Abstract
Fructansucrases (FSs), including levansucrases and inulosucrases, are enzymes that synthesize fructose polymers from sucrose by the direct transfer of the fructosyl moiety to a growing polymer chain. These enzymes, particularly the single domain fructansucrases, also possess an important hydrolytic activity, which may account for as much as 70 to 80% of substrate conversion, depending on reaction conditions. Here, we report the construction of four chimeric levansucrases from SacB, a single domain levansucrase produced by Bacillus subtilis. Based on observations derived from the effect of domain deletion in both multidomain fructansucrases and glucansucrases, we attached different extensions to SacB. These extensions included the transitional domain and complete C-terminal domain of Leuconostoc citreum inulosucrase (IslA), Leuconostoc mesenteroides levansucrase (LevC), and a L. mesenteroides glucansucrase (DsrP). It was found that in some cases the hydrolytic activity was reduced to less than 10% of substrate conversion; however, all of the constructs were as stable as SacB. This shift in enzyme specificity was observed even when the SacB catalytic domain was extended only with the transitional region found in multidomain FSs. Specific kinetic analysis revealed that this change in specificity of the SacB chimeric constructs was derived from a 5-fold increase in the transfructosylation kcat and not from a reduction of the hydrolytic kcat, which remained constant.
INTRODUCTION
There are several examples in nature of new enzymes that have evolved through the acquisition of one or more structural domains, providing the structural framework for the evolution of entirely new functions (1). This mechanism is particularly evident in the amylase family; it has been proposed that CGTases, which mainly bear a transferase activity, evolved from hydrolytic alpha-amylase by the incorporation of two additional domains (9). Enzymes also commonly acquire domains with functions that are complementary to the catalytic activity, such as substrate binding (cellulases) (18). Additional activities may also evolve through this mechanism, such as in a multifunctional glycosyl hydrolase isolated from a cow rumen microbial consortium. This enzyme bears two independent catalytic domains: one for the hydrolysis of mannan and a second domain for the hydrolysis of xylan and glucan (17). Another example is glucansucrase from Leuconostoc mesenteroides NRRL B-1299, which requires a second catalytic domain for dextran branching (2).
Fructansucrases (FSs) are enzymes that synthesize fructose polymers by direct transfer of the sucrose fructosyl moiety to a growing polymer chain, such as inulin in inulosucrases (EC 2.4.1.9) or levan in levansucrases (EC 2.4.1.10) (27). These enzymes also possess an important hydrolytic activity that may account for as much as 70 to 80% of substrate conversion, depending on reaction conditions. These enzymes can also transfer fructose to an acceptor molecule added to the reaction medium (6). The FSs belong to family 68 of glycoside hydrolases (GH68), according to the classification of carbohydrate-active enzymes (http://www.cazy.org/). Most FSs consist of a 45- to 64-kDa single domain with a five-bladed β-propeller fold that encloses a funnel-like central cavity, where most of the conserved catalytic residues are located (15). Recently, a subfamily of multidomain fructansucrases (MD-FSs) that have acquired structural domains from the N- and C-terminal regions of glucansucrases (GSs) has been described in Gram-positive Leuconostoc spp. (16). These FSs include inulosucrase (IslA) from Leuconostoc citreum (19, 22) and levansucrases (LevS, LevC, and LevL) from L. mesenteroides (16, 20). Although these enzymes are not as stable as some Gram-negative bacterial FSs, their total hydrolysis substrate conversion is as low as 25%, making them efficient fructan producers compared to single domain FSs, such as SacB (19).
Through the construction of truncated versions of IslA, we have recently demonstrated that the acquired regions in MD-FSs, particularly the C-terminal region, confer new properties to the inulosucrase IslA (8). Structural analysis with intrinsic fluorescence confirmed that the acquired domains in IslA interact with the catalytic domain, resulting in a new conformation that renders the enzyme more stable and generates a switch in specificity from a hydrolytic to a transglycosylase mechanism. Interestingly, when a single domain FSs (designated IslA4) was constructed by deletion of the additional domains in IslA, an enzyme with a 4-fold increase in global catalytic activity was obtained (kcat from 25 to 105 s−1), with a considerable reduction of the transferase specificity. However, these deletions also caused a 4-fold decrease in stability. Actually, these modifications were evident after deletion of the transitional region, which joins the catalytic and the C-terminal domain, as only slight changes in stability and specificity were observed in the absence of the C-terminal domain (8). As a consequence, because the constructed single domain inulosucrase (IslA4) hydrolyzed 70% of the sucrose, it behaves more like a fructosidase than a fructansucrase. In terms of specificity and structural architecture (45% identity), IslA4 approaches SacB, the levansucrase from Bacillus subtilis, which is stable and also exhibits a high hydrolytic activity. SacB has been extensively studied as a robust biocatalyst for the synthesis of levan. In this context, different strategies have been proposed to reduce the hydrolytic activity via the limitation of water availability for hydrolysis, such as the use of organic solvents (5), enzyme immobilization (7), or cross-linked enzyme aggregates (24).
