Abstract
Escherichia coli isolates were recovered from the National Antimicrobial Resistance Monitoring System retail meat program and examined for antimicrobial susceptibility. Retail meat samples (n = 11,921) from four U.S. states collected during 2002 to 2008, consisting of 2,988 chicken breast, 2,942 ground turkey, 2,991 ground beef, and 3,000 pork chop samples, were analyzed. A total of 8,286 E. coli isolates were recovered. The greatest numbers of samples contaminated with the organism were chicken (83.5%) and turkey (82.0%), followed by beef (68.9%) and pork (44.0%). Resistance was most common to tetracycline (50.3%), followed by streptomycin (34.6%), sulfamethoxazole-sulfisoxazole (31.6%), ampicillin (22.5%), gentamicin (18.6%), kanamycin (8.4%), amoxicillin-clavulanic acid (6.4%), and cefoxitin (5.2%). Less than 5% of the isolates had resistance to trimethoprim, ceftriaxone, ceftiofur, nalidixic acid, chloramphenicol, and ciprofloxacin. All isolates were susceptible to amikacin. Compared to beef and pork isolates, the poultry meat isolates had a greater percentage of resistance to all tested drugs, with the exception of chloramphenicol, to which pork isolates had the most resistance. More than half of the turkey isolates (56%) were resistant to multidrugs (≥3 classes) compared to 38.9% of chicken, 17.3% of pork, and 9.3% of beef isolates. The blaCMY gene was present in all ceftriaxone- and ceftiofur-resistant isolates. The cmlA, flo, and catI genes were present in 45%, 43%, and 40% of chloramphenicol-resistant isolates, respectively. Most nalidixic acid-resistant isolates (98.5%) had a gyrA mutation in S83 or D87 or both, whereas only 6.7% had a parC mutation in either S80 or E84. The results showed that E. coli was commonly present in the retail meats, and antimicrobial resistance profiles differed according to the animal origin of the isolates.
INTRODUCTION
Escherichia coli is a commensal bacterium in humans and animals and has a wide range of hosts. It is commonly present in the environment and is considered an indicator of fecal contamination in food and water. E. coli can acquire, maintain, and transmit resistance genes from other organisms in the environment. Due to its ubiquity in humans and animals and its role as a pathogenic and commensal organism, E. coli has become one of the microorganisms that are commonly resistant to antimicrobials.
The level of antimicrobial resistance in E. coli represents a useful indicator of resistance dissemination in bacterial populations and of selective pressure imposed by the antimicrobials used in treatment of food animals and humans (2, 25, 28, 30). Sáenz et al. (25) showed that the frequency of resistance to different antimicrobials in E. coli differed according to the source of the isolates. E. coli isolates from broilers were resistant to ciprofloxacin (CIP) (38%) and gentamicin (GEN) (40%) compared to 0% resistance to both drugs among E. coli isolates from healthy human volunteers versus 16% and 8% resistance among E. coli isolates from human clinical specimens. Kikuvi et al. (22) reported that E. coli isolates from chicken had higher resistance than E. coli isolates from pigs and cattle.
The emergence of antimicrobial resistance among E. coli strains of animal origin has important public health implications. Several studies showed that drug-resistant E. coli infections in humans were caused by strains from animals and that those infectious agents harbored the same mobile resistance genes as were found in diverse bacterial species from a variety of animal sources (17, 19, 20, 35). Johnson et al. investigated antimicrobial-resistant E. coli isolates from humans and poultry products in Minnesota and Wisconsin (20) and demonstrated that phylogenetic and virulence markers of antimicrobial-susceptible human isolates differed considerably from those of antimicrobial-resistant isolates from humans and poultry. However, antimicrobial-resistant isolates from humans were similar to those from poultry whereas antimicrobial-susceptible and antimicrobial-resistant isolates from poultry were largely indistinguishable. Those researchers concluded that some antimicrobial-resistant E. coli isolates from humans may have originated from poultry, whereas antimicrobial-resistant E. coli isolates from poultry likely were derived from susceptible E. coli strains in poultry. A similar study conducted by Vieira et al. (34) showed that resistance in E. coli isolates from poultry and pigs was highly correlated with resistance in E. coli isolates from human bloodstream samples. The study supported the hypothesis that a large proportion of resistant E. coli isolates causing bloodstream infections in humans may be derived from food sources.
