Abstract
Postharvest growth of Vibrio vulnificus in oysters can increase risk of human infection. Unfortunately, limited information is available regarding V. vulnificus growth and survival patterns over a wide range of storage temperatures in oysters harvested from different estuaries and in different oyster species. In this study, we developed a predictive model for V. vulnificus growth in Eastern oysters (Crassostrea virginica) harvested from Chesapeake Bay, MD, over a temperature range of 5 to 30°C and then validated the model against V. vulnificus growth rates (GRs) in Eastern and Asian oysters (Crassostrea ariakensis) harvested from Mobile Bay, AL, and Chesapeake Bay, VA, respectively. In the model development studies, V. vulnificus was slowly inactivated at 5 and 10°C with average GRs of −0.0045 and −0.0043 log most probable number (MPN)/h, respectively. Estimated average growth rates at 15, 20, 25, and 30°C were 0.022, 0.042, 0.087, and 0.093 log MPN/h, respectively. With respect to Eastern oysters, bias (Bf) and accuracy (Af) factors for model-dependent and -independent data were 1.02 and 1.25 and 1.67 and 1.98, respectively. For Asian oysters, Bf and Af were 0.29 and 3.40. Residual variations in growth rate about the fitted model were not explained by season, region, water temperature, or salinity at harvest. Growth rate estimates for Chesapeake Bay and Mobile Bay oysters stored at 25 and 30°C showed relatively high variability and were lower than Food and Agricultural Organization (FAO)/WHO V. vulnificus quantitative risk assessment model predictions. The model provides an improved tool for designing and implementing food safety plans that minimize the risk associated with V. vulnificus in oysters.
INTRODUCTION
Vibrio vulnificus is a Gram-negative, halophilic, motile, curved bacterium found in marine and estuarine environments. It can be isolated from seawater, sediments, plankton, and shellfish, especially oysters harvested from warm, brackish waters (28, 31, 38, 40). Vibrio vulnificus is a cause of food-borne illness associated with the consumption of raw oysters. Food-borne infections often progress rapidly into primary septicemia characterized by formation of edematous skin lesions on limbs. The mortality rate for those with septicemia is approximately 34.8% (5, 21, 23, 26, 27, 29, 35). Fatalities may occur within 24 h, especially in individuals with hepatic disease and immune-compromising conditions (18). In healthy people, V. vulnificus can cause illness within 16 h, resulting in vomiting, diarrhea, and abdominal pain. More extreme cases of infection cause fever, chills, decreased blood pressure (septic shock), and blistering skin lesions (5, 16). Approximately 96 food-borne cases occur in the United States annually; however, the U.S. Centers for Disease Control and Prevention (CDC) estimates that only half of cases are reported (5, 35).
More than 900 V. vulnificus infections were reported between 1988 and 2006 from Gulf Coast states, where most cases occur. In response, the Interstate Shellfish Sanitation Conference (ISSC) proposed to introduce new risk management practices intended to reduce the number of cases of V. vulnificus by 60% (22). The U.S. Food and Drug Administration proposed a plan to the ISSC in 2009 to mandate postharvest processing for all Gulf Coast oysters in an effort to eliminate V. vulnificus cases, but this plan met resistance and has not been implemented (17, 19). With respect to issues of risk mitigation, the United Nations Food and Agricultural Organization (FAO) and World Health Organization (WHO) previously conducted a risk assessment for V. vulnificus in oysters. In this assessment, they identified data gaps that increase the uncertainty of risk estimates (15). One of the identified data gaps was the lack of predictive models for the growth and survival of V. vulnificus in postharvest oysters over a wide range of commercially relevant storage temperatures.
Levels of V. vulnificus in Gulf Coast and Chesapeake Bay (CB) oysters follow a seasonal distribution and have been reported in a number of studies (12, 30, 31, 36, 38, 40). The highest concentrations at harvest (∼1,000 V. vulnificus cells per gram) are reported during the warmer months of April through October in the Gulf Coast and Chesapeake Bay and then decline through fall and winter to <10 cells per gram. In a previous study, V. vulnificus was not recovered in environmental samples (water, sediments, plankton, and oysters) collected from the Chesapeake Bay in the winter (40). Motes et al. (30) conducted a comprehensive survey of V. vulnificus levels in oysters from three major Gulf Coast and two Atlantic estuaries at weekly intervals for 15 months. Water temperature and salinity were found to influence V. vulnificus levels in oysters at harvest. Cook (7, 8) investigated growth of V. vulnificus in postharvest oysters from the Gulf Coast and found that at temperatures of <13°C, V. vulnificus failed to multiply. Vibrio vulnificus densities were found to be substantially higher in oysters held at temperatures of >18°C for 12 to 30 h compared to levels at harvest (7, 8). However, these studies were limited to a single region, and the effects of storage temperatures and time were not investigated systematically as storage temperature was ambient and not controlled.
