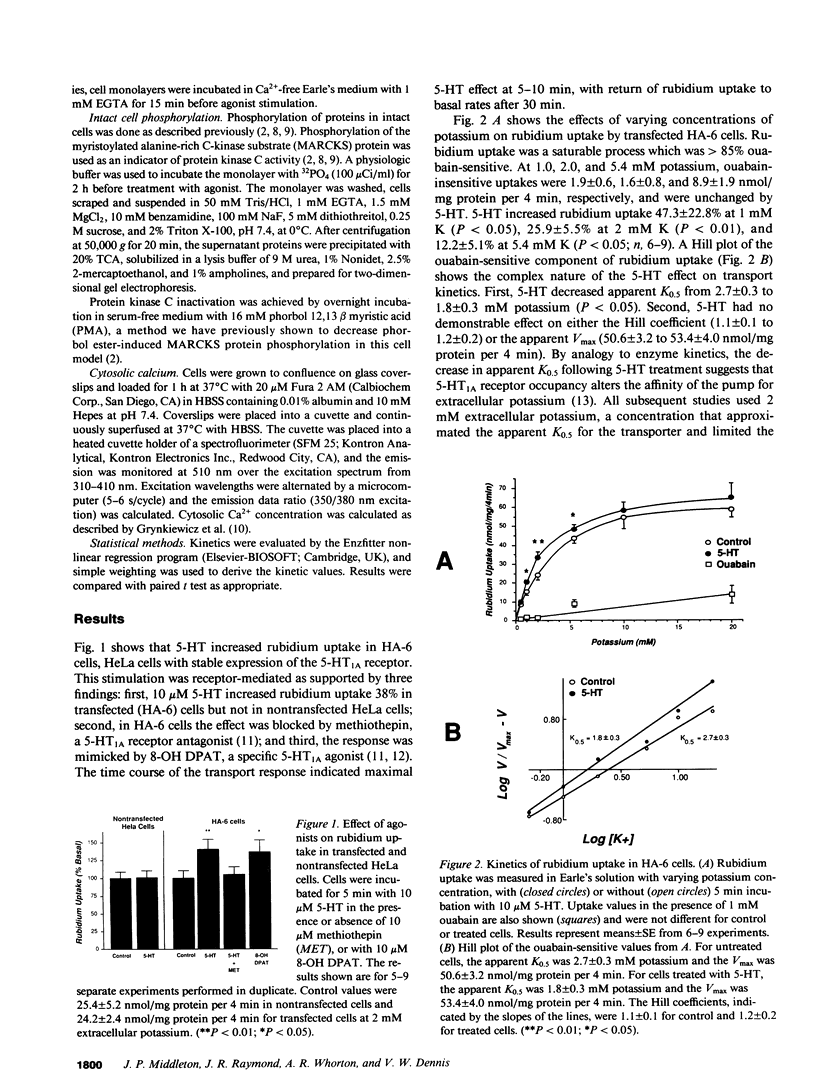
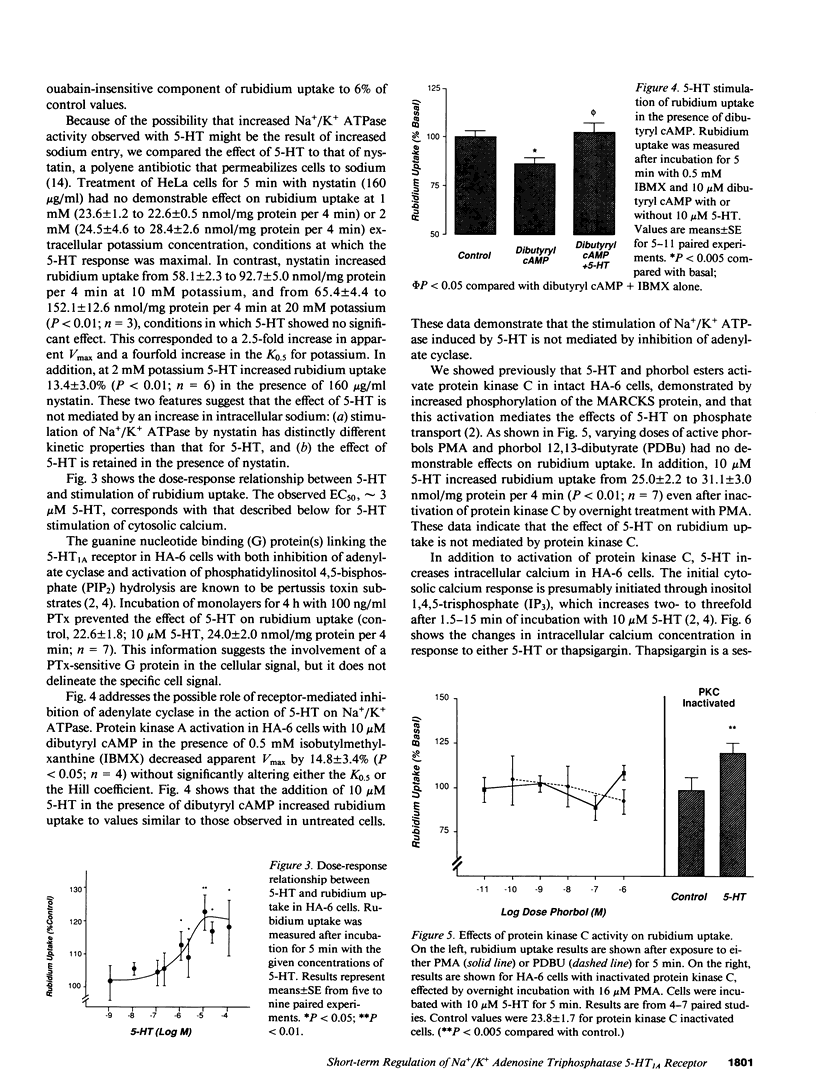
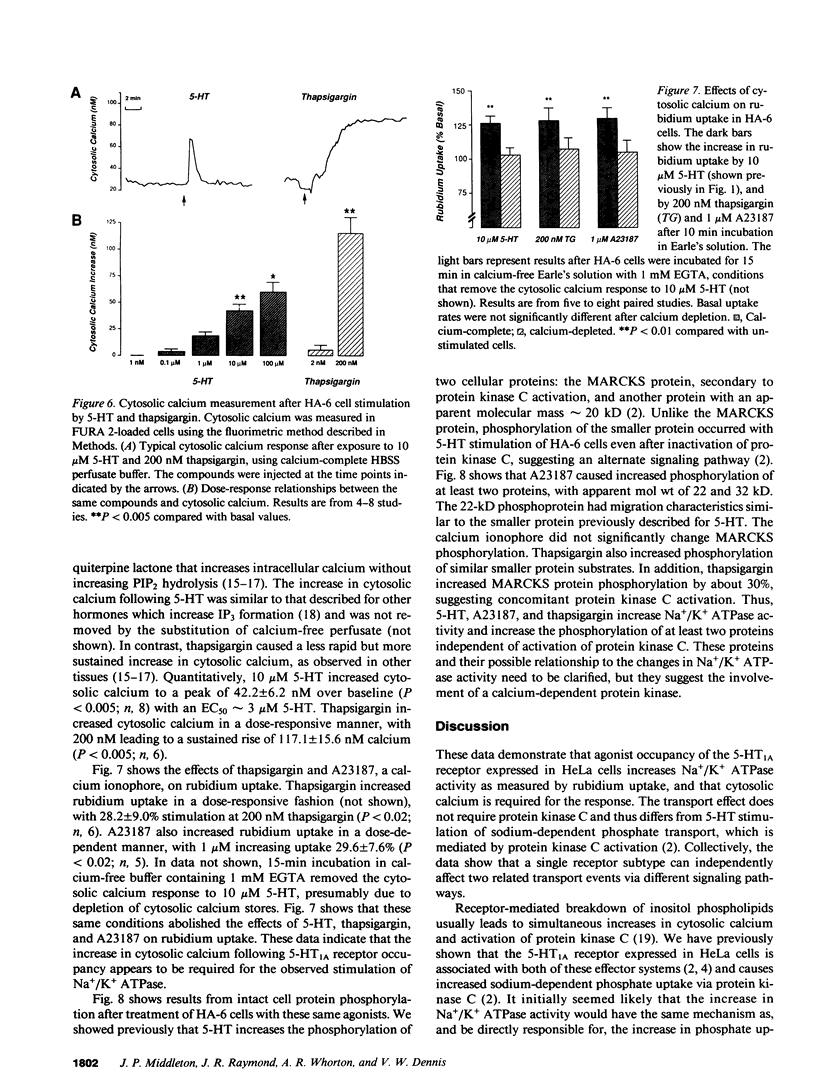
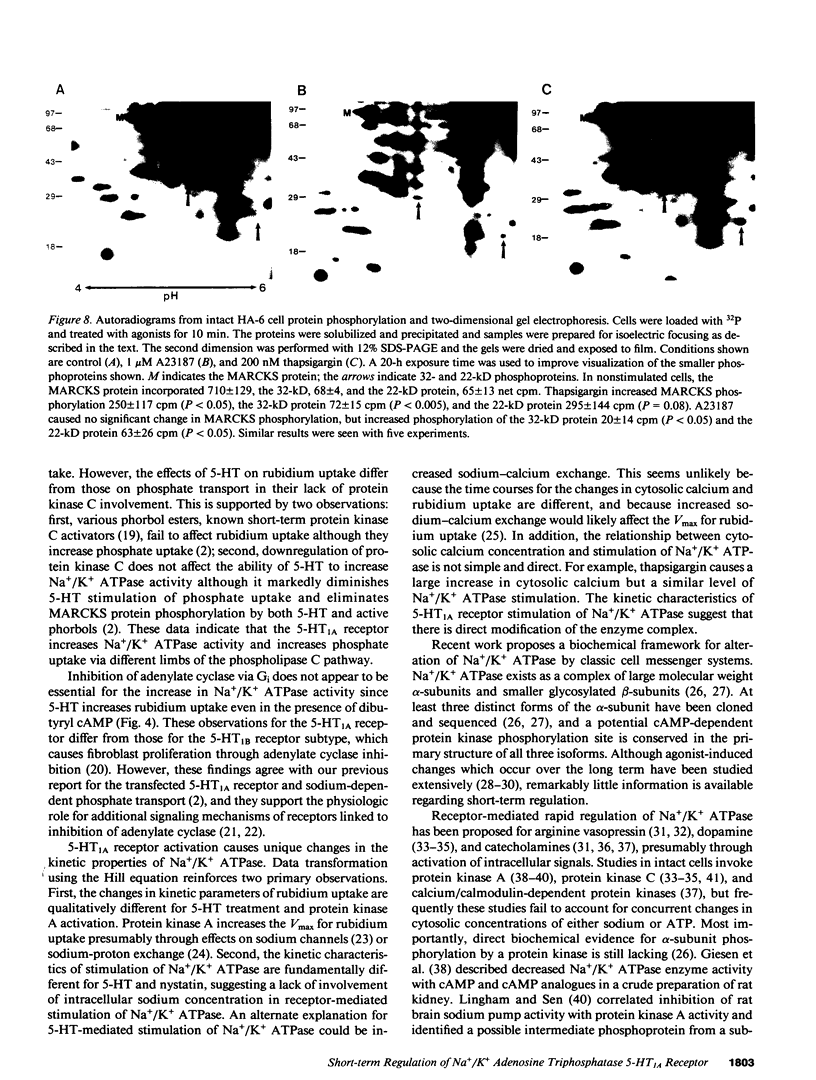
Agonist occupancy of the cloned human serotonin (5-HT)1A receptor expressed in HeLa cells stimulates Na+/K+ ATPase activity as assessed by rubidium uptake. The purpose of the study was to determine which of the receptor-associated signaling mechanisms was responsible for this effect. 5-HT stimulated Na+/K+ ATPase 38% at 2 mM extracellular potassium, an effect characterized by a decrease in apparent K0.5 from 2.8 +/- 0.3 to 1.8 +/- 0.3 mM potassium without a significant change in apparent Vmax. The EC50 for the transport effect was approximately 3 microM 5-HT. The response was pertussis toxin-sensitive but did not involve inhibition of adenylate cyclase, as stimulation of Na+/K+ ATPase by 5-HT was observed in the presence of excess dibutyryl cAMP. Protein kinase C was not required for the response since short-term incubation with the phorbol esters phorbol 12 myristate, 13 acetate (PMA) and phorbol 12,13-dibutyrate (PDBu) did not mimic the 5-HT effect. Moreover, 5-HT increased Na+/K+ ATPase activity after inactivation of protein kinase C by overnight incubation with PMA. 5-HT and the sesquiterpene lactone thapsigargin increased cytosolic calcium in this cell model, and the EC50 for 5-HT corresponded with that for stimulation of Na+/K+ ATPase. Both thapsigargin and A23187, a calcium ionophore, also increased Na+/K+ ATPase activity in a dose-responsive fashion. The response to 5-HT, thapsigargin, and A23187 was blocked by conditions that removed the cytosolic calcium response. By two-dimensional gel electrophoresis, we established evidence for a calcium-sensitive but protein kinase C-independent signaling pathway. We conclude that the 5-HT1A receptor, which we have previously shown to stimulate phosphate uptake via