Due to its natural presence in the gut of animals and easy acquisition of antimicrobial resistance, E. coli has been selected as a sentinel organism in antimicrobial resistance monitoring programs worldwide. In the United States, the Food & Drug Administration (FDA) National Antimicrobial Resistance Monitoring System (NARMS) tracks antimicrobial resistance in food-borne pathogenic and commensal bacteria from different sources along the food supply chain. The objectives of this study were to determine the prevalence, antimicrobial susceptibility, and mechanisms of selected resistance phenotypes of E. coli isolates from raw retail meats purchased in the United States during 2002 to 2008.
MATERIALS AND METHODS
Retail meat sampling.
Meat samples were purchased at retail outlets and cultured for E. coli at participating public health laboratories in Georgia (GA), Maryland (MD), Oregon (OR), and Tennessee (TN). Each site obtained up to 40 retail meats per month from grocery stores as follows: 10 samples each of chicken breast (CB) with skin on, ground turkey (GT), ground beef (GB), and pork chops (PC). A convenience sampling approach was used from 2002 to 2004 in which samples were collected from grocery stores in close proximity to the testing sites. A stratified random sampling scheme has been used since 2005 whereby samples were collected from a geographically coded list of grocery stores within ZIP codes representing highly populated areas for each site. The ZIP codes were partitioned into quadrants, and grocery stores were randomly selected for sample collection using SAS version 9.1.3 software. For each sample, the test sites logged the store name, lot number (if available), sell-by date, purchase date, and laboratory processing date.
Microbiological analysis.
In each laboratory, samples were refrigerated at 4°C and were processed no later than 96 h after purchase. Retail meat and poultry packages were kept intact until they were aseptically opened in the laboratory at the start of examination. For chicken and pork cuts, one piece of meat was examined. For ground beef and turkey products, 25-g portions were used. Each sample was placed in a sterile plastic bag with 225 ml of buffered peptone water, and the bags were vigorously shaken. Rinsate (50 ml) from each sample was transferred to a sterile flask for isolation and identification of E. coli. Double-strength MacConkey broth (50 ml) was then added, and the contents were mixed thoroughly and incubated at 35°C for 24 h.
From each flask, one loop of overnight broth culture was streaked onto a Levine's eosin methylene blue (EMB) agar plate and incubated at 35°C for 24 h. When typical E. coli colonies were present, one well-isolated colony was selected and streaked onto a Trypticase soy agar plate supplemented with 5% defribrinated sheep blood (Remel, Lenexs, KS). After indole and oxidase tests, E. coli isolates were subsequently frozen at −60 to −80°C in Brucella broth with 20% glycerol and shipped in cryovials on dry ice to the laboratory of the FDA Administration Center for Veterinary Medicine (FDA-CVM). Upon arrival, every isolate was streaked for purity on a blood agar plate (BAP) before being confirmed as E. coli by the use of a Vitek microbial identification system (bioMérieux, Hazelwood, MO). Bacteria were stored in Trypticase soy broth containing 15% glycerol at −80°C until use. All bacterial media were obtained from Becton Dickinson and Company, Franklin Lakes, NJ, unless otherwise specified.
Antimicrobial susceptibility testing.
