Abstract
Erythroderma may reveal a cutaneous T cell lymphoma (CTCL) and various erythrodermic inflammatory dermatoses (EID), and histopathological diagnosis is often difficult. The aim of this study was to determine whether morphological parameters, β-catenin and JunB, previously shown to be expressed by CTCL cells, the epidermal CD8:CD3 ratio, and CD30 expression may help for histopathological diagnosis of erythroderma, especially for the differential diagnosis between lymphomas and EID. We retrospectively reviewed a series of 47 skin biopsies from patients with erythroderma (18 CTCL and 29 EID). Etiological diagnosis in each case was established using all clinical, biological and histopathological results. At global blinded assessment of HES stained slides, a correct diagnosis of the underlying cause of erythroderma was made using only morphologic criteria in 31% of cases. A correct differential diagnosis between lymphoma and EID was done with certainty in 57% of cases. Various morphologic and phenotypic parameters were then recorded and we compared their frequency in the CTCL versus the EID group. With the exception of atypical lymphocytes, the moderate to high density of dermal infiltrates and Pautrier’s microabcesses, only found in CTCL, no morphologic parameter was found to be specific of CTCL, although single lymphocytes epidermotropism, telangiectasias and slight lymphocytic dermal infiltrate were significantly more frequent in EID. A low (<10%) CD8:CD3 ratio in the epidermal lymphocytic infiltrate and dermal CD30+ lymphocytes were significantly more frequent in CTCL. JunB expression by lymphocytes was specific of CTCL, but was inconstant in our series (17%). We found β-catenin expression in a minority of cases from both the CTCL and EID groups. Among EID, dermal suprapapillary thinning was specific of psoriasis. Neutrophils exocytosis and edema of papillary dermis were significantly more frequent in psoriasis, and spongiosis was more frequent in eczema. In conclusion, few morphological and phenotypical parameters are helpful in making a differential diagnosis between erythrodermic CTCL and EID using paraffin embedded skin biopsies.
Keywords: erythroderma, Sézary syndrome, histopathology, CD30, ß-catenin, JunB.
INTRODUCTION
Erythroderma may result from several causes, including cutaneous T-cell lymphomas (CTCL), especially Sézary syndrome (SS), and several erythrodermic inflammatory diseases (EID), mostly psoriasis, drug-eruptions or atopic dermatitis.1 It is difficult to establish the underlying cause in practice, since clinical and histological aspects are most often not enough specific. Especially, it is necessary to differentiate benign EID from SS, which is an aggressive lymphoma and requires an early and appropriate management.2 Erythroderma can present with a large variety of histologic patterns. However, there have been only few series detailing the histologic features of erythroderma. The identification of a monoclonal T-cell population has been proved to be of high value for the diagnosis of CTCL,3–7 but is not fully specific, unless a similar T-cell clone is found in the blood and skin.8 New markers of SS have been described in the past ten years using flow cytometry studies on circulating CD4+ T cells (loss of CD26, CD7 and CD49d) 9–12, and molecular studies in sorted circulating T cells or skin samples (Twist, EphA4 and T-plastin).13,14 Notably, CD158k/KIR3DL2 was shown to be highly specific for malignant CD4+ T cells, allowing diagnosis of SS in the blood using flow cytometry and in the skin using RT-PCR.15–17 Among new markers of SS, only β-catenin and JunB have been shown to be suitable for immunohistochemistry on paraffin embedded skin biopsies. Beta-catenin is a ubiquitously cytoplasmic protein that acts as a component of the homotypic cell-cell adhesion apparatus. It is also a critical member of the Wnt signaling pathway, which plays an important role in embryonic development and tumorigenesis.18–21 Interestingly, a recent study has shown that β-catenin is expressed in high grade CTCL.22 Indeed, immunohistochemical analysis demonstrated β-catenin expression in a majority of SS samples (70%) and in a proportion of mycosis fungoides. JunB is a member of the Jun family of proteins that are components of the AP-1 transcription factor complex. AP-1 members are involved in cell proliferation and apoptosis, thus contributing to oncogenesis.23 Recent studies have shown that JunB is expressed in various lymphomas, especially systemic and cutaneous CD30+ lymphomas, including lymphomatoid papulosis and cutaneous anaplastic T-cell lymphoma.24 In addition, in a recent study, amplification of the JunB gene was found in lymphocytes from patients with SS and mycosis fungoides (MF), and immunohistochemical analysis showed nuclear expression of JunB in the majority of SS (91%) SS and MF (50%) samples.25 This suggested that JunB may help to make a differential diagnosis between SS and EID in routine practice. We therefore conducted a retrospective study in order to determine whether morphologic parameters, lymphocyte phenotyping, including the epidermal CD8:CD3 ratio, CD30, and the recently described markers β-catenin and JunB may help for the diagnosis of erythroderma with paraffin embedded skin biopsies, especially to make a differential diagnosis between CTCL and EID.
MATERIALS AND METHODS
Patients and material selection
We retrospectively included 47 biopsies specimens from the 45 adult patients who presented in the Department of Dermatology of our institution with a clinical diagnosis of erythroderma between september 1996 and november 2007. There were 28 males and 17 females, with a median age of 70 years (range 36–96 years). In each case, the final etiological diagnosis of erythroderma was etablished according to all the clinical, biological, morphological and immunohistochemical datas obtained previously to the study, including T-cell clonality results and CD158k/KIR3DL2, shown to be a marker of SS in circulating CD4+ T-cells16,26 and in erythrodermic skin.17 Final diagnoses were CTCL in 18 patients, including 14 Sézary syndrome, 3 erythrodermic mycosis fungoide and 1 secondary cutaneous lymphoma. 29 patients had a benign erythrodermic inflammatory dermatosis (EID): drug eruption (n=5), psoriasis (n=9), eczema (n=5). The remaining patients (n=10) had pityriasis rubra pilaris, myelodysplastic syndrome, dermatomyositis, irritative dermatitis or idiopathic erythroderma (n=6).