protein kinase C, stimulates Na+/K+ ATPase via a calcium-dependent mechanism. This provides evidence for regulation of two separate transport processes by a single receptor subtype via different signaling mechanisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A., Bertorello A., Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987 Jan;252(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Winslow J. W., Peralta E. G., Peterson G. L., Schimerlik M. I., Capon D. J., Ramachandran J. An M2 muscarinic receptor subtype coupled to both adenylyl cyclase and phosphoinositide turnover. Science. 1987 Oct 30;238(4827):672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berthon B., Capiod T., Claret M. Effects of noradrenaline, vasopressin and angiotensin on the Na-K pump in rat isolated liver cells. Br J Pharmacol. 1985 Sep;86(1):151–161. doi: 10.1111/j.1476-5381.1985.tb09445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorello A., Aperia A. Na+-K+-ATPase is an effector protein for protein kinase C in renal proximal tubule cells. Am J Physiol. 1989 Feb;256(2 Pt 2):F370–F373. doi: 10.1152/ajprenal.1989.256.2.F370. [DOI] [PubMed] [Google Scholar]
- Bertorello A., Aperia A. Regulation of Na+-K+-ATPase activity in kidney proximal tubules: involvement of GTP binding proteins. Am J Physiol. 1989 Jan;256(1 Pt 2):F57–F62. doi: 10.1152/ajprenal.1989.256.1.F57. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Nemenoff R. A., Avruch J. Preliminary characterization of a heat-stable protein from rat adipose tissue whose phosphorylation is stimulated by insulin. Biochem J. 1982 Jun 15;204(3):817–824. doi: 10.1042/bj2040817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Cyclic AMP-dependent phosphorylation of the alpha subunit of the sodium channel in synaptic nerve ending particles. J Biol Chem. 1984 Jul 10;259(13):8210–8218. [PubMed] [Google Scholar]