Antimicrobial MICs were determined using a Sensititre automated antimicrobial susceptibility system in accordance with the instructions of the manufacturer (Trek Diagnostic Systems, Cleveland, OH). Initially, all isolates were tested using a panel designed for NARMS, which included ceftriaxone (AXO), ceftiofur (TIO), amoxicillin-clavulanic acid (AMC), ampicillin (AMP), cefoxitin (FOX), ciprofloxacin (CIP), nalidixic acid (NAL), amikacin (AMI), gentamicin (GEN), streptomycin (STR), kanamycin (KAN), sulfisoxazole (FIS), trimethoprim-sulfamethoxazole (COT), doxycycline (DOX), tetracycline (TET), and chloramphenicol (CHL). A total of 273 TIOr strains were tested with a secondary panel of ß-lactam antimicrobials that included aztreonam (AZT), cefquinome (CQN), imipenem (IMI), cefepime (FEP), piperacillin-tazobactam (P/T), ceftazidime (TAZ), ceftazidime-clavulanic acid (T/C), cefotaxime (FOT), and cefotaxime-clavulanic acid (F/C). Results were interpreted in accordance with CLSI criteria (8), with the exception of streptomycin (resistance breakpoint ≥ 64 μg/ml) and cefquinome (resistance breakpoint ≥ 32 μg/ml). E. coli ATCC 25922, Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 29213, and Pseudomonas aeruginosa ATCC 27853 were used as quality control organisms in the antimicrobial MIC determinations.
DNA isolation, PCR, and DNA sequencing.
A total of 273 TIOr isolates were screened for the presence of blaCMY, blaTEM, blaSHV, blaOXA, and blaCTX-M and 172 CHLr isolates were screened for the presence of cmlA, flo, catI, catII, and catIII genes by PCR using previously published methods (4, 6, 21, 26, 33, 43). A total of 269 Nalr E. coli isolates were screened for the presence of qnrA, qnrB, qnrS, and acc(6′)-Ib-cr genes and analyzed for quinolone resistance-determining region (QRDR) point mutations in gyrA and parC. The primers and PCR conditions were as previously reported (11, 14, 24). DNA templates were prepared using MoBio Ultraclean DNA isolation kits (MoBio Laboratories, Inc., Carlsbad, CA) following the manufacturer's instructions. Amplifications were carried out using 200 ng of DNA template, 250 μM (each) deoxynucleoside triphosphates, 1.5 mM MgCl2, 50 pmol of each primer, and 1 U of AmpliTaq Gold Taq polymerase (Applied Biosystems, Foster City, CA). The amplified products were separated by gel electrophoresis on 1.0% agarose gels and stained with ethidium bromide. Each PCR product was sequenced using an ABI 3730 automated sequencer (Applied Biosystems). Sequences were analyzed using the BLAST network service of the National Center for Biotechnology Information. Each gene was identified by comparison to sequences in the GenBank database (accession numbers AF252855, AY816215, EU418734, DQ415372, DQ319067, AY737250, EU979560, J02967, Y16784, X92506, EU279427, EU093091, EU391634, and GQ438248).
Statistical analysis.
Statistical analyses were conducted using SAS version 9.2 software (SAS Institute Inc., Cary, NC). One-way analysis of variance (ANOVA) with Tukey's multiple-comparison test was applied to evaluate statistically significant differences in the prevalences and antimicrobial resistances of E. coli isolates recovered from each meat type. P values of <0.05 were considered statistically significant. The Cochran-Armitage trend test was used for trend analysis of prevalence and antimicrobial resistance over time.
RESULTS
Prevalence of E. coli in retail meats.
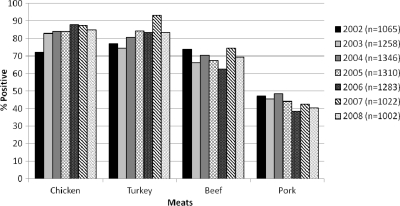
From 2002 to 2008, a total of 11,921 meat samples, collected in GA, MD, OR, and TN, were examined for the presence of E. coli. The sample set consisted of 2,988 chicken breast, 2,942 ground turkey, 2,991 ground beef, and 3,000 pork chop samples. Overall, 69.5% (n = 8,286) of the 11,921 retail meat samples were positive for E. coli, with the highest prevalence in chicken breast samples (n = 2,494; 83.5%), followed by ground turkey (n = 2,412; 82.0%), ground beef (n = 2,061; 68.9%), and pork chops (n = 1,319; 44%) (Fig. 1). There were significantly higher isolation rates from poultry meats than from beef and pork (P < 0.05). No significant differences in E. coli isolation rates were found by month within a sampling site over the 7 years of the study, although some months had slightly higher isolation rates than others (data not shown). However, E. coli isolation rates were significantly different among the states (P < 0.05). Over the testing time period, GA maintained the highest positive isolation rate (mean of 81.1%), followed by MD (72.8%), TN (68.1%), and OR (53.1%).