Histopathology and immunohistochemistry: techniques and parameters analysed
Hematoxylin-eosin-safran (HES) stained slides and formalin fixed, paraffin embedded blocks were retrieved from the archive material of our department of Pathology. Immunohistochemistry was applied to 3 micrometer-thick sections, using monoclonal antibodies to CD3, CD8, CD30 (Dako, SA) and JunB (C-11, Santa-Cruz biotechnology, 1:50) and β-catenin (CAT 5H10, Zymed laboraties, 1:100). We used a standard avidin-biotin-peroxydase method with diaminobenzidine (DAB) chromogen, using the Nexes immunostainer (Ventana, Tucson AZ), after antigen retrieval by heat in the appropriate buffer. Immunostaining of β-catenin was done manually with the horse-radish-peroxydase method and revealed by aminoethylcarbazole (AEC). Briefly, 3 μm sections were deparaffinized in xylene for 30 min and rehydrated through graded ethanol. Sections were first heated at 98°C for 30 min in a citrate buffer solution to retrieve the antigen. Endogenous peroxydase activity was blocked for 15 min with 3% hydrogen peroxydase. Nonspecific antibody binding was blocked for 10 min by incubation with protein block serum free (Vector laboratories, vectastain universal, ABC KIT). After a serie of washes, the sections were incubated with primary mouse monoclonal antibody at room temperature in a humidity chamber for 2 hours. The primary antibody was visualized using an anti-mouse HRP system with AEC as the chromogen. Sections were then counterstained by hematoxylin. Positive immunoreactivity of keratinocytes was used as an internal positive control for both β-catenin at the cell membrane22 and JunB in the nuclei.25 The immunostaining of JunB was considered to be positive when it was at least as positive as suprabasal keratinocytes. In addition, as controls, we analyzed JunB immunostaining in a series of CD30+ lymphomas, retrieved from the archive material of our Department of Pathology, which are all expected to be positive for JunB expression, according to the results obtained by Rassidakis et al.24
All HES slides were reviewed blindly together by 2 dermatopathologists (CR and NO). First, a global assessment of each HES stained slide was made, and a diagnosis based on the histopathology alone was done. The morphologic aspects were considered to be either characteristic for one diagnostic, for several diagnostic or non specific. Then the following morphologic parameters were systematically recorded, according to precedent studies that focused on the histopathologic aspects of CTCL or erythroderma.27–31: 1) aspect of cornified layer: normal, orthokeratosis, parakeratosis; 2) aspect of granulous layer: normal or thickened, 3) epidermal changes: psoriasiform hyperplasia (regular hyperplasia with extensive lamellar parakeratosis), irregular hyperplasia, atrophic epidermis, suprapapillary thinning, spongiosis (diffusely enlarged intercellular spaces, marked confluent spongiosis (“pre-vesicles”), or constituted vesicles), number of necrotic keratinocytes and of basal mitoses per millimeter, 4) aspect of dermo-epidermal junction: normal, focal interface dermatitis, widespread interface dermatitis, presence of melanophages in the papillary dermis; 5) presence or absence of intra-epidermal cells: lymphocytes exocytosis with evaluation of epidermotropic lymphocytes (single lymphocytes, basilar lymphocytes, pagetoid epidermotropism, Pautrier’s microabcess), exocytosis of eosinophils and neutrophils, corneal/subcorneal pustules; 6) presence or absence of atypical lymphocytes (lymphocytes with enlarged nuclei, lymphocytes with enlarged hyperconvoluted nuclei suggesting Sézary cells and large atypical lymphocytes); 7) dermal infiltrate : localization (superficial, superficial and deep, or deep dermis, hypodermis), architecture (band-like, perivascular, diffuse), and density (low, i.e. scattered lymphocytes, intermediate or high i.e. cohesive sheets of lymphocytes) and components (eosinophils, neutrophils); 8) dermal changes: vasculitis, papillary dermal fibrosis, telangiectasias, edema of papillary dermis, and increased number of papillary capillaries reflecting angiogenesis.
Immunohistochemical parameters analysed were as follows: the presence of CD30+, β-catenin+ and JunB+ lymphocytes in both the dermis and epidermis were recorded. The density of positive cells was categorized as low (0–10% positive lymphocytes), intermediate (>10%–50%) or strong (>50%–100%). The epidermal CD8:CD3 ratios were evaluated in all cases. A semi-quantitative analysis was done, in which we categorized these ratios into 3 groups: 1 (0–10%), 2 (>10%–50%), 3 (>50%–100%).
Statistical analysis
Statistical correlations used the Chi-2 test and the Mann-Whitney U-test (Statview, abacus concept). We first compared the CTCL and the EID groups. Each morphologic and immunohistochemistry parameter was studied. Then, we evaluated the morphological parameters that are usually considered to be of diagnostic value in the histopathologic diagnosis of psoriasis, eczema and drug eruption to determine whether they may be useful in the context of erythroderma. Differences were considered to be statistically significant at P < 0.05.
RESULTS
Comparison of the diagnoses made with histopathology alone with the final diagnosis
Comparison of the diagnoses based on pathologic findings with the final diagnoses revealed that a correct differential diagnosis between lymphoma and EID was done with certainty in 57% of cases. Two patients with a benign form of erythroderma showed a histologic picture that suggested CTCL, because of a suspect epidermotropism. Considering all etiological diagnoses, skin biopsy analysis alone yielded a correct diagnosis in 31% of cases and a non specific aspect in 27%. In the remaining cases, the final diagnosis was only suspected (20/47, 42%). Diagnosis of CTCL was more easy (61% of certain diagnosis) than diagnosis of EID. In the EID group, the aspect was actually considered non specific in 41% of cases, and the correct diagnosis was only suspected among other diagnoses in 45% of cases.