Based on the structural features identified for MD-FSs, the objective of this study was to design efficient and stable fructansucrases introducing the high transferase efficiency of MD-FSs, such as IslA, into the highly stable single domain fructansucrase SacB, through the incorporation of an extension on its C-terminal domain. The explored extensions of SacB include the transitional domain and whole C-terminal domain of L. citreum inulosucrase (Tn-IslA and C-IslA, respectively), the transitional domain and whole C-terminal domain of L. mesenteroides levansucrase (Tn-LevC and C-LevC, respectively), and the C-terminal domain of the L. mesenteroides glucansucrase DsrP (C-DsrP).
MATERIALS AND METHODS
Bacterial strains, plasmids, and gene construction.
In all cases, Escherichia coli plasmid DNA was isolated with the High Pure plasmid isolation kit (Roche Diagnostics GmbH, Germany). Gene amplifications were carried out by PCR on a DNA RoboCycler gradient 96 (Stratagene, La Jolla, CA) using Expand High Fidelity polymerase (Roche Diagnostics GmbH, Germany). DNA fragments were isolated from agarose gels by means of the Qiagen gel extraction kit (Qiagen, Inc., Chatsworth, CA) according to the manufacturer's instructions.
The sequences encoding the chimeric levansucrases were constructed using the sequential PCR amplification methodology as described previously (10). All chimeras were constructed using a forward primer corresponding to the 5′ end of the gene (sacB Fw, 5′-CAT GCC ATG GGG AAG CCA TAT AAG GAA ACA TAC GGC-3′), while the reverse primers were designed to include a 16-nucleotide sequence complementary to the 5′ end of the fragment encoding the C-terminal regions of DsrP, LevC, and IslA. The sequences of these reverse primers were as follows: sacB Rv-dsrP, 5′-ATC ATC TCC CCA TTT GTT AAC TGT TAA TTG TCC TTG-3′; sacB Rv-levC, 5′-ACC AAC TAA TTC TTT GTT AAC TGT TAA TTG TCC TTG-3′; and sacB Rv-islA, 5′-ATA TGT CAT CCC TTT GTT AAC TGT TAA TTG TCC TTG-3′. In all cases, sacB from the pET-sacB vector was used as template (23).
Subsequently, two primers were designed in order to amplify each of the sequences encoding the DsrP, LevC, and IslA C-terminal regions. The forward primer contained a sequence complementary to the SacB 3′ end and a sequence complementary to the 3′ end corresponding to each C-terminal domain. For DsrP, pBAD-dsrP (21) was used as the template, with the following primer sequences: dsrP C-Fw, 5′ACA GTT AAC AAA GGG ATG ACA TAT TAT TCT ACA AGT 3′, and dsrP C-Rv, 5′CCG CTC GAG GCT TTT AAT CAG CTC TCC AGA ATT 3′. IslA was amplified from pCRIS (19) using the primers islA C-Fw, 5′ ACA GTT AAC AAA TGG GGA GAT GAT CAC TCT ATA G 3′, and islA C-Rv, 5′CCG CTC GAG AGC TTG CAA AGC ACG CTT ATC AAT 3′. Finally, LevC was amplified from plevC (20) with the forward primer levC C-Fw, 5′ACA GTT AAC AAA GAA TTA GTT GGT ACA AAA GCT ACT 3′, and the reverse primer levC C-Rv, 5′ TCC ATC GAG CTC ACG TAA GTA ATA TGT GCC ATC A 3′.
Two additional chimeras were constructed exclusively with the transitional regions (Tn) of IslA and LevC, which are defined as the region between the catalytic and the C-terminal domains (discussed later). For these regions, the forward primer sequences were the same as above (islA C-Fw and levC C-Fw), while the reverse primer sequences were islA Tn-Rv, 5′CCG CTC GAG AGC ATT TAA ATC GCG TGA AAA GCT 3′, and levC Tn-Rv, 5′CCG CTC GAG ATC CGT TAC ATC TTG TAA GTA ACC 3′, respectively.