Fig 1.
Prevalence of E. coli recovered from retail meats in 2002 to 2008. A total of 11,921 retail meat samples, consisting of 2,988 chicken breast, 2,942 ground turkey, 2,991 ground beef, and 3,000 pork chop samples, were analyzed during 2002 to 2008. A total of 8,286 E. coli isolates (2,494 from chicken breast, 2,412 from ground turkey, 2,061 from ground beef, and 1,319 from pork chops) were recovered.
Antimicrobial susceptibility.
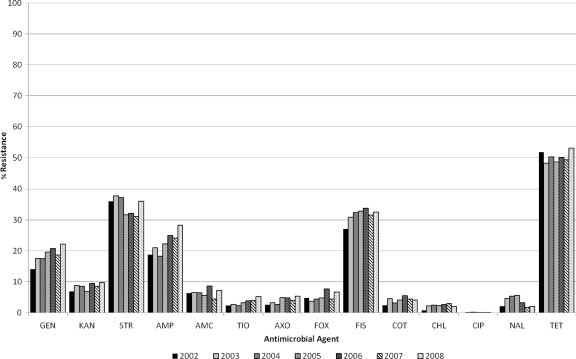
From 2002 to 2008, the E. coli isolates from retail meats displayed resistance most frequently to DOX/TET (48.3% to 53.0%), followed by STR (31.1% to 37.2%), FIS (27.1% to 33.8%), AMP (18.3% to 28.3%), GEN (14.1% to 22.2%), KAN (6.9% to 9.7%), AMC (4.4% to 8.7%), FOX (3.7% to 7.7%), COT (2.4% to 5.5%), AXO (2.5% to 5.4%), NAL (1.7% to 5.6%), TIO (2.3% to 5.3%), CHL (0.8% to 3.0%), and CIP (0.0% to 0.2%) (Fig. 2). All isolates were susceptible to AMI.
Fig 2.
Antimicrobial resistance trends of E. coli isolated from retail meats in 2002 to 2008. A total of 8,286 E. coli isolates, including 1,065 in 2002, 1,258 in 2003, 1,346 in 2004, 1,310 in 2005, 1,283 in 2006, 1,022 in 2007, and 1,002 in 2008, were tested. The testing was done by broth microdilution using amoxicillin-clavulanic acid (AMC), amikacin (AMI), ampicillin (AMP), cefoxitin (FOX), ceftriaxone (AXO), chloramphenicol (CHL), ciprofloxacin (CIP), gentamicin (GEN), kanamycin (KAN), nalidixic acid (NAL), streptomycin (STR), sulfamethoxazole-sulfisoxazole (SMX/FIS), tetracycline (TET), trimethoprim-sulfamethoxazole (COT), and ceftiofur (TIO).