Comparison of erythrodermic lymphoma with inflammatory dermatoses
Results of the morphologic study are summarized in Table 1 and Figure 1. Results of immunohistochemistry are summarized in Table 2 and Figure 2.
TABLE 1.
Histologic features of erythroderma observed in 47 skin biopsies
| Feature | CTCL (n=18) | Drug (n=5) | Psoriasis (n=9) | Eczema (n=5) | Other (n=10) | EID (n=29) | Total (n=47) | P (Chi-2) CTCL vs EID |
|---|---|---|---|---|---|---|---|---|
| Epidermal changes | ||||||||
| Orthokeratosis | 5 (28%) | 1 (20%) | 2 (22%) | 0 | 2 (20%) | 5 (17%) | 10 (21%) | 0.618 |
| Parakeratosis | 7 (39%) | 1 (20%) | 7 (77%) | 4 (80%) | 5 (50%) | 17 (59%) | 24 (51%) | 0.307 |
| Thickened granulous layer | 1 (6%) | 0 | 1(11%) | 1 (20%) | 0 | 2 (7%) | 3 (6%) | >0.9 |
| Psoriasiform hyperplasia | 3 (17%) | 0 | 4 (44%) | 3 (60%) | 1 (10%) | 8 (28%) | 11 (23%) | 0.608 |
| Irregular hyperplasia | 6 (33%) | 4 (80%) | 2 (22%) | 2 (40%) | 3 (30%) | 11 (38%) | 17 (36%) | >0.9 |
| Atrophic epidermis | 2 (11%) | 1 (20%) | 0 | 0 | 1 (10%) | 2 (7%) | 4 (9%) | >0.9 |
| Suprapapillary thinning | 0 | 0 | 2 (22%) | 0 | 0 | 2 (7%) | 2 (4%) | 0.688 |
| Presence of spongiosis | 5 (28%) | 1 (20%) | 4 (44%) | 4 (80%) | 6 (60%) | 15 (52%) | 20 (43%) | 0.188 |
| Marked spongiosis (pre-vesicles) | 0 | 0 | 0 | 3 (60%) | 2 (20%) | 5 (17%) | 5 (11%) | 0.165 |
| Vesicles | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Number of necrotic keratinocytes per mm | 0.34 | 0.07 | 0 | 0,12 | 0.57 | 0,19 | 0,22 | 0.353 |
| Number of mitoses per mm (mean) | 0,68 | 0.98 | 1.80 | 2.15 | 0.73 | 1,42 | 1.27 | 0.099 |
| Dermo-epidermal junction | ||||||||
| Focal interface dermatitis | 6 (33%) | 1 (20%) | 2 (22%) | 3 (60%) | 2 (20%) | 8 (28%) | 14 (30%) | >0.9 |
| Widespread interface dermatitis | 1 (6%) | 0 | 0 | 0 | 2 (20%) | 2 (7%) | 3 (6%) | >0.9 |
| Melanophages | 3 (17%) | 0 | 0 | 0 | 2 (20%) | 2 (7%) | 5 (11%) | 0.562 |
| Presence of exocytosis | 14 (78%) | 4 (80%) | 6 (67%) | 4 (80%) | 4 (40%) | 18 (62%) | 32 (68%) | 0.419 |
| Single lymphocytes epidermotropism | 5 (28%) | 4 (80%) | 6 (67%) | 2 (40%) | 4 (40%) | 16 (55%) | 21 (45%) | 0.006 |
| Basilar lymphocytes | 1 (6%) | 0 | 0 | 0 | 0 | 0 | 1 (2%) | - |
| Pagetoid epidermotropism | 0 | 0 | 0 | 2 (40%) | 0 | 2 (7%) | 2 (4%) | 0.581 |
| Pautrier’s microabcess | 8 (44%) | 0 | 0 | 0 | 0 | 0 | 8 (17%) | 0.004 |
| Exocytosis of eosinophils | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Exocytosis of isolated neutrophils | 1 (6%) | 0 | 1 (11%) | 1 (20%) | 0 | 2 (7%) | 3 (6%) | 0.680 |
| Corneal/subcorneal pustules | 2 (11%) | 1 (20%) | 6 (67%) | 1 (20%) | 1 (10%) | 9 (31%) | 11 (23%) | 0.222 |
| Atypical lymphocytes | 12 (66%) | 1 (20%) | 0 | 0 | 0 | 1 (3%) | 13 (28%) | 0.014 |
| Circonvoluted nuclei | 4 (22%) | 0 | 0 | 0 | 0 | 0 | 4 (9%) | - |
| Dermal infiltrate | ||||||||
| Superficial infiltrate | 18 (100%) | 5 (100%) | 9 (100%) | 5 (100%) | 10 (100%) | 28 (100%) | 47 (100%) | - |
| Superficial and deep infiltrate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Deep infiltrate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Hypodermic infiltrate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Band like infiltrate | 13 (72%) | 1 (20%) | 5 (56%) | 1 (20%) | 5 (50%) | 12 (41%) | 25 (53%) | 0.164 |
| Perivascular infiltrate | 5 (28%) | 4 (80%) | 4 (44%) | 4 (80%) | 5 (50%) | 17 (59%) | 22 (47%) | 0.077 |
| Diffuse infiltrate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Slight density | 1 (6%) | 4 (80%) | 7 (78%) | 2 (40%) | 7 (70%) | 20 (69%) | 21 (44%) | <0.001 |
| Moderate density | 12 (67%) | 1 (20%) | 2 (22%) | 3 (60%) | 3 (30%) | 9 (31%) | 21 (45%) | 0.036 |
| High density | 5 (28%) | 0 | 0 | 0 | 0 | 0 | 5 (11%) | 0.011 |
| Eosinophils | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Neutrophils | 1 (6%) | 1 (20%) | 3 (33%) | 2 (40%) | 3 (30%) | 9 (31%) | 10 (2%) | 0.086 |
| Dermal changes | ||||||||
| Vasculitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Papillary dermal fibrosis | 7 (39%) | 1 (20%) | 0 | 3 (60%) | 1 (10%) | 5 (17%) | 12 (26%) | 0.188 |
| Telangiectasias | 8 (44%) | 3 (60%) | 8 (89%) | 5 (100%) | 8 (80%) | 24 (83%) | 32 (68%) | 0.015 |
| Edema of papillary dermis | 4 (22%) | 1 (20%) | 7 (78%) | 3 (60%) | 5 (50%) | 16 (55%) | 20 (43%) | 0.054 |
| Angiogenesis | 11 (61%) | 1 (20%) | 5 (56%) | 5 (100%) | 3 (30%) | 14 (48%) | 25 (53%) | 0.573 |
Results are expressed in percentage within each category of erythroderma. CTCL, cutaneous T cell lymphoma; EID, erythrodermic inflammatory dermatoses.