The SacB amplification products containing complementary sequences of dsrP, levC, and islA, together with the corresponding Tn and C-terminal amplified regions, were used in a second PCR with equimolar concentrations of each coding sequence: sacB and C-islA, sacB and Tn-islA, sacB and C-levC, sacB and Tn-levC, and sacB and C-dsrP. Finally, the resulting fusion genes were reamplified using sacB Fw and the corresponding reverse primer of each chimeric construct: dsrP C-Rv, islA C-Rv, islA Tn-Rv, levC C-Rv, and levC Tn-Rv.
Cloning, expression, and purification.
Following PCR amplification, the constructed chimeric genes were cloned into the pBAD/Thio-TOPO vector, under the ara promoter, and transformed into TOP10 E. coli. The expressed proteins, SacB C-IslA, SacB Tn-IslA, SacB C-LevC, SacB Tn-LevC, and SacB C-DsrP, included thioredoxin and a His tag fused to the N- and C-terminal regions, respectively, while SacB wild-type (WT) constructs containing the His tag, with and without thioredoxin, were used as controls. The transformed strains were cultured overnight at 37°C in 50 ml YT2X medium, including 100 μg/ml ampicillin, and used to inoculate 950 ml of the same medium. When an optical density at 600 nm (OD600) of 0.6 was reached, the temperature was reduced to 20°C, and expression of the recombinant proteins was induced by the addition of 0.02% (wt/vol) of l-arabinose. Finally, at 1.8 OD600, the cells were harvested by centrifugation (10 min at 4°C and 4,600 × g), and the resulting pellet was washed twice with 50 mM phosphate buffer (pH 6.5). The cells were then suspended in 5 ml of the same buffer containing a protease inhibitor cocktail (complete, EDTA-free) and lysed at 900 lb/in2 in a French press. Cell debris was removed by centrifugation (30 min at 4°C and 10,000 × g), and the supernatant was assayed for activity.
Although all proteins included a His tag, purification with a Ni column was not possible. Therefore, the chimeras were purified from inclusion bodies by means of a purification kit containing the BugBuster protein extraction reagent (product no. 70584-4; Novagen). The inclusion bodies were resuspended in 10 volumes of 6 M guanidinium chloride (GdnHCl) at pH 6.5. Samples were subsequently dialyzed at 4°C with stirring against 5 volumes of 3 M, 2 M, 1 M, and 0.5 M GdnHCl, including 200 mM arginine and 1 mM CaCl2 in the last GdnHCl solution. The proteins were finally dialyzed against 50 mM phosphate buffer (pH 6.5) containing 1 mM CaCl2. The purity of the resulting fused protein was verified by 10% SDS-PAGE.
FS activity assay.
For all enzymes (SacB and fusion proteins), the initial rates were measured at 37°C in 50 mM phosphate buffer (pH 6.5) in the presence of 293 mM sucrose and 1 mM CaCl2. The global activity (G) was measured by following the reducing sugars released from sucrose with the 3,5-dinitrosalicylic acid method (DNS) (25). One global activity unit (U) is defined as the amount of enzyme that produces 1 μmol of reducing sugars (measured as glucose) per minute. Specific activity is reported as U/mg of protein. The protein concentration was determined by the Bradford method (3), using the Bio-Rad reagent and bovine serum albumin (BSA) as a standard.
Zymograms of the levansucrase activity of the chimeras were carried out in SDS-PAGE gels. After electrophoresis, the gels were preincubated in 50 mM potassium phosphate buffer (pH 6.5) containing 1% Tween 80 to eliminate the denaturizing agent. In situ polymer synthesis was then induced by overnight incubation in 150 mM sucrose in the same buffer.
The transglycosylase and hydrolase activity ratios (H/T) were determined by measuring the amount of glucose and fructose released in each reaction. The amount of glucose reflects the total amount of sucrose utilized during the reaction (total activity). Fructose is the result of hydrolytic activity, whereas the difference between the glucose and fructose concentration corresponds to the amount of fructose used for fructan synthesis and is therefore the result of the transfructosylation activity. H/T reaction ratio in the presence of xylose and maltose as acceptors was determined following the same procedure. Sugars involved in these experiments (xylose, maltose, fructose, glucose, and sucrose) were quantified by high-pressure liquid chromatography (HPLC) with a Waters 600E system controller (Waters Corp., Milford, MA) equipped with a refractive index detector (Waters 410) and a carbohydrate column (4.6 × 250 mm) kept at 35°C, using acetonitrile-water (75:25) as the mobile phase at 1.2 ml/min.
