Abstract
The person-to-person transmission of influenza virus, especially in the event of a pandemic caused by a highly virulent strain of influenza, such as H5N1 avian influenza, is of great concern due to widespread mortality and morbidity. The consequences of seasonal influenza are also substantial. Because airborne transmission appears to play a role in the spread of influenza, public health interventions should focus on preventing or interrupting this process. Air disinfection via upper-room 254-nm germicidal UV (UV-C) light in public buildings may be able to reduce influenza transmission via the airborne route. We characterized the susceptibility of influenza A virus (H1N1, PR-8) aerosols to UV-C light using a benchtop chamber equipped with a UVC exposure window. We evaluated virus susceptibility to UV-C doses ranging from 4 to 12 J/m2 at three relative humidity levels (25, 50, and 75%). Our data show that the Z values (susceptibility factors) were higher (more susceptible) to UV-C than what has been reported previously. Furthermore, dose-response plots showed that influenza virus susceptibility increases with decreasing relative humidity. This work provides an essential scientific basis for designing and utilizing effective upper-room UV-C light installations for the prevention of the airborne transmission of influenza by characterizing its susceptibility to UV-C.
INTRODUCTION
Seasonal influenza is a major cause of morbidity and mortality in the United States and throughout the world. Each year, influenza accounts for about 3 million hospital days, 31 million outpatient visits, and $10 billion in excess costs in the United States. These consequences are most predominant in the very young and the elderly (22). As demonstrated by the 2009 H1N1 influenza pandemic, influenza can spread rapidly in populations, and the development of vaccines can take months to accomplish (11). Furthermore, the use of nonpharmaceutical interventions appears to have had limited effectiveness (11). In the event of the pandemic spread of highly virulent strains of influenza, such as H5N1 bird flu, which has recently shown a mortality rate of approximately 60%, effects could be disastrous (5, 26). For these reasons, effective interventions to prevent the transmission of influenza are needed.
Contemporary science continues to study and understand the modes used by influenza virus to spread from person to person. There is debate about whether influenza virus is transmitted via fine aerosols that are airborne, through exposure to large ballistic droplets, or contact with fomites (3, 29). Understanding the route of transmission is critical for implementing the best control strategies. There is considerable evidence for the airborne transmission of influenza via fine aerosols: influenza aerosols have been shown to remain infective in laboratory experiments; the aerosol transmission of influenza has also been shown to occur in animal and human volunteer studies (1, 17); influenza nucleic acids have been associated with fine particles in the exhaled breath of persons infected with influenza virus (2, 7, 15, 16); and human epidemiology studies have associated influenza transmission with airborne routes (20, 23). As such, interventions to prevent influenza transmission must go beyond the traditional dogma of cough etiquette, hand hygiene, and social distancing. Due to their very low terminal settling velocity, infectious fine particles, wafted by air currents, would be expected to remain airborne for hours. One intervention proposed to be effective is UV irradiation, also called UV-C light, emitted from specialized lamps placed near the ceiling.
The germicidal effect of light in the UV-C electromagnetic spectrum (specifically 254-nm light) has been recognized for some time. The susceptibility of microorganisms to UV-C light varies and has been summarized in previous investigations (14). Microorganism susceptibility to UV-C light is traditionally thought to follow first-order kinetics according to the equation FR = CUV/CNo UV = e−ZD, where FR is the fraction remaining, CUV is microorganism concentration with UV exposure, CNo UV is microorganism concentration without UV exposure, Z is the susceptibility parameter expressed in m2/J, and D is the dose of UV-C in J/m2 (4, 8, 18). These susceptibility parameters, or Z values, do not appear to be static but vary with environmental conditions, such as relative humidity (RH) (12, 13, 18). There is also evidence that the susceptibility of microorganisms to UV-C light does not necessarily follow first-order kinetics (18). Thus, it is necessary to determine Z values in a defined and controlled experimental system to predict UV-C effectiveness, determine the amount of UV-C energy required for intervention, and develop models of UV-C effectiveness.
Traditionally, UV-C light has been used to sanitize air using UV-C light emitted by lamps located in the upper portions of rooms (8). High-intensity light restricted to the upper part of the room by special louvered fixtures inactivates microorganisms as they circulate through the room via air currents. Due to the louvered fixtures, the UV levels in the lower, occupied part of the room remain at safe levels (25). The aim of our investigation was to determine Z values for influenza virus aerosols at low, medium, and high relative humidity.