FIGURE 1. Morphological features in representative samples of erythrodermic CTCL and inflammatory dermatoses.
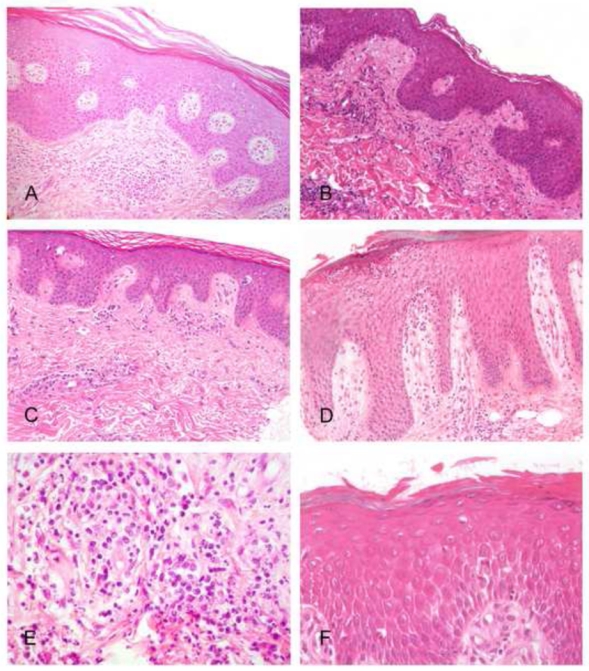
A, C and E show examples of erythrodermic CTCL, while B, D, F are from the EID group. A, In this Sézary syndrome, skin biopsy did not allow a definite diagnosis, only showing a perivascular lymphocytic infiltrate without atypical lymphocyte, with an overlying hyperplastic and parakeratotic epidermis with slight spongiosis. B, In this erythrodermic drug reaction sample, a common perivascular lymphocytic infiltrate is seen, while no interface dermatitis and no apoptotic keratinocytes are identified. C, This Sézary syndrome sample show a very discrete infiltrate in the dermis, but atypical lymphocytes and Pautrier’s microabcesses can be identified in the epidermis, constiting of aggregates of atypical lymphocytes showing enlarged circonvoluted hyperchromatic nuclei (arrow), allowing histopathological diagnosis. D, This sample from a psoriatic erythrodermic patient shows an irregular epidermal hyperplasia with a multilocular subcorneal pustule. E, Atypical lymphocytes admixed with neutrophils are present within the infiltrate of this erythrodermic CTCL sample. F, Confluent spongiosis (pre-vesicles) is seen in this skin biopsy from an erythrodermic allergic contact dermatitis.
TABLE 2.
Immunohistochemistry results
| Feature | CTCL (n=18) | EID (n=29) | P (Chi-2) |
|---|---|---|---|
| Epidermal CD8/CD3 < 10% | 10 (56%) | 6 (21%) | 0.03 |
| Epidermal CD8/CD3: 10–50% | 1 (6%) | 9 (33%) | 0.086 |
| Epidermal CD8/CD3 >50% | 2 (17%) | 5 (19%) | >0.9 |
| CD30+ lymphocytes | 14 (78%) | 11 (38%) | 0.009 |
| JunB+ lymphocytes | 3(17%) | 0 | 0.049 |
| β-catenin+ lymphocytes | 5 (28%) | 9 (31%) | >0.99 |
CTCL, cutaneous T cell lymphoma; EID, erythrodermic inflammatory dermatoses
FIGURE 2. Representative immunohistochemical results for CD30, beta-catenin and JunB expression in erythrodermic CTCL and inflammatory dermatoses.