For two chimeric levansucrases, the individual kinetic constants were determined through the analysis of initial reaction rate measurements carried out at pH 6.5 and 37°C in sucrose solutions up to 350 mM, containing 1 mM CaCl2. All experiments were performed in triplicate. After addition of the enzyme, samples were withdrawn at 3 min intervals to perform the sugar analysis. For all enzymes (SacB and fusion proteins), stability was assayed by measuring the residual activity of the stored proteins at 40°C in 50 mM phosphate buffer (pH 6.5) containing 1 mM CaCl2. All experiments were performed in triplicate.
RESULTS AND DISCUSSION
Design of SacB chimeras.
Based on a homology analysis of the C-terminal domain of MD-FSs produced by ClustalW (13), nine GW repeats were identified in IslA with 20% identity to GW modules already described in internalin (InlB) and amidase (Ami) from Listeria monocytogenes (12). The GW modules are involved in anchoring the protein to the cell wall through interactions with lipoteichoic acids; each module is independently folded and retains its functionality (4, 14). The C-terminal domain of IslA behaves in a similar manner, as demonstrated by direct binding assays (8). Additionally, two GW modules also constitute the transitional region between the catalytic and the C-terminal domains in IslA, as described by Olivares-Illana et al. (19). Interestingly, removal of this particular region causes IslA to lose its transferase activity, as demonstrated by Del Moral et al. (8) through the characterization of truncated IslA constructs. Furthermore, we were also able to identify eight CW motifs (ngWikdn.gnWYYfdsdGkm) in the C-terminal region of MD levansucrases (LevS, LevC, and LevL), which are similar to those found in toxins from Clostridium and Streptococcus spp. (28). These consensus sequences are also involved in anchoring the proteins to the cell wall (11). As in IslA, the transitional region between the catalytic and the C-terminal domains of MD levansucrases also contains two CW motifs.
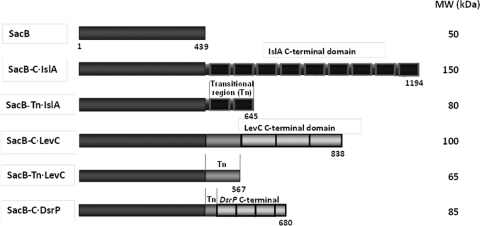
In order to explore if the single domain levansucrase SacB could acquire MD-FS properties through the addition of recombinant domains, four SacB chimeric levansucrases were designed and constructed: the first two are fusions of the C-terminal region (nine GW modules) and the transitional region (two GW modules) of IslA, the inulosucrase from L. citreum CW28, and are referred to as SacB C-IslA and SacB Tn-IslA, respectively; two chimeras contained the C-terminal region (eight CW motifs) and the transitional region (two CW motifs) of LevC levansucrase from L. mesenteroides ATCC 8293 and are designated SacB C-LevC and SacB Tn-LevC, respectively (Fig. 1). In all of these constructs, we included an 8-amino-acid region found between the catalytic domain and the first GW or CW motifs in IslA and LevC, respectively. Finally, a construct was made by fusion of SacB with a segment of the C-terminal region of DsrP, a glucansucrase from L. mesenteroides IBT-PQ (21) that has 30% identity with the C-terminal region of MD-FSs (also illustrated in Fig. 1). All chimeric constructs were cloned into pBAD/Thio-TOPO and successfully expressed in E. coli TOP10 cells, as shown in Fig. 2 by SDS-PAGE analysis. After extraction of the chimeric proteins, the activity was identified through zymograms. Although the chimeric constructions were the main and most abundant proteins, several activity bands associated with the fructan production were present. These additional activity bands of lower molecular weight than the expected chimers were the result of chimer proteolysis, a common problem associated to MD-FSs production (19, 16, 20). In spite of this limited proteolysis, a modification of the crude chimer specificity was observed (Fig. 3A). For a proper kinetic characterization, the chimers were purified from inclusion bodies (see below).
Fig 1.
Illustration of the chimeric levansucrase constructs. SacB, B. subtilis levansucrase; SacB C-IslA, SacB fusion with the C-terminal region of IslA; SacB Tn-IslA, SacB fusion with the transitional region of IslA; SacB C-LevC, SacB fusion with the C-terminal region of LevC; SacB Tn-LevC, SacB fusion with the transitional region of LevC; SacB C-DsrP, SacB fusion with the C-terminal region of DsrP.
Fig 2.