Estimating Vibrio growth over a wide range of temperatures is facilitated by the development and application of predictive models. Predictive modeling has been used to describe the population dynamics of a number of pathogenic and spoilage bacteria in food (34, 37, 41). Viability rates determined from primary models can be translated into secondary models to estimate bacterial viability under dynamic environmental conditions, such as those during harvest, transport, processing, and storage.
The D model developed by Baranyi and coworkers is a widely used primary growth model (1–3), while the Ratkowsky secondary model is preferred to describe the square root of the specific growth rate (SGR) as a function of temperature (1). However, these predictive models must be validated with independent data before they can be recognized as support tools in food safety risk management (1).
Although there have been several studies on environmental levels of V. vulnificus as a function of seawater temperature and salinity (30, 31, 36), comprehensive predictive models have not been developed and validated for the growth of V. vulnificus in postharvest oyster shellstock at all temperatures relevant to commercial and consumer handling practices. The primary objectives of this study were to develop a predictive model for the growth of V. vulnificus in postharvest shellstock of Eastern oysters (Crassostrea virginica) as a function of storage temperature and to validate the model against model-independent data collected from Eastern and Asian (C. ariakensis) oysters. Additionally, possible residual effects of harvest temperature, salinity, season, and region on V. vulnificus growth rates were evaluated.
MATERIALS AND METHODS
Oyster collection and storage for modeling the growth of V. vulnificus.
Eastern oysters were collected in spring, summer, and fall of 2008 from Chesapeake Bay, MD. Temperature and salinity were measured in the top 0.5 m of the water column with a Hydrolab Quanta multiparameter unit (model 85; Hach Environmental Instruments, Loveland, CO). Immediately after harvest, oyster samples were bagged and placed in insulated chests. Bubble-wrap was placed between the oyster bags and ice bags to prevent direct contact with ice and water. Shipping temperature was monitored using data loggers (ACR Systems, Inc., Data Logger Store, Contoocook, NH) inside the oyster and in the cooler to verify that temperature did not exceed 13°C (the minimum temperature for V. vulnificus growth) during transport to the laboratory. All microbiological analyses were initiated within 24 h of sample collection (13). Upon arrival at the laboratory, 0-h samples were analyzed. Then oysters were placed in plastic trays along with data loggers, and stored at 5, 10, 15, 20, 25, and 30°C for selected time intervals.
Sample preparation.
Oysters were scrubbed with a stiff brush under running tap water to remove mud and then placed on absorbent paper to drain excess liquid. Two sets of six oysters were shucked, and meats were mixed with an equal volume of sterile phosphate-buffered-saline (PBS) (7.65 g NaCl, 0.724 g of anhydrous Na2HPO4, 0.21 g of KH2PO4 [Sigma, St. Louis, MO] per liter [pH 7.4]). Next, the mixture was blended at high speed for 90 s in a sterile Waring blender jar (12, 20). A 1/10 (wt/wt) dilution of oyster homogenate was prepared by mixing 20 g of homogenate and 80 g of PBS. Additional 1/10 dilutions were prepared in PBS on a volume/volume basis.
Bacteriological analyses.
The concentration of V. vulnificus was determined by the three-tube most probable number (MPN) procedure described in the FDA Bacteriological Analytical Manual (25) using alkaline peptone water (APW) with 1% NaCl as the enrichment medium. Dilutions of oyster homogenate were inoculated into APW and incubated at 35°C for 18 to 24 h. A loopful (∼10 μl) of APW from the top 1 cm of turbid tubes was streaked on modified cellobiose-polymyxin-colistin (mCPC) agar, and the plates were incubated at 39°C for 18 to 24 h. Cellobiose-fermenting (yellow) colonies were considered presumptive for V. vulnificus. Three colonies were picked from each mCPC plate and transferred to individual wells of 96-well plates containing 100 μl of APW using sterile wooden applicator sticks. After overnight incubation at 35°C of the 96-well plates, a 48-prong replicator was used to transfer the growth in each well to T1N3 (1% tryptone and 3% NaCl [pH 7.2]) agar plates.