MATERIALS AND METHODS
Virus preparation.
A suspension of influenza virus (A/PR/8/34 H1N1), which was purchased from Advanced Biotechnologies Inc. (Columbia, MD), was thawed, divided into single-use portions, and stored at −80°C until needed.
Virus infectivity assay.
A fluorescent focus reduction assay was used to enumerate numbers of infective viruses and has been described previously (9, 28). Briefly, infected Madin-Darby canine kidney (MDCK) cells (ATTC CCL-34) containing influenza A nucleoproteins were labeled with influenza A virus nucleoprotein antibody (Abcam, Cambridge, MA) and subsequently labeled with rhodamine-labeled goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). The number of cells having resulting fluorescent foci (measured in fluorescent focus units [FFU]) was counted using an Olympus CKX-41 inverted fluorescence microscope (200× total power; Olympus, Center Valley, PA). The number of FFU per sample was computed based on dilution factors and the fraction of the well counted.
Benchtop UV-C aerosol exposure chamber.
Influenza aerosols were generated by adding 0.075 ml of undiluted influenza virus and 75 ml of buffer (Dulbecco's phosphate-buffered saline with calcium and magnesium containing 0.1% bovine serum albumin) into a high-output extended aerosol respiratory therapy (HEART) nebulizer (Westmed, Tucson, AZ) and pressurizing at 69 kPa. The nebulizer output was mixed with dry or humidified air (to achieve the desired RH level) in a 7.5-liter chamber prior to delivery to the aerosol exposure chamber. The details of the one-pass, dynamic aerosol test system were described previously (18). RH and temperature in the chamber (measured via an Omega RH32 temperature and relative humidity meter [Omega Engineering Inc., Stamford, CT]) were measured prior to entry into the UV-C exposure section. UV-C was generated by six 36-W low-pressure mercury lamps (254-nm light; Lumalier, Memphis, TN), and screens were placed between the UV-C light source and the exposure window to attenuate UV-C output and dose within the exposure chamber. UV-C irradiance in the chamber was measured through a fused quartz port in the bottom of the UV-C exposure section of the chamber using an IL 1400A UV light meter (International Light, Peabody, MA). The irradiance levels used were based on initial influenza virus dose-response experiments performed in our chamber and the detection limits of our infectivity assay. The UV-C dose was computed by multiplying the UV-C irradiance by the exposure time. The exposure time was computed by dividing the volume of the chamber by the airflow rate.
Air was drawn through the chamber by a pump at 25 liters/min through a manifold attached to 2 SKC Biosamplers (SKC Inc., Eighty Four, PA), each operating at 12.5 liters/min. Each Biosampler contained 20 ml of virus buffer (Dulbecco's phosphate-buffered saline with calcium and magnesium containing 0.1% bovine serum albumin). A HEPA filter was connected after the samplers to remove fugitive aerosols before the airstream entered the pump. When sampling was not in progress, the aerosol-laden airstream running through the chamber was bypassed around the samplers, and the 25-liter/min flow was directed to the HEPA filter. The entire apparatus was set up inside a 6-foot class II biosafety cabinet and maintained under negative pressure with respect to the cabinet interior.
The nebulizer was run for 20 min before sampling to ensure that concentrations within the chamber had stabilized. Samples were collected by passing the entire chamber airflow through the Biosamplers for a period of 15 min. Sample pairs were collected that consisted of a sample with the UV-C lights on followed by a control sample with the UV-C lights off. Triplicate sample pairs were collected for combinations of UV-C dose (ranging from 4 to 12 J/m2) and RH (25 to 27%, 50 to 54%, and 81 to 84%). After each sampling the BioSamplers were removed from the chamber, the volume of collection liquid was measured, and virus collection fluid was stored at 4°C for a maximum of 3 h prior to performing the infectivity assay. The BioSamplers were decontaminated with 10% bleach, rinsed with 70% ethanol, and dried before reusing.
The fraction of virus surviving for each sample pair was calculated by dividing the number of FFUs per sample with the UV-C lights on by the number FFUs per sample with the UV-C lights off.