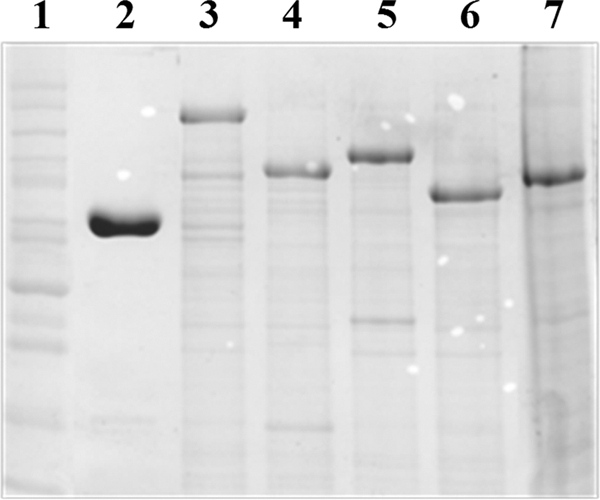
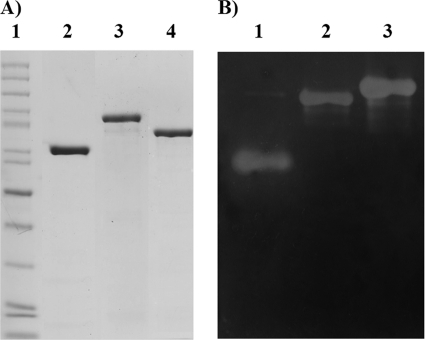
SDS-PAGE analysis (Coomassie blue stain) of chimeric levansucrases in the E. coli TOP10 crude cell extract. Molecular weight control (lane 1), SacB (lane 2), SacB C-IslA (lane 3), SacB Tn-IslA (lane 4), SacB C-LevC (lane 5), SacB Tn-LevC (lane 6), and SacB C-DsrP (lane 7).
Fig 3.
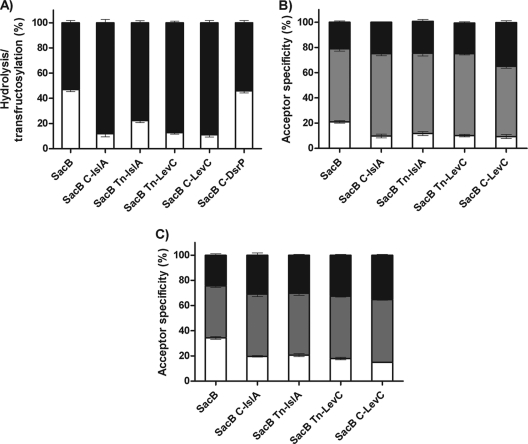
Hydrolysis, transfructosylation, and acceptor specificity of B. subtilis SacB and the chimeric levansucrases. The reactions were carried out with sucrose as substrate (A), sucrose with xylose as an acceptor (B), and sucrose with maltose as an acceptor (C). The proportion of fructosyl residues transferred to sucrose or levan (black), to xylose or maltose (gray), or to water (empty) was analyzed after 80% sucrose conversion. All reactions were carried out using a concentration of 120 g/liter of substrate and acceptor and 0.5 U/ml of enzyme.
Biochemical characterization of chimeric levansucrases.
To determine the effect of each C-terminal extension on the stability of SacB, the half-lives of the five chimeric levansucrases were determined at 40°C. These results suggest that the additional regions did not significantly change the half-life of SacB (72 min). While a slight decrease of 10 min in the half-lives of SacB C-LevC and SacB C-DsrP was observed, the half-life of SacB C-IslA increased by 8 min. Del Moral et al. (8) report a large decrease in stability when the IslA transitional region is eliminated from the enzyme. However, as SacB is already a stable fructansucrase, it is not surprising that a significant increase in stability did not occur.
The effect of additional domains on the SacB H/T ratio was examined at the optimum pH and temperature conditions and using sucrose as substrate. The results shown in Fig. 3A indicate that an inversion in the H/T ratio occurs in SacB C-IslA and SacB Tn-IslA, as a result of a considerable increase in transglycosylase activity. Additionally, in the case of SacB C-LevC and SacB Tn-LevC, the transglycosylase activity was increased to 90% of the total activity. Interestingly, in the smallest constructs containing only the transitional region (SacB Tn-IslA and SacB Tn-LevC), the hydrolytic activity is reduced to the same levels found in constructs containing the complete C terminus (SacB C-IslA and SacB C-LevC). These results suggest that the additional regions, particularly the transitional region, interact with the catalytic core and decrease the hydrolysis of the enzyme, either by affecting the accessibility of the catalytic water molecules to the active site or improving the acceptor specificity of the fructan growing chains. Finally, changes in the transglycosylase activity of SacB C-DsrP were not observed (Fig. 3A), demonstrating that the 25% identity between DsrP and the C terminus of MD-FSs is not related to the molecular determinants responsible for the increase in transferase properties, as the repeats in DsrP are CW motifs.