After overnight incubation at 35°C, colony lifts were prepared using Whatman no. 541 paper as described by Kaysner and DePaola (25). Briefly, bacterial colonies were lysed, and the DNA was fixed to the filters by treatment with alkaline lysis buffer, ammonium acetate neutralization, and proteinase K. Alkaline phosphatase-labeled DNA probes (DNA Technology, Aarhus, Denmark) targeting the species-specific V. vulnificus hemolysin gene (vvhA) were used to confirm the identity of suspected isolates as V. vulnificus (14, 25, 39). The filters were hybridized with the probe at 55°C for 1 h, followed by washing and colorimetric development according to the manufacturer's instructions (Roche Diagnostics, Indianapolis, IN). Positive (V. vulnificus strain) and negative (Vibrio parahaemolyticus tlh+ and tdh+ strains) controls were included with each hybridization experiment. Probe-positive colonies (purple-brown) were counted, and V. vulnificus MPN/g oyster were calculated using a three-tube MPN table.
Primary and secondary model development.
The dynamic model described by Baranyi and Roberts (2) was used to fit curves to kinetic data and to estimate lag phase duration (LPD [h]), maximum population density (MPD [log MPN/g]), and growth rate (GR [log MPN/h]) using DMFit curve-fitting software (kindly provided by J. Baranyi, Institute of Food Research, Norwich, United Kingdom). GRs were converted to the square root of the log GR (SGR) for secondary models. The same dynamic model was also used to fit nonlinear inactivation kinetics. The Ratkowsky two-parameter square-root model was fit as the secondary growth model using Table Curve 2D (SPSS, Inc., Chicago, IL) (32).
Validation of the secondary model.
The Ratkowsky secondary model for V. vulnificus growth in Chesapeake Bay (CB) Eastern oysters was validated against observed V. vulnificus growth kinetics in Eastern oysters collected from the Cedar Point Reef in the Mississippi Sound near Mobile Bay (MB), AL, in the spring, summer, and fall of 2007. In addition, the model was compared to V. vulnificus growth rates in Asian oysters collected from CB, VA, during the summer of 2008. The samples were collected, shipped, and analyzed with the same protocols used to produce the primary model above.
Model performance was measured by calculating bias (Bf) and accuracy (Af) factors (33). Analysis of covariance (ANCOVA) was used to evaluate possible effects of harvest temperature, salinity, and harvest region on the growth of V. vulnificus in oysters. ANCOVA was conducted using the PROC GLM procedure in SAS (Statistic Analysis Software version 9.1, SAS Institute, Cary, NC). Differences were considered statistically significant at P < 0.05.
RESULTS
Primary model parameters for growth/inactivation of V. vulnificus in oysters.
Viability kinetics of natural populations of V. vulnificus in multiple collections of oysters harvested from the CB during spring, summer, and fall were measured at 5, 10, 15, 20, 25, and 30°C at selected time intervals. Analyses were continued until oyster shells visibly gaped. Samples were stored up to 504 h (21 days) at 5 and 10°C, up to 336 h (14 days) at 15 and 20°C, and up to 168 h (7 days) at 25 and 30°C. Representative curves fitted to kinetic data are shown in Fig. 1 (inactivation) and Fig. 2 (growth) for CB and MB Eastern oysters. Primary growth/inactivation parameters for CB are summarized in Table 1.
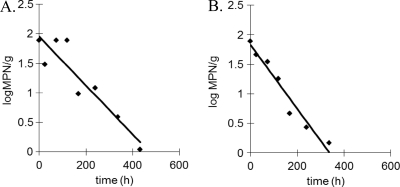
Fig 1.
Representative inactivation profiles of V. vulnificus at 5°C (A) and 10°C (B) in Eastern oysters from Chesapeake Bay.
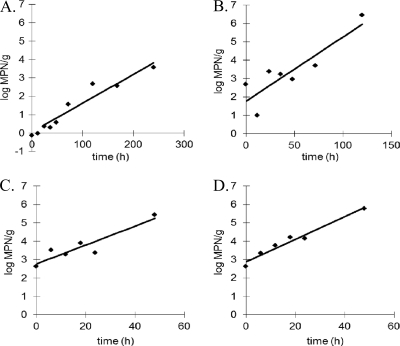
Fig 2.
Representative growth profiles of V. vulnificus at 15°C (A), 20°C (B), 25°C (C), and 30°C (D) in Eastern oysters from Chesapeake Bay and Mobile Bay.
Table 1.