Linear regression through the origin was used to estimate the Z values for each level of humidity. The −log(FR) was our outcome variable, and the dose level was our response. The estimated coefficient of the regression line then was our estimate of the Z value. The bootstrap method was used to determine whether the estimated Z values differed. Given any two Z values that were tested, we bootstrapped 1,000 pairs of resampled data sets from the original data at each humidity level, reran the regression models, and calculated the difference between the estimated coefficients. This gave us an estimate of the distribution of the difference between the coefficients. We calculated a P value for each difference by using this distribution to test the hypothesis that the true difference is equal to 0. Statistical analysis was performed using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
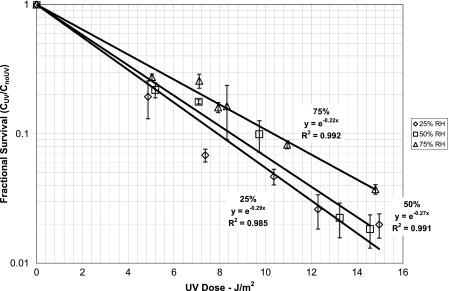
The average airborne concentration of influenza virus in the chamber as measured by the focus assay was 5.62 × 103 FFU per liter of air, which corresponded to 2,636 FFU per well on the 96-well plate. The fractional survival of influenza aerosols was measured in triplicate for combinations of three ranges of relative humidity (low, 25 to 27%; medium, 50 to 54%; and high, 81 to 84%) and 6 UV doses ranging from 4.9 to 15.0 J/m2 (Fig. 1). Using our experimental system, we measured influenza reductions as low as 98.2% by comparing samples with the UV light on to subsequent samples control samples with the UV light off. The coefficients of variation between triplicate experiments ranged from 0.04 to 0.45 with a median of 0.13. The fractional survival of influenza aerosolized at low (25 to 27%), medium (50 to 54%), or high (81 to 84%) RH is shown in Fig. 1. We calculated Z values of 0.29, 0.27, and 0.22 m2/J for low, medium, and high relative humidity levels, respectively (Table 1). Generally, as the RH increased the Z value decreased. The dose-response relationship appears linear when the log of the fractional survival is plotted against the UV-C dose and reflects first-order decay. Linear regression models fit the data well, as reflected by R2 values, which were at least 0.95. Bootstrap results of the 1,000 samples show that all Z values are significantly different from each other (P < 0.0001).
Fig 1.
UV-C susceptibilities of influenza virus aerosols at 25 to 27%, 50 to 54%, and 81 to 84% RH (each data point is the average from triplicate experimental trials; error bars denote one standard deviation).
Table 1.
Estimated Z values for influenza aerosols determined at low, medium, and high relative humidity
| RH range (%) | Estimated Z value | 95% confidence interval |
R2 | |
|---|---|---|---|---|
| Lower | Upper | |||
| 25–27 | 0.29 | 0.27 | 0.31 | 0.985 |
| 50–54 | 0.27 | 0.26 | 0.31 | 0.991 |
| 81–84 | 0.22 | 0.21 | 0.23 | 0.992 |
DISCUSSION
The Z values determined for influenza aerosols in our study suggest that influenza will be effectively inactivated during exposure to upper-room UV-C. The Z value reported for influenza virus is within the range of those reported for Mycobacterium tuberculosis. Upper-room UV-C has been shown to be highly effective in laboratory studies and has been proposed to control exposures to tuberculosis (6, 12, 21, 24, 27). The influenza Z values were much higher (i.e., influenza is more susceptible to UV-C) than those reported for hardy spores, such as those from Bacillus anthracis (Table 2) (4, 14).
Table 2.
Z values reported for various microorganismsa
| Microorganism | Z value (m2/J) |
|---|---|
| Influenza A virus | 0.15 |
| Mycobacterium tuberculosis | 0.10–0.48 |
| Bacillus anthracis | 0.031 |
Data are from Kowalski et al. (14).
The Z values reported herein all are higher than those reported previously for influenza virus aerosols in a review by Kowalski et al. (14), which was based on research by Jensen (10), and suggest an increased efficacy of UV-C for deactivating influenza virus. Jensen's experiments were not designed to measure a Z value for influenza, and in fact he did not calculate a Z value (18). However, by making assumptions that were not explicitly supported by Jensen's description of his experiments, Kowalski was able to calculate a Z value of 0.15 m2/J (18). The differences in Z values between our data and previously reported data, although modest, may correspond to large differences in virus survival. For example, for a dose of 6 J/m2 a Z value of 0.15 m2/J would result in a 60% reduction, while a Z value of 0.25 would result in a 77% reduction.