In order to analyze the effect of the additional regions on SacB acceptor specificity, xylose and maltose were studied experimentally as acceptor molecules, and all of the constructs were assayed in the presence of sucrose. As shown in Fig. 3B and C, xylose is a better acceptor of the fructosyl residue than maltose, as demonstrated by the large amounts of accumulated xylose fructoside. However, this property is present in native SacB and does not result from the additional domains in the chimeras. In general, only a slight shift in acceptor reaction specificity was observed in all of the constructed chimeric levansucrases, resulting in decreases of approximately 15% of the hydrolytic activity, with a concomitant increase in the transfer to both acceptors and the polymer. This slight change in specificity is more evident in the reaction with maltose, which is a worse acceptor than xylose. In conclusion, although the presence of the additional domains decreases hydrolytic activity and increases the transferase reaction, the acceptor specificity is not modified, as the fructoside yield in the presence of high specificity acceptors, such as xylose, is the same for all constructs. Similar effects have been observed when SacB is assayed in the presence of organic solvents, where a reduction of the hydrolysis/transglucosylation ratio is observed but not an increase in the transfer activity to acceptors (5).
Catalytic efficiencies of SacB Tn-IslA and SacB Tn-LevC.
All chimeric constructs resulted in highly active and stable enzymes with reduced hydrolytic activity. Accordingly, the smaller chimeras SacB Tn-IslA and SacB Tn-LevC, which contain only the transitional region, were selected for further characterization, including kinetic behavior and reaction specificity. The chimeric levansucrases were refolded after separation and purification from inclusion bodies as described in Materials and Methods. Single bands were obtained from the purified enzymes, as observed in Fig. 4.
Fig 4.
SDS-PAGE analysis (Coomassie blue stain) and zymogram of the purified chimeric levansucrases. (A) Purified enzymes used in the biochemical characterization. Molecular weight control (lane 1), SacB (lane 2), SacB Tn-IslA (lane 3), and SacB Tn-LevC (lane 4). (B) Zymogram of the purified chimers using sucrose as substrate. SacB (lane 1), SacB Tn-LevC (lane 2), and SacB Tn-IslA (lane 3).
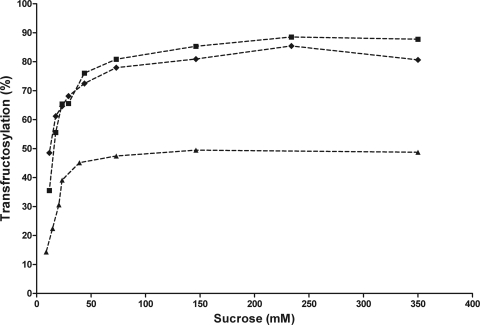
The effect of the substrate concentration on reaction specificity for both constructs was studied in reactions with up to 350 mM sucrose. Both the initial rate of sucrose hydrolysis and the initial rate of the transferase reaction were measured and compared to the values observed for SacB. Figure 5 demonstrates that even low substrate concentrations, which favor hydrolytic reactions (26), yielded high transferase activity for both chimeric levansucrases. The kinetic parameters were derived from Eadie-Hofstee type plots and are reported in Table 1, along with the values observed for SacB (no differences were found with kinetic parameter values obtained by nonlinear regression or Lineweaver-Burk-type plots). Interestingly, the Km and kcat values for hydrolysis of chimeric levansucrases were found to be very similar to those obtained for B. subtilis SacB. These results suggest that, as far as the affinity for water as acceptor of the fructosyl residue is concerned, the catalytic efficiencies of the chimeric levansucrases are similar to that of SacB, with both reactions proceeding at the same rate. However, when considering the transferase reaction (fructose transferred to the polymer), the kcat value of the chimeric enzymes was significantly increased (from 48 s−1 to 115 s−1 for SacB Tn-IslA and to 238 s−1 for SacB Tn-LevC), while the Km value remained the same. Therefore, although a similar turnover number is observed for hydrolysis, more substrate molecules are used for transfructosylation per unit time, resulting in an overall decease in hydrolytic activity. More specifically, while SacB uses 1.45 mol of sucrose for transfer reactions per mol of hydrolyzed sucrose, this kcat ratio for SacB Tn-IslA is increased to 3.24. Finally, SacB Tn-LevC is the most efficient construct, with a kcatT/kcatH ratio (mol of substrate transferred per mol of hydrolyzed substrate) of 6.54. Furthermore, the similarity in the kinetic parameters indicates that the additional regions in these chimeric levansucrases do not affect the accessibility of the catalytic water molecules to the active site, but rather these regions increase the affinity of the enzyme for the fructan acceptor molecule. Based on these results and the structural analysis obtained by intrinsic fluorescence of IslA from Del Moral et al. (8), we suggest that the transitional region modifies the catalytic domain, producing a structural adjustment that increases affinity for the polymer acceptor molecule and the transferase efficiency. The construction of chimeric enzymes may become a rational strategy to modify single domain fructansucrases or mutants to increase the efficiency and reduce substrate loss by hydrolysis, without affecting the enzyme stability.