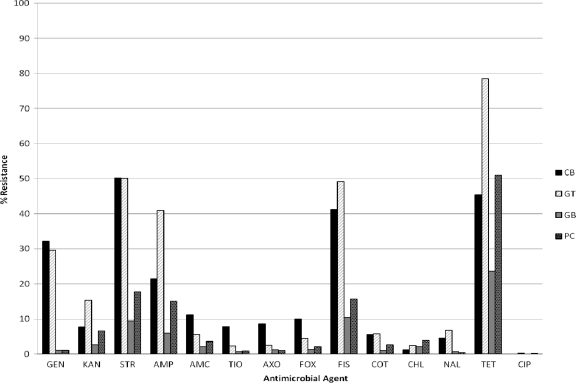
Resistance profiles of the E. coli isolates differed significantly by the type of meat from which they were isolated. The poultry meat isolates had greater resistance to most tested drugs compared to pork and beef isolates (Fig. 3 and Table 1). Notably, the resistance of CB isolates was significantly greater to AMC (11.2%; P < 0.0001), FOX (10%; P < 0.0001), AXO (8.6%; P < 0.0001), and TIO (7.8%; P < 0.0001), and the resistance of GT isolates was significantly greater to KAN (15.3%; P < 0.0001), AMP (40.9%; P < 0.0001), FIS (49.2%; P < 0.0001), TET (78.5%; P < 0.0001), and CIP (0.3%; P = 0.0047). PC isolates had higher resistance to CHL (3.9%) compared to other meat isolates; the resistance level was significantly higher than that of CB isolates (1.2%; P = 0.0068) but not significantly higher than those of GT (2.5%) and GB (2.1%) isolates. In terms of multidrug resistance (MDR [resistance to ≥3 antimicrobial classes]), GT isolates had significantly higher MDR (56%) than CB (38.9%), PC (17.3%), and GB (9.3%) isolates. GT isolates also had the highest percentage of resistance to ≥5 antimicrobial classes (7.6%) compared to CB (6.6%), PC (2.6%), and GB (1.6%) isolates. An MDR-AmpC profile (resistance to AMP, CHL, STR, FIS, TET, and TIO plus other ß-lactams) was detected in E. coli isolates from all four types of meats, with the highest number in CB samples (data not shown). A total of 273 TIOr isolates were tested for resistance to an additional seven ß-lactam antimicrobials and screened (using T/C and F/C) for the presence of extended-spectrum ß-lactamases (ESBL). All were susceptible to P/T, FEB, CQN, FOT, and IMI. Low resistance to AZT (0.7%) and TAZ (7.0%) was seen. No ESBL-producing E. coli isolates were detected.
Fig 3.
Antimicrobial resistance profiles of E. coli recovered from different meat types. A total of 8,286 E. coli isolates, including 2,494 from chicken breast (CB), 2,412 from ground turkey (GT), 2,061 from ground beef (GB), and 1,319 from pork chops (PC), were tested. The testing was done by broth microdilution using amoxicillin-clavulanic acid (AMC), amikacin (AMI), ampicillin (AMP), cefoxitin (FOX), ceftriaxone (AXO), chloramphenicol (CHL), ciprofloxacin (CIP), gentamicin (GEN), kanamycin (KAN), nalidixic acid (NAL), streptomycin (STR), sulfamethoxazole-sulfisoxazole (SMX/FIS), tetracycline (TET), trimethoprim-sulfamethoxazole (COT), and ceftiofur (TIO).
Table 1.