- Doods H. N., Boddeke H. W., Kalkman H. O., Hoyer D., Mathy M. J., van Zwieten P. A. Central 5-HT1A receptors and the mechanism of the central hypotensive effect of (+)8-OH-DPAT, DP-5-CT, R28935, and urapidil. J Cardiovasc Pharmacol. 1988 Apr;11(4):432–437. doi: 10.1097/00005344-198804000-00008. [DOI] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Lohse M. J., Kobilka B. K., Caron M. G., Lefkowitz R. J. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988 Sep 22;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Regan J. W., Cotecchia S., Lefkowitz R. J., Caron M. G. Effector coupling mechanisms of the cloned 5-HT1A receptor. J Biol Chem. 1989 Sep 5;264(25):14848–14852. [PubMed] [Google Scholar]
- Fischer C. A., Aprison M. H. Determination of nanomole levels of 5-hydroxytryptophan, 5-hydroxytryptamine, and 5-hydroxyindoleacetic acid in the same sample. Anal Biochem. 1972 Mar;46(1):67–84. doi: 10.1016/0003-2697(72)90396-x. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Johnsen D. E., Campanile C. P. Evidence for the role of phosphorylase kinase, protein kinase C, and other Ca2+-sensitive protein kinases in the response of hepatocytes to angiotensin II and vasopressin. J Biol Chem. 1984 Mar 10;259(5):3283–3292. [PubMed] [Google Scholar]
- Giesen E. M., Imbs J. L., Grima M., Schmidt M., Schwartz J. Modulation of renal ATPase activities by cyclic AMP. Biochem Biophys Res Commun. 1984 Apr 30;120(2):619–624. doi: 10.1016/0006-291x(84)91300-7. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Henley J. M. Epinephrine-stimulated maintained rubidium efflux from guinea pig hepatocytes may involve alpha 1- and alpha 2-adrenoceptors. Mol Pharmacol. 1985 Nov;28(5):431–435. [PubMed] [Google Scholar]
- Hughes B. A., Miller S. S., Joseph D. P., Edelman J. L. cAMP stimulates the Na+-K+ pump in frog retinal pigment epithelium. Am J Physiol. 1988 Jan;254(1 Pt 1):C84–C98. doi: 10.1152/ajpcell.1988.254.1.C84. [DOI] [PubMed] [Google Scholar]