Fig 5.
Effect of the sucrose concentration on the transfructosylation activity of chimeric levansucrases relative to hydrolysis: SacB (▲), SacB Tn-IslA (♦), and SacB Tn-LevC (■). Products were determined after 24 h of reaction at pH 6.5 and 30°C and with 0.5 U/ml enzyme.
Table 1.
Michaelis-Menten kinetic parameters of B. subtilis levansucrase SacB and the chimeric levansucrases
| Enzyme | Parametersa |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| KmH (mM) | KmT (mM) | KmG (mM) | kcatH (s−1) | kcatT (s−1) | kcatG (s−1) | kcatH/KmH (s−1 mM−1) | kcatT/KmT (s−1 mM−1) | kcatG/KmG (s−1 mM−1) | |
| SacB (WT) | 11.6 ± 0.24 | 38.3 ± 0.76 | 21.5 ± 1.21 | 33.3 ± 0.87 | 48.4 ± 6.7 | 87.3 ± 2.89 | 2.87 | 1.26 | 4.06 |
| SacB Tn-IslA | 13.2 ± 1.65 | 72.6 ± 2.03 | 58.7 ± 4.64 | 35.6 ± 3.54 | 115.6 ± 16.9 | 166.8 ± 11.25 | 2.7 | 1.59 | 2.84 |
| SacB Tn-LevC | 13.9 ± 2.24 | 102.5 ± 6.78 | 88.25 ± 4.33 | 36.4 ± 1.02 | 238.3 ± 21.5 | 280.1 ± 10.89 | 2.6 | 2.33 | 3.17 |
H, hydrolytic; T, transferase activities; and G, global.
ACKNOWLEDGMENTS
This project was supported by PAPIIT-UNAM no. IN212311 and Consejo Nacional de Ciencia y Tecnología (CONACyT) 81637.
We thank Maria Elena Rodriguez and Fernando Gonzalez for technical assistance.
Footnotes
Published ahead of print 13 January 2012
REFERENCES
- 1. Bashton M, Chothia C. 2007. The generation of new protein functions by the combination of domains. Structure 5:85–99 [DOI] [PubMed] [Google Scholar]
- 2. Bozonnet S, et al. 2002. Molecular characterization of DSR-E, an alpha-1,2 linkage-synthesizing dextransucrase with two catalytic domains. J. Bacteriol. 184:5753–5761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 4. Braun L, et al. 1997. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol. 25:285–294 [DOI] [PubMed] [Google Scholar]
- 5. Castillo E, Lopez-Munguia A. 2004. Synthesis of levan in water-miscible organic solvents. J. Biotechnol. 114:209–217 [DOI] [PubMed] [Google Scholar]
- 6. Chambert R, Treboul G, Dedonder R. 1974. Kinetic studies of levansucrase of Bacillus subtilis. Eur. J. Biochem. 41:285–300 [DOI] [PubMed] [Google Scholar]
- 7. Chambert R, Petit-Glatron MF. 1993. Immobilisation of levansucrase on calcium phosphate gel strongly increases its polymerase activity. Carbohydrate Res. 244:129–136 [DOI] [PubMed] [Google Scholar]
- 8. Del Moral S, Olvera C, Rodriguez ME, Lopez-Munguía A. 2008. Functional role of the additional domains in inulosucrase (IslA) from Leuconostoc citreum CW28. BMC Biochem. 9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. del-Rio G, Morett E, Soberon X. 1997. Did cyclodextrin glycosyltransferases evolve from alpha-amylases? FEBS Lett. 416:221–224 [DOI] [PubMed] [Google Scholar]
- 10. Elion EA, Marina P, Yu L. 2007. Constructing recombinant DNA molecules by PCR. In Ausubel FM, et al. (ed), Current protocols in molecular biology. John Wiley and Sons, New York, NY: [DOI] [PubMed] [Google Scholar]
- 11. Fernández-Torneo C, López R, García E, Giménez-Gallego G, Romero A. 2001. A novel solenoid fold in the cell wall anchoring domain of the pneumococcal virulence factor LytA. Nat. Lett. 8:1020–1024 [DOI] [PubMed] [Google Scholar]
- 12. Jonquières R, Bierne H, Fiedler F, Gounon P, Cossart P. 1999. Interaction between the protein InlB of Listeria monocytogenes and lipoteichoic acid: a novel mechanism of protein association at the surface of Gram-positive bacteria. Mol. Microbiol. 34:902–914 [DOI] [PubMed] [Google Scholar]