Inactivation/growth parameters for V. vulnificus in Chesapeake Bay oysters stored at 5 to 30°Ca
| Temp (°C) | GR (log MPN/h) |
LPD (h) |
MPD (log MPN/g) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Fall | Spring | Summer | Fall | Spring | Summer | Fall | |
| 5 | −0.002 (0.001) | −0.007 (0.0008) | ND | ND | ND | ND | ND | ND | ND |
| 10 | −0.004 (0.0007) | −0.005 (0.0006) | −0.004 (0.0004) | ND | ND | ND | ND | ND | ND |
| 15 | 0.016 (0.002) | 0.028 (0.007) | ND | ND | ND | ND | ND | 5.7 (0.1) | ND |
| 20 | 0.049 (0.002) | ND | 0.035 (0.018) | 30 (2.2) | ND | ND | 5.5 (0.1) | 5.8 (0.1) | 6.1 (0.3) |
| 25 | 0.091 (0.077) | 0.098 (0.057) | 0.073 (0.052) | 25.2 (22.8) | ND | ND | 4.0 (0.4) | 6.0 (0.2) | 5.5 (0.4) |
| 30 | 0.064 (0.010) | 0.095 (0.018) | 0.121 (0.038) | ND | ND | ND | ND | 7.1 (0.3) | 6.0 (0.4) |
GR, growth rate; LPD, lag phase duration for growth or duration of shoulder for inactivation; MPD, maximum population density; ND, not determined. Values in parentheses are the standard errors.
Vibrio vulnificus was inactivated in CB Eastern oysters at 5 and 10°C, with estimated average inactivation rates of −0.005 and −0.004 log MPN/h, respectively (Table 1). In contrast, V. vulnificus replicated in CB Eastern oysters stored at 15, 20, 25, and 30°C, with average GRs of 0.022, 0.042, 0.087, and 0.093 log MPN/h, respectively. The highest MPD (7.1 log MPN/g) was observed at 30°C for oysters harvested during summer (Table 1). Lag phase was inconsistent among trials and was only observed at 20 and 25°C for spring samples (Table 1).
Secondary model for V. vulnificus growth in oysters.
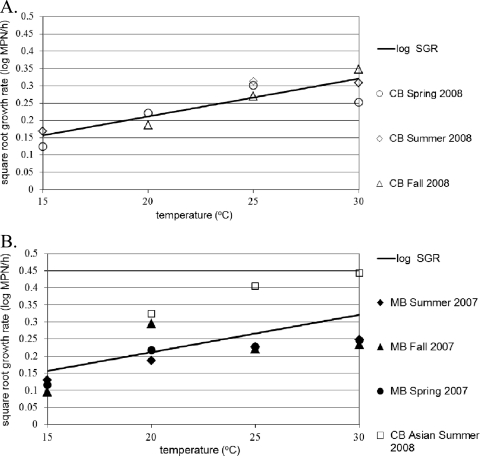
The square root model (32) was used to describe growth rate as a function of temperature and is shown in Fig. 3. Growth was not observed at 5 and 10°C; therefore, these temperatures were not included in the model. The equation for the square root model is √log rate = b × (T − To), where b = 0.0109, To = 0.7005, T = temperature in °C, r2 = 0.77, and standard error of fit = 0.037. The standard errors for b and To were 0.002 and 4.54, respectively.
Fig 3.
(A) Secondary model of V. vulnificus square root of the log growth rate (SGR) in Chesapeake Bay (CB) Eastern oysters stored at 15 to 30°C, with model-dependent data. (B) Comparison of V. vulnificus log SGRs in Eastern oysters from Mobile Bay (MB) and CB Asian oysters.
Overall, the CB V. vulnificus growth model predicted higher GR compared to MB in Eastern oysters and markedly lower GR for Asian oysters (Fig. 3B; Table 2).
Table 2.
Predicted and observed log growth rates for V. vulnificus in Eastern and Asian oysters harvested from Mobile Bay and Chesapeake Bay
| Storage temp (°C) | Log GR (log MPN/h)a |
||
|---|---|---|---|
| Predicted | Observed |
||
| CB Asian oysters | MB Eastern oysters | ||
| 15 | 0.024 | ND | 0.013 (0.001) |
| 20 | 0.045 | 0.100 (0.02) | 0.056 (0.02) |
| 25 | 0.070 | 0.160 (0.02) | 0.051 (0.007) |
| 30 | 0.103 | 0.200 (0.02) | 0.059 (0.008) |
CB, Chesapeake Bay; MB, Mobile Bay; ND, not determined due to inactivation rather than growth. Values in parentheses are the standard errors. The standard error for the model prediction is 0.0014.
Model performance and comparison with the FAO/WHO model.
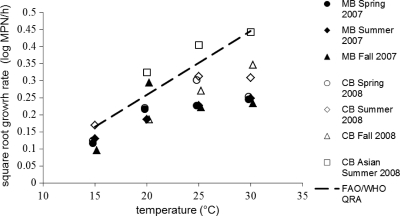
Model performance was measured (Bf and Af). Independent data for MB Eastern oysters and CB Asian oysters showed that Bf and Af were 1.67 and 1.98 and 0.29 and 3.40, respectively. Figure 4 shows the SGR for CB and MB oysters compared to the FAO/WHO quantitative risk assessment (QRA) model (16). Generally, SGR estimates for CB and MB Eastern oysters held at 25 and 30°C were found to be consistently lower than those predicted by the FAO/WHO (16) V. vulnificus QRA model. GRs in the FAO/WHO QRA were based on observations in studies by Cook (7, 8).