- Ikehara T., Yamaguchi H., Sakai T., Miyamoto H. Kinetic parameters and mechanism of active cation transport in HeLa cells as studied by Rb+ influx. Biochim Biophys Acta. 1984 Sep 5;775(3):297–307. doi: 10.1016/0005-2736(84)90184-6. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E. Receptors linked to inhibition of adenylate cyclase: additional signaling mechanisms. FASEB J. 1988 Aug;2(11):2686–2695. doi: 10.1096/fasebj.2.11.2840317. [DOI] [PubMed] [Google Scholar]
- Lingham R. B., Sen A. K. Regulation of rat brain (Na+ +K+)-ATPase activity by cyclic AMP. Biochim Biophys Acta. 1982 Jun 14;688(2):475–485. doi: 10.1016/0005-2736(82)90359-5. [DOI] [PubMed] [Google Scholar]
- Lynch C. J., Wilson P. B., Blackmore P. F., Exton J. H. The hormone-sensitive hepatic Na+-pump. Evidence for regulation by diacylglycerol and tumor promoters. J Biol Chem. 1986 Nov 5;261(31):14551–14556. [PubMed] [Google Scholar]
- Miyamoto H., Ikehara T., Yamaguchi H., Hosokawa K., Yonezu T., Masuya T. Kinetic mechanism of Na+, K+, Cl--cotransport as studied by Rb+ influx into HeLa cells: effects of extracellular monovalent ions. J Membr Biol. 1986;92(2):135–150. doi: 10.1007/BF01870703. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Thoren P., Wilczynski E. A., Victor R. G., Mark A. L. Serotonergic mechanisms mediate renal sympathoinhibition during severe hemorrhage in rats. Am J Physiol. 1988 Sep;255(3 Pt 2):H496–H502. doi: 10.1152/ajpheart.1988.255.3.H496. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pollock A. S., Warnock D. G., Strewler G. J. Parathyroid hormone inhibition of Na+-H+ antiporter activity in a cultured renal cell line. Am J Physiol. 1986 Feb;250(2 Pt 2):F217–F225. doi: 10.1152/ajprenal.1986.250.2.F217. [DOI] [PubMed] [Google Scholar]