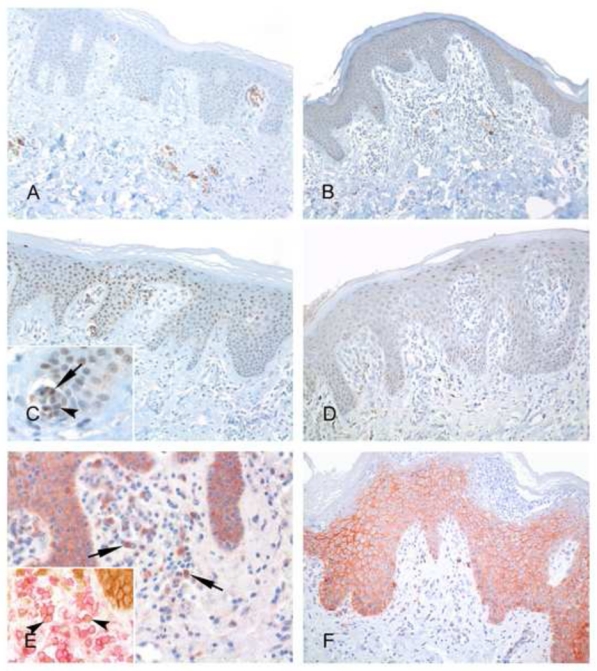
A, C and E show examples of CTCL, while B, D, F are from the EID group. A, Clusters of CD30+ atypical lymphocytes are present around dermal vessels in this Sézary syndrome skin sample. B, Presence of scattered CD30+ lymphocytes is evidenced in this example of erythrodermic psoriasis. C, A subset of atypical lymphocytes within Pautrier’s microabcesses show a positive nuclear staining for JunB (inset, arrows), with a similar staining in the surrounding keratinocytes, used as a positive control. D, In this EID sample, no JunB positive lymphocytes are seen, whereas a distinctive staining of keratinocytes nuclei is evidenced. E, Few β-catenin positive lymphocytes can be demonstrated in this Sézary syndrome sample (arrows), whereas keratinocytes expressing this antigen at their membrane can be seen in the overlying epidermis, used as a positive control. Most β-catenin positive (brown) lymphocytes are T-cells, as shown by double immunostaining experiments with CD3 (red) (inset, arrowheads). F, In this pustular erythrodermic psoriasis, no β-catenin positive lymphocytes are seen.
We compared the CTCL and the EID groups for each morphological parameter recorded. The presence of Pautrier’s microabcesses (44%, P =0.004), atypical lymphocytes (66%, P = 0.014), a high (28%, P =0.011), or intermediate (67%, P=0.036 ) density lymphocytic infiltrate were significantly associated with diagnosis of CTCL. Pautrier’s microabcesses (Fig. 1C), atypical lymphocytes and a dense dermal infiltrate were found only in CTCL (Fig. 1E). In addition, in one case of CTCL we observed presence of basilar lymphocytes, a feature that was never found in any EID sample. Fibrosis of papillary dermis and lymphocyte epidermotropism were not significantly associated with diagnosis of erythrodermic CTCL. By contrast, single lymphocytes epidermotropism (P=0.006), a low density lymphocytic dermal infiltrate (P<0.0001) and telangiectasias (P=0.015) were significantly more frequent in EID.
Regarding immunohistochemical parameters, an epidermal CD8:CD3 ratio <10% was seen in most CTCL (56% versus 21% in EID, P = 0.032). In all cases, the density of JunB+, β-catenin+ and CD30+ lymphocytes was low (<10% of lymphocytes). CD30+ lymphocytes, when present, were found in the dermal infiltrates. CD30 expression was not discriminant between CTCL and EID but was significantly more frequent in CTCL (14/18, 78% versus 11/29, 38% in EID, P = 0.009) (Figs. 2A, 2B). Interestingly, a proportion of CD30+ lymphocytes in CTCL were morphologically atypical, suggesting that at least a proportion of positive cells were neoplastic (Fig. 2A). β-catenin expression in lymphocytes was rare and when positive, it was inconspicuous, mainly found in the cytoplasm and heterogeneous within the dermal infiltrates. β-catenin positivity was not discriminant (P>0.999) between CTCL (5/18, 28%) and EID (9/29, 31%). Using a double immunostaining with CD3 (red), we confirmed that β-catenin positive cells (brown) within the infiltrate were T lymphocytes (Fig. 2F). JunB expression was found only in CTCL cases but with a very low sensitivity in this serie with only 3 positive cases (17% of CTCL, P=0.049). Interestingly we noticed an heterogeneous expression within the neoplastic cells, even in a single Pautrier’s microabcess (Fig. 2C, inset). In 2 of the 3 JunB+ CTCL cases, CD30 was also expressed.
Analysis of the sub-groups of inflammatory dermatoses : psoriasis, eczema and drug eruptions
We then considered separately the subgroups of EID, including psoriasis, eczema and drug eruption. Suprapapillary thinning was an inconstant finding but was found only in psoriasis (2/9, 22%, P = 0.038). Exocytosis of neutrophils was significantly associated with a diagnosis of psoriasis (78% versus 18% of other erythroderma, P = 0.006), as well as subcorneal pustules (67% versus 13%, P = 0.003) (Fig. 1D) and edema of papillary dermis (P=0.044). Psoriasiform hyperplasia was frequently observed but not significantly associated with psoriasic erythroderma (P=0.129). Similarly, the number of mitoses per mm was not increased in erythrodermic psoriasis as compared to other groups (P = 0.059). Marked and confluent spongiosis (pre-vesicles) was significantly associated with eczema (60% versus 5% of other erythroderma, P = 0.002) (Fig. 1F) whereas constituted vesicles appeared to be rare. Parakeratosis (P=0.365), oedema of papillary dermis (P=0.714), irregular hyperplasia (P>0.999) and single lymphocyte epidermotropism (P=0.888) were frequently observed but not significantly associated with eczematous erythroderma. Pagetoid epidermotropism appeared pathognomonic of eczematous erythroderma (2/5, 40%). Unexpectedly, the total number of apoptotic keratinocytes was not significantly increased in erythrodermic drug reactions (0.07 apoptotic bodies per mm versus 0.26 in other causes, P = 0.719) (Table 1). Focal interface dermatitis (P>0.999) and eosinophils in dermal infiltrate were not significantly associated with drug-induced erythroderma.