Fig 4.
Chesapeake Bay (CB) and Mobile Bay (MB) square root of the log growth rate estimates compared to FAO/WHO quantitative risk assessment (QRA) (dashed line).
Effect of harvest seawater temperature and salinity on V. vulnificus growth rate.
Table 3 shows the average water temperature and salinity of harvest water and the initial counts of V. vulnificus in CB and MB oysters at harvest. Mobile Bay water temperature ranged from 17.4 to 36.3°C, and salinities ranged from 18.9 to 28.7 ppt. In Chesapeake Bay, water temperature ranged from 16.4 to 26.2°C and salinities ranged from 8.6 to 17.3 ppt. Mean water temperature at harvest was higher in MB during the summer (33.2 ± 2.4°C) than that in CB (25.5 ± 0.6°C) (Table 3). Mean V. vulnificus levels in CB oysters were 0.78 ± 0.9 log MPN/g in samples collected in the spring, while the highest levels (4.47 ± 0.4 log MPN/g) were found in oysters collected from CB during summer. Relative to the expectation based on storage temperature alone, growth rates did appear to be depressed at low (CB) and high (MB) salinities, but these observed differences were not statistically significant. In addition, there was no significant relationship between deviations from expected growth rates versus water temperature at harvest.
Table 3.
Water temperature, salinity at harvest, and V. vulnificus initial countsa
| Site (oyster type) | Season | Water temp (°C) | Salinity (ppt) | V. vulnificus initial count (log MPN/g) |
|---|---|---|---|---|
| Chesapeake Bay (Eastern) | Spring | 17.3 ± 0.7 | 9.1 ± 0.4 | 0.78 ± 0.9 |
| Summer | 25.5 ± 0.6 | 12.6 ± 1.3 | 4.47 ± 0.4 | |
| Fall | 18.3 ± 1.7 | 16.4 ± 1.1 | 3.10 ± 0.6 | |
| Chesapeake Bay (Asian) | Summer | 22.8 ± 2.7 | 21.5 ± 5.1 | 3.15 ± 1.2 |
| Mobile Bay (Eastern) | Spring | 27.6 ± 1.0 | 23.9 ± 4.4 | 1.97 ± 0.6 |
| Summer | 33.2 ± 2.4 | 22.7 ± 2.5 | 2.40 ± 0.4 | |
| Fall | 21.0 ± 4.2 | 23.3 ± 1.9 | 1.49 ± 1.0 |
The values shown are means ± standard deviations.
DISCUSSION
This study is the most comprehensive investigation to date on the postharvest growth and survival of V. vulnificus in live shellstock oysters. Experimental oysters were collected from two regions in the United States with relatively high risk of V. vulnificus infection and stored at temperatures that represent a range of potential industry and consumer practices. Additionally, the study included the most relevant major commercial oyster species (C. virginica) and an oyster species (Crassostrea ariakensis) that has been proposed for culture in CB due to disease resistance.
At 5 and 10°C, temperatures below the minimum growth temperature for V. vulnificus, its levels decreased to nondetectable (<0.03 MPN/g) (11) after 432 h (18 days) in both oyster species. Vibrio vulnificus counts in CB oysters decreased on average of 2.21 log MPN/g at 5°C and 1.82 log MPN/g at 10°C. In MB oysters, V. vulnificus levels decreased an average of 1.33 and 1.17 log MPN/g at 5 and 10°C, respectively. However, V. vulnificus levels significantly increased after 24 h by 1 to 3 log MPN/g in oysters stored at 15 to 30°C. These results are consistent with an earlier study where V. vulnificus decreased during storage at 10°C, but increased up to 2 log cycles after 1 day of storage at 22°C and 30°C (9).
The highest concentration of the microbial population in an environment is termed the maximum population density (MPD) (37). In general, the MPD increased with storage temperature and reached a peak of 7.1 log MPN/g in the summer oysters stored at 30°C, but the MPD was lower at 30°C in the fall, 6.0 log MPN/g. At 15°C, the MPD in the summer was 5.7 log MPN/g. The MPD is much lower for the spring than the other seasons.