The Z values for influenza virus were statistically different as a function of RH. These differences showing that influenza aerosols are less susceptible to UV at higher RH than at lower RH have been noted in previous UV susceptibility studies for other organisms (18, 19). In climate-controlled, indoor environments, high RH values near 75% may be unusual, but in areas without environmental controls UV effectiveness may decrease in the presence of elevated RH. In these instances where UV-C may be a cost-effective intervention to reduce influenza exposure, more UV-C energy may be required.
In recent studies of pox virus susceptibility to UV-C light, the decay was not linear (18) but rather fit a model where a log-normal distribution of susceptibilities described virus susceptibility. These differences may be due to the differences between pox viruses and influenza virus. Pox viruses are large DNA viruses with a high degree of structure, whereas influenza viruses are smaller, simpler, RNA viruses. UV-C light may have different interactions with nucleic acids to form photodimers, with thiamine found in DNA and uracil found in RNA, and this may be the source of the difference in dose-response.
The hospital environment is a location where the unchecked transmission of influenza virus infection may lead to a hospital-associated disease outbreak (26, 27). Despite increasing attention to hospital infection control precautions in recent years, the exposure of health care workers, patients, and visitors to influenza and other respiratory infections remains a substantial problem (28). Among the most formidable hospital-associated outbreaks is one involving a highly virulent virus, such as the H5N1 virus currently circulating in Eurasia that is known to cause death in an alarming fraction of its victims (29). To accommodate a surge in patients needing health care during a severe pandemic, hospitals are expected to house infected patients in groups consisting of those with confirmed or suspected pandemic infection. In this scenario, patients may be held in close proximity to each other on a designated ward (section) of the facility. Limited numbers of negative-pressure isolation rooms will be available, producing a crowded environment with substantial airflow limitations. Upper-room UV-C light could be utilized in this and many other pandemic scenarios for air disinfection. Lindsley et al. and Balchere et al. detected influenza virus nucleic acid associated with fine particles in an emergency department and throughout a health care facility (2, 15). Although the presence of nucleic acid does not confirm the presence of infectious viruses, it is strong evidence that viruses are being released into the air. The use of upper-room UV-C light would provide cost-effective air disinfection in settings where it would not be feasible to use engineering controls, such as increased ventilation rates or filtration (4). Furthermore, the use of upper-room UV would not be limited to controlling the spread of influenza virus, as it would also control the spread of other airborne infectious agents (many of which are susceptible to UV-C light) (14).
Relative humidity is one important variable which may influence the germicidal activity of UV-C light against viruses. Nebulization method, sampling time, aerosol concentration, and dose are other factors which may play an important role in the germicidal activity of UV-C light. The characterization of these factors was beyond the scope of our study but warrants future investigation.
Conclusions.
By characterizing the susceptibility of influenza virus to UV-C exposure, this work provides an essential scientific basis for designing effective upper-room UV-C installations for the prevention of influenza virus infection transmitted from person to person via an airborne route. The Z values determined for influenza virus were higher (i.e., greater susceptibility to UV-C) than those previously reported. Additionally, UV-C effectiveness was shown to decrease with increasing relative humidity.
ACKNOWLEDGMENTS
We thank Jinyize Wang for her work in the laboratory.
The opinions expressed in this article are those of the authors and do not necessarily reflect the opinions or positions of the Department of Veterans Affairs, the Office of Public Health, or the National Center for Occupational Health and Infection Control.