DISCUSSION
One of the most important challenge facing a dermatopathologist when evaluating skin biopsies from erythrodermic patients lies in the distinction between benign and malignant causes. Previous studies have already highlighted the difficulties for making a definitive diagnosis with paraffin embedded skin biopsies in this context.28,29,31,32 In the past ten years, new phenotypic and molecular markers of malignant Sézary cells were published, including loss of CD26 and CD7, expression of Twist, EphA4, T-plastin, and CD158k/KIR3DL2, the latter allowing diagnosis in skin biopsies with quantitative RT-PCR.15–17 In addition, among the new markers, only β-catenin and JunB have been proved to be suitable for immunohistochemistry on paraffin embedded skin biopsies,22,25 but there has been no additional study to date that evaluated these markers in a series of erythroderma.
Our findings indicate that the diagnosis of the underlying cause of erythroderma was made (31%) or suspected (42%) by histopathologic examination in only 73% of cases, when microscopic patterns more or less reproduced those seen in conventional manifestations of the underlying diseases. Those results are in keeping with precedent studies (Table 3) supporting the view that skin biopsy is a useful procedure in the evaluation of patients with erythroderma, but remains unsufficient to make a correct diagnosis in all instances. According to a previous study from Walsh et al,32 morphological identification of CTCL, psoriasis and spongiotic dermatitis as underlying causes of erythroderma was more successful than that of drug eruption or other causes. In present study, diagnosis of CTCL is more easily confirmed by microscopy alone (61% of certitude of the diagnosis in this group). In Table 3, we reported main results from this study and previous publications on the diagnosis of erythroderma with skin biopsy.
TABLE 3.
Main results from this study and previous publications on the diagnosis of erythroderma with skin biopsy
| Feature | Zip and al, 1993 (29) | Walsh and al, 1994 (32) | Trotter and al, 1997 (28) | current study |
|---|---|---|---|---|
| Histopathological diagnosis of underlying cause | 66% | 53% | NA | 73% |
| Correct diagnosis of CTCL | 50% | 60% | 61% | 61% |
| Epidermotropism in CTCL | 50% | NA | NA | 78% |
| Pautrier’s microabcesses in CTCL | NA | NA | 20% | 44% |
| Moderate to high density of infiltrate in CTCL | 75% | NA | NA | 95% |
| Epidermal hyperplasia in CTCL | 50% | NA | 35% | 50% |
| Neutrophils in dermal infiltrate in psoriasis | 69% | NA | NA | 33% |
| Superficial perivascular infiltrate in psoriasis | 100% | NA | NA | 44% |
| Epidermal hyperplasia in psoriasis | 94% | NA | NA | 66% |
| Dilated blood vessels in papillary dermis of psoriasis | 81% | NA | NA | 89% |
| Suprapapillary thinning in psoriasis | 69% | NA | NA | 22% |
| Exocytosis of neutrophils in psoriasis | 63% | NA | NA | 78% |
| Spongiosis in eczema | 79% | NA | NA | 80% |
| Epidermal hyperplasia in eczema | 71% | NA | NA | 100% |
| Superficial perivascular infiltrate in eczema | 86% | NA | NA | 80% |
| Moderate to high density of infiltrate in eczema | 71% | NA | NA | 60% |
| Moderate to high density of infiltrate in drug-induced erythroderma | 83% | NA | NA | 20% |
| Epidermal hyperplasia in drug-induced erythroderma | 33% | NA | NA | 80% |
CTCL, cutaneous T cell lymphoma; NA, not available
In precedent morphological studies, Zip et al29 and Sentis et al33 reported that when Pautrier’s microabcesses are present, a diagnosis of cutaneous T-cell lymphoma can be rendered without equivocation. In our study, the presence of atypical dermal lymphocytes and a moderate to dense dermal lymphocytic infiltrate were also more frequently observed in CTCL. As for Pautrier’s microabcesses these features however may lack. Similarly, only one case of CTCL showed basilar lymphocytes (lymphocytes aligned within the basal layer). This is in agreement with Kohler et al. who reported that this feature was more frequent in limited patch/plaque lesions of MF than in erythrodermic CTCL 31. In addition, we show that the identification of numerous dilated vessels favours a diagnosis of EID.
Bellei et al. demonstrated β-catenin expression in 31% of MF and 70% of SS samples using immunohistochemistry. In this study none of the 10 investigated inflammatory dermatitis expressed β-catenin, suggesting that this marker is specific for CTCL diagnosis.22 In our study, we found that β-catenin expression was rare and not discriminant between CTCL (5/18, 28%) and EID (9/29, 31%), and was actually seen in T-cells, according to double immunohistochemistry results in representative samples. Similarly, CD30 expression was not discriminant between CTCL and EID, although the finding of CD30+ lymphocytes was significantly more frequent in CTCL. It is already documented that CD30+ lymphocytes can be found in reactional lymphocytic infiltrates. In addition, as shown in a recent study, CD30+ lymphocytes in reactional condition can be large cells, simulating atypical lymphocytes of CTCL.34 Such findings further suggest that CD30 is of limited value in making a diagnosis of CTCL. Interestingly, according to Bellei et al., the deregulation of β-catenin appears to correlate with CD30 expression.22 In our study it is noticeable that all CTCL positive for β-catenin also comprised CD30+ lymphocytes. The expression of CD30 can be induced on normal blood B and T cells by mitogens and viruses, indicating that CD30 is associated with activation of lymphoid cells. The positivity of β-catenin in minor T-cell subsets in erythroderma might therefore correlate with the presence of activated T-cells, which is expected in both inflammatory and lymphomatous erythroderma. Sézary cells indeed are neoplastic activated helper T-cells.22 In the present study, JunB expression was specific of CTCL but with a very low sensitivity (17% of CTCL) and with a very heterogeneous pattern among atypical lymphocytes. Such results are contrasting with previous studies in which JunB expression was mentioned in up to 91% of SS and 50% of MF.25 Interestingly, in 2 of the 3 JunB+ CTCL cases, we noticed that CD30 was also expressed. This result may suggest that JunB and CD30 expression in these CTCL are linked, such as in Reed-Sternberg cells in Hodgkin’s lymphoma, in which CD30 expression was shown to be induced by JunB through an interaction with the CD30 gene promoter.24,35 The discordance between our results and results obtained previously is difficult to explain. Such discordance has recently been found regarding the NAV3 deletion gene, shown to be a specific marker of MF and SS neoplastic cells36 and later found to be rather a rare and non specific event. 37 Multicentric and comparative studies on larger series of cases are thus needed to better evaluate the real sensitivity and specificity of these markers.