According to Baranyi and Roberts (2), the history of the cells affects the transition period during which the bacteria reach the exponential phase. In the case of death curves, this is frequently referred to as “shoulder”; with growth curves it is called the “lag.” While no “shoulder” was observed in CB oysters stored at 5 and 10°C, a “shoulder” at 232 h was observed in MB spring samples stored at 10°C. This “shoulder” may have been an artifact, possibly due to a significant temperature drop in oysters during shipping. The average temperature of the shipping container and oysters during the transportation period was below 4°C for more than 15 h. No LPD was observed in MB oysters stored at 15, 20, 25, and 30°C. There were LPDs of 30 and 25 h in the spring CB samples stored at 20 and 25°C. The average shipping container temperature in spring was 11.1°C, and the oyster temperature was 10.5°C. Longer LPDs may be attributed to low water temperature at harvest or to extended exposure of oysters to low temperatures during shipping. However, based on ANCOVA, no correlation was found between holding temperatures and GRs (data not shown). These results suggest that shipping container temperature and transportation period had no effect on the GRs of V. vulnificus in oysters.
The Ratkowsky square root model provided a good fit for CB Eastern oyster data as observed SGR values at 15, 20, 25, and 30°C were within 0.02 log MPN/h of model predictions (Fig. 3A). However, the SGR for V. vulnificus in MB oysters was systematically overpredicted, except at 20°C. For CB Asian oysters stored at 20°C and above, the mean model prediction was markedly less than the observed SGR (Fig. 3B).
The observed SGR at 30°C showed higher variation than the other storage temperatures. This could indicate that 30°C is close to the maximum growth rate for V. vulnificus and/or an effect from oyster host defenses and endogenous microflora. However, higher storage temperatures were not tested. If there are risk management scenarios in which temperatures greater than 30°C are relevant to stored live oysters, then further studies are warranted. This may also require a different model to fit convex SGR patterns.
Recent findings by Chase and Harwood (6) demonstrate that the growth rate for biotype 1 strains is greater than that for less common biotypes 2 and 3. Therefore, this adds additional confidence that the model proposed will be fail-safe for V. vulnificus at the species level.
A more specific comparison of the CB Eastern oyster model performance was performed by measuring the factors Bf and Af. The Bf and Af for model-dependent data were 1.02 and 1.25. The model-independent (validation) data for MB Eastern and CB Asian oysters showed Bf and Af of 1.67 and 1.98 and 0.29 and 3.40, respectively, demonstrating that the model provides higher predictions for growth rate that may offer more safe estimations with regard to reducing risk for Eastern but not Asian oysters. Given the variability in observed V. vulnificus growth rates among experiments with Eastern oysters, the more rapid growth in Asian oysters should be interpreted cautiously. The growth rates observed in the current study for Asian oysters are similar to those published in the FAO/WHO QRA based on experiments with Eastern oysters (15).
The average water temperature and salinity in CB were lower than those in MB. There were lower V. vulnificus initial counts in spring, but no correlation was found between growth rates versus water temperature at harvest. Vibrio vulnificus can grow at 13°C and a salinity range of 0.8 to 35 ppt. The highest concentration of V. vulnificus can be found at water temperatures of 20 to 30°C and salinity of 5 to 25 ppt (7, 12, 24, 26, 30).
Vibrio vulnificus growth rates were found to be variable, particularly at 25°C and 30°C. Although no significant correlation between growth rates and harvest conditions was identified, growth rates did appear to be depressed at the low- and high-salinity values observed in the current study, which were generally in the optimal range for V. vulnificus. The deviation from expected was small (i.e., near zero) around 20 ppt salinity, which is consistent with salinity levels found to be favorable for V. vulnificus in previous environmental surveys (30) and the optimal salinity of 17 ppt reported in the FAO/WHO V. vulnificus QRA. Wright et al. (40) reported that the increased V. vulnificus concentrations in CB were correlated with lower salinities (8 to 16 ppt).
A comparison of the estimated growth rate in CB oysters with the FAO/WHO QRA shows higher growth rate estimates at 25 and 30°C than in the current study. The FAO/WHO model was based on observations made at 18°C and 23 to 34°C in studies by Cook (7, 8), where the growth rates were estimated to be 0.025 and 0.175 log CFU/h, respectively. The growth model used in the QRA was the 3-phase linear growth model for microbial risk assessment advocated by Buchanan et al. (4), and in these studies, upon which the FAO/WHO model is based, only oysters harvested from the U.S. Gulf coast were used in the postharvest growth trials (15). Overall, V. vulnificus growth appears to vary greatly from one lot of oysters to the next, especially when stored at warm ambient temperatures typical of industry practices during the higher-risk season for this organism. The estimates in the FAO/WHO QRA are fail-safe compared to those obtained in the current study and can be used to develop more protective controls. However, the distribution of growth rates in the current study should be considered in future risk assessments to more accurately estimate exposure.