Footnotes
Published ahead of print 6 January 2012
REFERENCES
- 1. Alford RH, Kasel JA, Gerone PJ, Knight V. 1966. Human influenza resulting from aerosol inhalation. Proc. Soc. Exp. Biol. Med. 122:800–804 [DOI] [PubMed] [Google Scholar]
- 2. Blachere FM, et al. 2009. Measurement of airborne influenza virus in a hospital emergency department. Clin. Infect. Dis. 48:438–440 [DOI] [PubMed] [Google Scholar]
- 3. Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. 2007. Transmission of influenza A in human beings. Lancet Infect. Dis. 7:257–265 [DOI] [PubMed] [Google Scholar]
- 4. Brickner PW, et al. 2003. The application of ultraviolet germicidal irradiation to control transmission of airborne disease: bioterrorism countermeasure. Public Health Rep. 118:99–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dawood FS, et al. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605–2615 [DOI] [PubMed] [Google Scholar]
- 6. Escombe AR, et al. 2009. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 6:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fabian P, et al. 2008. Influenza virus in human exhaled breath: an observational study. PLoS One 3:e2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. First M, Rudnick SN, Banahan KF, Vincent RL, Brickner PW. 2007. Fundamental factors affecting upper-room ultraviolet germicidal irradiation–part I. Exp. J. Occup. Environ. Hyg. 4:321–331 [DOI] [PubMed] [Google Scholar]
- 9. Hartshorn KL, et al. 2007. Reduced influenza viral neutralizing activity of natural human trimers of surfactant protein D. Respir. Res. 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jensen MM. 1964. Inactivation of airborne viruses by ultraviolet irradiation. Appl. Microbiol. 12:418–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelly HA, Priest PC, Mercer GN, Dowse GK. 2011. We should not be complacent about our population-based public health response to the first influenza pandemic of the 21st century. BMC Public Health 11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ko G, First MW, Burge HA. 2002. The characterization of upper-room ultraviolet germicidal irradiation in inactivating airborne microorganisms. Environ. Health Perspect. 110:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ko G, First MW, Burge HA. 2000. Influence of relative humidity on particle size and UV sensitivity of Serratia marcescens and Mycobacterium bovis BCG aerosols. Tuber. Lung Dis. 80:217–228 [DOI] [PubMed] [Google Scholar]
- 14. Kowalski WJ, Bahnfleth WP, Witham DL, Severin BF, Whittam TS. 2000. Mathematical modeling of ultraviolet germicidal irradiation for air disinfection. Quant. Microbiol. 2:249–270 [Google Scholar]
- 15. Lindsley WG, et al. 2010. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin. Infect. Dis. 50:693–698 [DOI] [PubMed] [Google Scholar]
- 16. Lindsley WG, et al. 2010. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One 5:e15100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lowen AC, Mubareka S, Tumpey TM, Garcia-Sastre A, Palese P. 2006. The guinea pig as a transmission model for human influenza viruses. Proc. Natl. Acad. Sci. U. S. A. 103:9988–9992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDevitt JJ, et al. 2007. Characterization of UVC light sensitivity of vaccinia virus. Appl. Environ. Microbiol. 73:5760–5766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McDevitt JJ, Milton DK, Rudnick SN, First MW. 2008. Inactivation of poxviruses by upper-room UVC light in a simulated hospital room environment. PLoS One 3:e3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McLean RL. 1961. Discussion after paper: the mechanism of spread of Asian influenza. Am. Rev. Respir. Dis. 83:36–38 [DOI] [PubMed] [Google Scholar]
- 21. Miller SL, Macher JM. 2000. Evaluation of a methodology for quantifying the effect of room air ultraviolet germicidal irradiation on airborne bacteria. Aerosol Sci. Technol. 33:274–295 [Google Scholar]
- 22. Molinari NA, et al. 2007. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25:5086–5096 [DOI] [PubMed] [Google Scholar]
- 23. Moser MR, et al. 1979. An outbreak of influenza aboard a commercial airliner. Am. J. Epidemiol. 110:1–6 [DOI] [PubMed] [Google Scholar]
- 24. Nardell EA. 1995. Interrupting transmission from patients with unsuspected tuberculosis: a unique role for upper-room ultraviolet air disinfection. Am. J. Infect. Control 23:156–164 [DOI] [PubMed] [Google Scholar]
- 25. Nardell EA, et al. 2008. Safety of upper-room ultraviolet germicidal air disinfection for room occupants: results from the Tuberculosis Ultraviolet Shelter Study. Public Health Rep. 123:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palese P. 2004. Influenza: old and new threats. Nat. Med. 10:S82–S87 [DOI] [PubMed] [Google Scholar]
- 27. Riley RL, Knight M, Middlebrook G. 1976. Ultraviolet susceptibility of BCG and virulent tubercle bacilli. Am. Rev. Respir. Dis. 113:413–418 [DOI] [PubMed] [Google Scholar]
- 28. Rudnick SN, McDevitt JJ, First MW, Spengler JD. 2009. Inactivating influenza viruses on surfaces using hydrogen peroxide or triethylene glycol at low vapor concentrations. Am. J. Infect. Control. 37:813–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tellier R. 2006. Review of aerosol transmission of influenza A virus. Emerg. Infect. Dis. 12:1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]