In conclusion, characterisation of erythroderma in skin biopsies is difficult morphologically, so that a definite diagnosis can be rendered in only a proportion of cases, one third in the present study. Only few histopathological features allow characterization of the underlying aetiology, which are Pautrier’s microabcesses, atypical lymphocytes with hyperconvoluted nuclei and a dense dermal infiltrate for CTCL. Among new markers of CTCL, JunB is not a sensitive feature but is specific for CTCL, whereas in this study, β-catenin expression was rare an inconspicuous in lymphocytes, and did not allow differential diagnosis between CTCL and erythrodermic inflammatory dermatoses. In light of these results, the search for new specific markers working in paraffin embedded tissue sections needs to be continued in order to improve the diagnostic value of skin biopsy in erythroderma.
References
- 1.Akhyani M, Ghodsi ZS, Toosi S, et al. Erythroderma: a clinical study of 97 cases. BMC Dermatol. 2005;5:1–5. doi: 10.1186/1471-5945-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–85. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 3.Curco N, Servitje O, Llucia M, et al. Genotypic analysis of cutaneous T-cell lymphoma: a comparative study of Southern blot analysis with polymerase chain reaction amplification of the T-cell receptor-gamma gene. Br J Dermatol. 1997;137:673–9. [PubMed] [Google Scholar]
- 4.Gorochov G, Bachelez H, Cayuela JM, et al. Expression of V beta gene segments by Sezary cells. J Invest Dermatol. 1995;105:56–61. doi: 10.1111/1523-1747.ep12312560. [DOI] [PubMed] [Google Scholar]
- 5.Guitart J, Kaul K. A new polymerase chain reaction-based method for the detection of T-cell clonality in patients with possible cutaneous T-cell lymphoma. Arch Dermatol. 1999;135:158–62. doi: 10.1001/archderm.135.2.158. [DOI] [PubMed] [Google Scholar]
- 6.Wood GS, Tung RM, Haeffner AC, et al. Detection of clonal T-cell receptor gamma gene rearrangements in early mycosis fungoides/Sezary syndrome by polymerase chain reaction and denaturing gradient gel electrophoresis (PCR/DGGE) J Invest Dermatol. 1994;103:34–41. doi: 10.1111/1523-1747.ep12389114. [DOI] [PubMed] [Google Scholar]
- 7.Witzens M, Möhler T, Willhauck M, et al. Detection of clonally rearranged T-cell-receptor gamma chain genes from T-cell malignancies and acute inflammatory rheumatic disease using PCR amplification, PAGE, and automated analysis. Ann Hematol. 1997;74:123–30. doi: 10.1007/s002770050269. [DOI] [PubMed] [Google Scholar]
- 8.Delfau-Larue MH, Laroche L, Wechsler J, et al. Diagnostic value of dominant T-cell clones in peripheral blood in 363 patients presenting consecutively with a clinical suspicion of cutaneous lymphoma. Blood. 2000;96:2987–92. [PubMed] [Google Scholar]
- 9.Jones D, Dang NH, Duvic M, et al. Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol. 2001;115:885–92. doi: 10.1309/U1Y6-J4AG-5M4M-7AYV. [DOI] [PubMed] [Google Scholar]
- 10.Scala E, Narducci MG, Amerio P, et al. T cell receptor-Vbeta analysis identifies a dominant CD60+ CD26− CD49d− T cell clone in the peripheral blood of Sézary syndrome patients. J Invest Dermatol. 2002;119:193–6. doi: 10.1046/j.1523-1747.2002.18194.x. [DOI] [PubMed] [Google Scholar]
- 11.Bernengo MG, Novelli M, Quaglino P, et al. The relevance of the CD4+ CD26− subset in the identification of circulating Sézary cells. Br J Dermatol. 2001;144:125–35. doi: 10.1046/j.1365-2133.2001.04014.x. [DOI] [PubMed] [Google Scholar]
- 12.Rappl G, Muche JM, Abken H, et al. CD4(+)CD7(−) T cells compose the dominant T-cell clone in the peripheral blood of patients with Sézary syndrome. J Am Acad Dermatol. 2001;44:456–61. doi: 10.1067/mjd.2001.110900. [DOI] [PubMed] [Google Scholar]
- 13.van Doorn R, Dijkman R, Vermeer MH, et al. Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sézary syndrome identified by gene expression analysis. Cancer Res. 2004;64:5578–86. doi: 10.1158/0008-5472.CAN-04-1253. [DOI] [PubMed] [Google Scholar]
- 14.Su MW, Dorocicz I, Dragowska WH, et al. Aberrant expression of T-plastin in Sezary cells. Cancer Res. 2003;63:7122–7. [PubMed] [Google Scholar]
- 15.Bagot M, Moretta A, Sivori S, et al. CD4(+) cutaneous T-cell lymphoma cells express the p140-killer cell immunoglobulin-like receptor. Blood. 2001;97:1388–91. doi: 10.1182/blood.v97.5.1388. [DOI] [PubMed] [Google Scholar]
- 16.Poszepczynska-Guigné E, Schiavon V, D’Incan M, et al. CD158k/KIR3DL2 is a new phenotypic marker of Sezary cells: relevance for the diagnosis and follow-up of Sezary syndrome. J Invest Dermatol. 2004;122:820–3. doi: 10.1111/j.0022-202X.2004.22326.x. [DOI] [PubMed] [Google Scholar]