- 13. Larkin MA, et al. 2007. ClustalW and ClustalX version 2. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 14. Marino M, Banerjee M, Jonquières R, Cossart P, Gosh P. 2002. GW domains of the Listeria monocytogenes invasion protein InlB are SH3-like and mediate binding to host ligands. EMBO J. 21:5623–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meng G, Fütterer K. 2003. Structural framework of fructosyl transfer in Bacillus subtilis levansucrase. Nat. Struct. Biol. 10:935–941 [DOI] [PubMed] [Google Scholar]
- 16. Morales-Arrieta S, Rodriguez ME, Segovia L, Lopez-Munguia A, Olvera-Carranza C. 2006. Identification and functional characterization of levS, a gene encoding for a levansucrase from Leuconostoc mesenteroides NRRL B-512 F. Gene 376:59–67 [DOI] [PubMed] [Google Scholar]
- 17. Palackal N, et al. 2007. A multifunctional hybrid glycosyl hydrolase discovered in an uncultured microbial consortium from ruminant gut. Appl. Microbiol. Biotechnol. 74:113–124 [DOI] [PubMed] [Google Scholar]
- 18. Prates JA, et al. 2001. The structure of the feruloyl esterase module of xylanase 10B from Clostridium thermocellum provides insights into substrate recognition. Structure 9:1183–1190 [DOI] [PubMed] [Google Scholar]
- 19. Olivares-Illana V, Lopez-Munguia A, Olvera C. 2003. Molecular characterization of inulosucrase from Leuconostoc citreum: a fructansucrase within a glucosyltransferase. J. Bacteriol. 185:3606–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olvera C, Centeno-Leija S, Lopez-Munguia A. 2007. Structural and functional features of fructansucrases present in Leuconostoc mesenteroides ATCC 8293. Antonie Van Leeuwenhoek 92:11–20 [DOI] [PubMed] [Google Scholar]
- 21. Olvera C, Fernandez-Vazquez JL, Ledezma-Candanoza L, Lopez-Munguia A. 2007. Role of the C-terminal region of dextransucrase from Leuconostoc mesenteroides IBT-PQ in cell anchoring. Microbiology 153:3994–4002 [DOI] [PubMed] [Google Scholar]
- 22. Ortiz-Soto ME, Olivares-Illana V, Lopez-Munguia A. 2004. Biochemical properties of inulosucrase from Leuconostoc citreum CW28 used for inulin synthesis. Biocatal. Biotransformation 22:275–281 [Google Scholar]
- 23. Ortiz-Soto ME, Rivera M, Rudiño-Piñera E, Olvera C, López-Munguía A. 2008. Selected mutations in Bacillus subtilis levansucrase semi-conserved regions affecting its biochemical properties. Protein Eng. Des. Sel. 21:589–595 [DOI] [PubMed] [Google Scholar]
- 24. Ortiz-Soto ME, Rudino-Pinera E, Rodriguez-Alegria ME, Lopez-Munguia A. 2009. Evaluation of cross-linked aggregates from purified Bacillus subtilis levansucrase mutants for transfructosylation reactions. BMC Biotechnol. 9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sumner JB, Howell ST. 1934. A method for determination of saccharasa activity. J. Biol. Chem. 108:51–54 [Google Scholar]
- 26. Tanaka T, Oi S, Yamamoto T. 1979. Synthesis of levan by levansucrase. Some factors affecting the rate of synthesis and degree of polymerization of levan. J. Biochem. 85:287–293 [DOI] [PubMed] [Google Scholar]
- 27. van Hijum SA, Kralj S, Ozimek LK, Dijkhuizen L, van Geel-Schutten IG. 2006. Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol. Mol. Biol. Rev. 70:157–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wren BW. 1991. A family of clostridial and streptococcal ligand-binding proteins with conserved C-terminal repeat sequences. Mol. Microbiol. 5:797–803 [DOI] [PubMed] [Google Scholar]