- Raymond J. R., Fargin A., Middleton J. P., Graff J. M., Haupt D. M., Caron M. G., Lefkowitz R. J., Dennis V. W. The human 5-HT1A receptor expressed in HeLa cells stimulates sodium-dependent phosphate uptake via protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21943–21950. [PubMed] [Google Scholar]
- Reznik V. M., Shapiro R. J., Mendoza S. A. Vasopressin stimulates DNA synthesis and ion transport in quiescent epithelial cells. Am J Physiol. 1985 Sep;249(3 Pt 1):C267–C270. doi: 10.1152/ajpcell.1985.249.3.C267. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- Schmidt A. W., Peroutka S. J. 5-Hydroxytryptamine receptor "families". FASEB J. 1989 Sep;3(11):2242–2249. doi: 10.1096/fasebj.3.11.2673898. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Hoyer D. Centrally acting hypotensive agents with affinity for 5-HT1A binding sites inhibit forskolin-stimulated adenylate cyclase activity in calf hippocampus. Br J Pharmacol. 1988 Nov;95(3):975–985. doi: 10.1111/j.1476-5381.1988.tb11728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuwen K., Magnaldo I., Pouysségur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988 Sep 15;335(6187):254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Lane L. K., Lingrel J. B. Amino-acid sequence of the beta-subunit of the (Na+ + K+)ATPase deduced from a cDNA. Nature. 1986 May 22;321(6068):429–431. doi: 10.1038/321429a0. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Active ion transport in the renal proximal tubule. I. Transport and metabolic studies. J Gen Physiol. 1984 Oct;84(4):601–622. doi: 10.1085/jgp.84.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Linnebjerg H., Bjerrum P. J., Knudsen J. B., Christensen S. B. The inflammatory and tumor-promoting sesquiterpene lactone, thapsigargin, activates platelets by selective mobilization of calcium as shown by protein phosphorylations. Biochim Biophys Acta. 1987 Jan 19;927(1):65–73. doi: 10.1016/0167-4889(87)90066-8. [DOI] [PubMed] [Google Scholar]