These results demonstrate that the model developed for the growth of V. vulnificus in CB American oysters is valid for and predictive of growth occurring in Eastern oysters harvested from two different estuaries (MB and CB), and likely other estuaries with similar environmental conditions, but not for Asian oysters. Growth rates were similar over the three seasons, except at 25 and 30°C, but were systematically lower than that predicted by the FAO/WHO V. vulnificus QRA at temperatures greater than 20°C. Given the high variability of growth rates at 25°C and 30°C in the current study, there are apparently factors affecting V. vulnificus growth that were beyond the scope of this investigation. A higher growth rate at any given temperature should also be considered in development of control measures in order to provide a more conservative public health stance for a specific oyster species. The results of this study will assist risk managers and the seafood industry in designing and implementing food safety plans to minimize the risk associated with V. vulnificus in oysters.
ACKNOWLEDGMENTS
We gratefully acknowledge C. White, M. Mudoh, J. Eckroade, K. Cephas, G. Rutto, J. Ngeno, P. Sang, R. Korir, S. Pagadala, T. Rippen, J. Schwarz, C. Brooks (University of Maryland Eastern Shore), R. McKay, K. Bassett, W. Beatty (Maryland Department of the Environment), and S. Allen (Virginia Institute of Marine Sciences) for their great contributions to this study. Tom Ross is acknowledged for advice with modeling. William Burkhardt is acknowledged for helpful review of the manuscript.
This project was supported by the National Research Initiative of the United States Department of Agriculture, Cooperative State Research, Education and Extension Service, grant 2006-35201-16644 and University of Maryland Eastern Shore Advanced Curriculum Technology-Based Instructional Opportunities Network (ACTION).
Footnotes
Published ahead of print 13 January 2012
REFERENCES
- 1. Baranyi J, Roberts TA. March 2004. Predictive microbiology—quantitative microbial ecology. Culture http://www.ifr.ac.uk/safety/comicro/Culture_25.pdf
- 2. Baranyi J, Roberts TA. 1994. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23:277–294 [DOI] [PubMed] [Google Scholar]
- 3. Baranyi J, Pin C, Ross T. 1999. Validating and comparing predictive models. Int. J. Food. Microbiol. 48:159–166 [DOI] [PubMed] [Google Scholar]
- 4. Buchanan RL, Whiting RC, Damert WC. 1997. When is simple good enough: a comparison of the Gompertz, Baranyi and three-phase linear models for fitting bacterial growth curves. Food Microbiol. 14:313–326 [Google Scholar]
- 5. Centers for Disease Control and Prevention 2009. Vibrio vulnificus general information. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nczved/divisions/dfbmd/diseases/vibriov/#type [Google Scholar]
- 6. Chase E, Harwood VJ. 2011. Comparison of the effects of environmental parameters on growth rates of Vibrio vulnificus biotypes I, II, and III by culture and quantitative PCR. Appl. Environ. Microbiol. 77:4200–4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cook DW. 1994. Effect of time and temperature on multiplication of Vibrio vulnificus in post harvest Gulf Coast shellstock oysters. Appl. Environ. Microbiol. 60:3483–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cook DW. 1997. Refrigeration of oyster shellstock: conditions which minimize the outgrowth of Vibrio vulnificus. J. Food Prot. 60:349–352 [DOI] [PubMed] [Google Scholar]
- 9. Cook DW, Ruple AD. 1989. Indicator bacteria and vibrionaceae multiplication in post-harvest shellstock oysters. J. Food Prot. 52:343–349 [DOI] [PubMed] [Google Scholar]
- 10. Reference deleted.
- 11. DePaola A, Jones JL, Woods J, Burkhardt W, III, et al. 2010. Bacterial and viral pathogens in live oysters: 2007 United States market survey. Appl. Environ. Microbiol. 76:2754–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DePaola A, Capers GM, Alexander D. 1994. Densities of Vibrio vulnificus in the intestines of fish from the U.S. Gulf Coast. Appl. Environ. Microbiol. 60:984–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DePaola A, et al. 2003. Analysis of Vibrio vulnificus from market oysters and septicemia cases for virulence markers. Appl. Environ. Microbiol. 69:4006–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DePaola A, et al. 1997. Evaluation of alkaline phosphatase-labeled DNA probe for enumeration of Vibrio vulnificus in Gulf Coast oysters. J. Microbiol. Methods 29:115–120 [Google Scholar]
- 15. FAO/WHO 2005. Risk assessment of Vibrio vulnificus in raw oysters: interpretative summary and technical report. Microbiology Risk Assessment Series no. 8. Food and Agriculture Organization of the United Nations, Rome, Italy, and World Health Organization, Geneva, Switzerland [Google Scholar]
- 16. Food and Drug Administration 2009. BBB—Vibrio vulnificus bad bug book: foodborne pathogenic microorganisms and natural toxins handbook. White Oak, MD: http://www.fda.gov/Food/FoodSafety/FoodborneIllness/FoodborneIllnessFoodbornePathogensNaturalToxins/BadBugBook/ucm070473.html [Google Scholar]