- 17.Ortonne N, Le Gouvello S, Mansour H, et al. CD158K/KIR3DL2 transcript detection in lesional skin of patients with erythroderma is a tool for the diagnosis of Sézary syndrome. J Invest Dermatol. 2008;128:465–72. doi: 10.1038/sj.jid.5701013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Provost E, Rimm DL. Controversies at the cytoplasmic face of the cadherin-based adhesion complex. Curr Opin Cell Biol. 1999;11:567–572. doi: 10.1016/s0955-0674(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 19.Miller JR, Hocking AM, Brown JD, et al. Mechanisms and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- 20.Barker N, Clevers H. Catenins, Wnt signaling and cancer. Bioassays. 2000;22:961–965. doi: 10.1002/1521-1878(200011)22:11<961::AID-BIES1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 22.Bellei B, Pacchiarotti A, Perez M, et al. Frequent beta-catenin overexpression without exon 3 mutation in cutaneous lymphomas. Mod Pathol. 2004;17:1275–1281. doi: 10.1038/modpathol.3800181. [DOI] [PubMed] [Google Scholar]
- 23.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 24.Rassidakis GZ, Thomaides A, Atwell C, et al. JunB expression is a common feature of CD30+ lymphomas and lymphomatoid papulosis. Mod Pathol. 2005;18:1365–1370. doi: 10.1038/modpathol.3800419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao X, Orchard G, Lillington DM, et al. Amplification and overexpression of JUNB is associated with primary cutaneous T-cell lymphomas. Blood. 2003;101:1513–1519. doi: 10.1182/blood-2002-08-2434. [DOI] [PubMed] [Google Scholar]
- 26.Ortonne N, Huet D, Gaudez C, et al. Significance of circulating T-cell clones in Sezary syndrome. Blood. 2006;107:4030–8. doi: 10.1182/blood-2005-10-4239. [DOI] [PubMed] [Google Scholar]
- 27.Massone C, Kodama K, Kerl H, et al. Histopathologic features of early (patch) lesions of Mycosis Fungoides. Am J Surg Pathol. 2005;29:550–560. doi: 10.1097/01.pas.0000153121.57515.c6. [DOI] [PubMed] [Google Scholar]
- 28.Trotter MJ, Whittaker SJ, Orchard GE, et al. Cutaneous histopathology of Sézary syndrome: a study of 41 cases with a proven circulating T-cell clone. J Cutan Pathol. 1997;24:286–291. doi: 10.1111/j.1600-0560.1997.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 29.Zip C, Murray S, Walsh NM. The specificity of histopathology in erythroderma. J Cutan Pathol. 1993;20:393–8. doi: 10.1111/j.1600-0560.1993.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 30.Kamarashev J, Burg G, Kempf W, et al. Comparative analysis of histological and immunohistological features in Mycosis Fungoides and Sézary syndrome. J Cutan Pathol. 1998;25:407–412. doi: 10.1111/j.1600-0560.1998.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 31.Kohler S, Kim YH, Smoller BR. Histologic criteria for the diagnosis of erythrodermic mycosis fungoides and Sézary syndrome: a critical reappraisal. J Cutan Pathol. 1997;24:292–297. doi: 10.1111/j.1600-0560.1997.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 32.Walsh NMG, Prokopetz R, Tron VA, et al. Histopathology in erythroderma: review of a series of cases by multiple observers. J Cutan Pathol. 1994;21:419–423. doi: 10.1111/j.1600-0560.1994.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 33.Sentis HJ, Willemze R, Scheffer E. Histopathologic studies in Sezary syndrome and erythrodermic mycosis fungoides : a comparison with benign forms of erythroderma. J Am Acad Dermatol. 1986;15:1217–26. doi: 10.1016/s0190-9622(86)70294-6. [DOI] [PubMed] [Google Scholar]
- 34.Werner B, Massone C, Kerl H, et al. Large CD30-positive cells in benign, atypical lymphoid infiltrates of the skin. J Cutan Pathol. 2008;35:1100–7. doi: 10.1111/j.1600-0560.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe M, Sasaki M, Itoh K, et al. JunB induced by Constitutive CD30-Extracellular Signal-Regulated Kinase ½ Mitogen-Activated Protein Kinase Signaling Activates the CD30 Promoter in Anaplastic Large Cell Lymphoma and Reed-Sternberg Cells of Hodgkin Lymphoma. Cancer Res. 2005;65:7628–7634. doi: 10.1158/0008-5472.CAN-05-0925. [DOI] [PubMed] [Google Scholar]
- 36.Karenko L, Hahtola S, Paivinen S, et al. Primary cutaneous T-cell lymphomas show a deletion or translocation affecting NAV3, the human UNC-53 homologue. Cancer Res. 2005;65:8101–8110. doi: 10.1158/0008-5472.CAN-04-0366. [DOI] [PubMed] [Google Scholar]
- 37.Marty M, Prochazkova M, Laharanne E, et al. Primary cutaneous T-cell lymphomas do not shown specific NAV3 gene deletion or translocation. J Invest Dermatol. 2008;128:2458–2466. doi: 10.1038/jid.2008.113. [DOI] [PubMed] [Google Scholar]