- 17. Food and Drug Administration 13 November 2009, posting date FDA statement on Vibrio vulnificus in raw oysters. News and event. FDA news release. Food and Drug Administration, White Oak, MD: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm90513.html [Google Scholar]
- 18. Food and Nutrition Board IOM 1991. Seafood Safety/Committee on Evaluation of the Safety of Fishery Products. National Academy Press, Washington, DC: [DOI] [PubMed] [Google Scholar]
- 19. Food Quality 24 November 2009, posting date FDA delays raw oyster ban: plan for post-harvest processing put off. Food Quality http://dmmsclick.wiley.com/share.asp?m=561qhjqdhxyhsgfw7gvm&f=h
- 20. Gooch J, DePaola A, Bowers JC, Marshall DL. 2002. Growth and survival of Vibrio parahaemolyticus in postharvest American oysters. J. Food Prot. 65:970–974 [DOI] [PubMed] [Google Scholar]
- 21. Hlady WG, Klontz KC. 1996. The epidemiology of Vibrio infections in Florida 1981–1993. J. Infect. Dis. 173:1176–1183 [DOI] [PubMed] [Google Scholar]
- 22. Interstate Shellfish Sanitation Conference 2011. Vibrio vulnificus risk management for oysters. 2009 guide for the control of molluscan shellfish. National Shellfish Sanitation Program (NSSP) guide, section II. Model ordinance chapter II. Risk management and risk assessment. Interstate Shellfish Sanitation Conference, Columbia, SC http://www.issc.org/NSSP [Google Scholar]
- 23. Jackson JK, Murphree RL, Tamplin ML. 1997. Evidence that mortality from Vibrio vulnificus infections results from single strain among heterogeneous populations in shellfish. J. Clin. Microbiol. 35:2098–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaspar CW, Tamplin ML. 1993. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl. Environ. Microbiol. 59:2425–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaysner CA, DePaola A. 2004. Vibrio. Bacteriological analytical manual online. Food and Drug Administration, White Oak, MD: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/UCM070830 [Google Scholar]
- 26. Kelly MT. 1982. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl. Environ. Microbiol. 44:820–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumamoto KS, Vukich DJ. 1998. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J. Emerg. Med. 16:61–66 [DOI] [PubMed] [Google Scholar]
- 28. La Valley KJ, DeAlteris J, Rice M, Gomez-Chiarri M. 2008. North Atlantic Vibrio vulnificus surveillance from postharvest oysters at a US shellfish processing facility. J. Foodservice 19:234–237 [Google Scholar]
- 29. Lipp EK, Rose JB. 1997. The role of seafood in foodborne diseases in the United States of America. Rev. Sci. Tech. 16:620–640 [DOI] [PubMed] [Google Scholar]
- 30. Motes ML, et al. 1998. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters. Appl. Environ. Microbiol. 64:1459–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oliver JD, Warner RA, Cleland DR. 1982. Distribution and ecology of Vibrio vulnificus and other lactose fermenting marine vibrio in coastal waters of the southern United States. Appl. Environ. Microbiol. 44:1404–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ratkowsky DA, Olley J, McMeekin TA, Ball A. 1982. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 149:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ross T. 1996. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 81:501–508 [DOI] [PubMed] [Google Scholar]
- 34. Ross T. 1999. Predictive food microbiology models in the meat industry, vii, p 196 Meat and Livestock Australia, North Sydney, Australia [Google Scholar]
- 35. Scallan E, et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tamplin ML, Rodrick GE, Blake NJ, Cuba T. 1982. Isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl. Environ. Microbiol. 44:1466–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamplin ML, Phillips R, Stewart TA, Luchansky JB, Kelley LC. 2008. Behavior of Bacillus anthracis strains Stern and Ames K0610 in sterile raw ground beef. Appl. Environ. Microbiol. 74:1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vanoy RW, Tamplin ML, Schwartz JR. 1992. Ecology of V. vulnificus in Galveston Bay oysters, suspended particular matter, sediment and sea water: detection by monoclonal antibody immunoassay-most probable number procedures. J. Ind. Microbiol. 9:219–234 [Google Scholar]
- 39. Wright AC, Jr, et al. 1993. Rapid identification of Vibrio vulnificus on nonselective media with an alkaline phosphatase-labeled oligonucleotide probe. Appl. Environ. Microbiol. 50:541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wright AC, et al. 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl. Environ. Microbiol. 62:717–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoon KS, et al. 2008. A model of the effect of temperature on the growth of pathogenic and non pathogenic Vibrio parahaemolyticus isolated from oysters in Korea. Food Microbiol. 25:635–641 [DOI] [PubMed] [Google Scholar]