Antimicrobial resistance of E. coil by meat type
| Antimicrobiala | Breakpoint (μg/ml) | % resistanceb |
|||
|---|---|---|---|---|---|
| CB (n = 2,494) | GT (n = 2,412) | GB (n = 2,061) | PC (n = 1,319) | ||
| GEN | ≥16 | 32.2 (A) | 29.6 (A) | 1.1 (B) | 1.1 (B) |
| KAN | ≥64 | 7.7 (A) | 15.3 (B) | 2.7 (C) | 6.6 (A) |
| STR | ≥64 | 50.2 (A) | 50.1 (A) | 9.5 (B) | 17.7 (C) |
| AMP | ≥32 | 21.5 (A) | 40.9 (B) | 6.0 (C) | 15 (A) |
| AMC | ≥32/16 | 11.2 (A) | 5.6 (B) | 2.2 (C) | 3.6 (B, C) |
| FOX | ≥32 | 10.0 (A) | 4.4 (B) | 1.3 (C) | 2.1 (C) |
| AXO | ≥4 | 8.6 (A) | 2.6 (B) | 1.2 (B) | 1.0 (B) |
| TIO | ≥8 | 7.8 (A) | 2.4 (B) | 0.7 (B) | 0.9 (B) |
| SMX/FIS | ≥512 | 41.2 (A) | 49.2 (B) | 10.5 (C) | 15.7 (D) |
| COT | ≥4/76 | 5.6 (A) | 5.8 (A) | 1.0 (B) | 2.7 (B) |
| CHL | ≥32 | 1.2 (A) | 2.5 (A, B) | 2.1 (A, B) | 3.9 (B) |
| CIP | ≥4 | 0.0 (A) | 0.3 (B) | 0.0 (A) | 0.1 (A) |
| NAL | ≥32 | 4.5 (A) | 6.9 (A) | 0.8 (B) | 0.4 (B) |
| TET | ≥16 | 45.4 (A) | 78.5 (B) | 23.7 (C) | 51.0 (D) |
All isolates were susceptible to amikacin (AMI) (resistance breakpoint > 64 μg/ml), gentamicin (GEN), kanamycin (KAN), streptomycin (STR), ampicillin (AMP), amoxicillin-clavulanic acid (AMC), cefoxitin (FOX), ceftriaxone (AXO), ceftiofur (TIO), SMX/FIS, sulfamethoxazole-sulfisoxazole; COT, trimethoprim-sulfamethoxazole; chloramphenicol (CHL), ciprofloxacin (CIP), nalidixic acid (NAL), and tetracycline (TET). CB, chicken breast; GT, ground turkey; GB, ground beef; PC, pork chops.
Entries with identical letters in parentheses indicate no statistically significant differences between those results. Statistical significance calculations are based on P ≤ 0.05.
Presence of resistance genes.
The mechanisms of resistance to expanded-spectrum cephalosporins, CHL, and fluoroquinolone (FQ) were investigated at the genetic level. All of the 273 TIOr/AXOr isolates contained blaCMY, as expected. In addition, 20% (n = 55) contained blaTEM. No isolates were found to carry blaSHV, blaOXA, or blaCTX-M. Thirty percent (n = 82) of TIOr/AXOr isolates were resistant to ≥9 antimicrobials, and 12% (n = 33) were MDR-AmpC. Some of the MDR-AmpC strains also had resistance to GEN, KAN, COT, and/or NAL. Among 172 CHLr isolates, 45% (n = 78), 43% (n = 74), and 40% (n = 68) carried cmlA, flo, and catI, respectively, whereas only 4.6% (n = 8) of isolates carried catII. catIII was not detected in any strains. Seven CHLr isolates carried three of the five CHL genes, and 42 carried two of the five CHL genes. Most of the CHLr isolates were coresistant to TET (96.5%, 166/172) and FIS (90%, 155/172); 44% (n = 76) were ACSSuT (i.e., resistant to AMP, CHL, STR, SMX/FIS, and TET), and 19.2% (n = 33) were MDR-AmpC resistant.
A total of 269 NALr E. coli isolates were screened for the presence of qnrA, qnrB, qnrS, and acc(6′)-Ib-cr genes and analyzed for QRDR point mutations in the gyrA and parC genes. Two qnr+ isolates were found among the NALr strains, one carrying qnrA and one with qnrB. Neither qnrS nor acc(6′)-Ib-cr was detected in any isolates. Additionally, most NALr E. coli isolates (98.5%) had a gyrA mutation(s) in S83 or D87 or both, whereas 6.7% had a parC mutation at either S80 or E84 (Table 2). More than half (59.8% and 59.5%) of the Nalr E. coli isolates had coresistance to FIS and STR, respectively, and 6.3% had coresistance to TIO and AXO.
Table 2.
Summary of QRDR mutations among 269 NALr E. coli isolatesa
| gyrA mutation | parC mutation | CIP phenotype | NAL phenotype | No. of isolates |
|---|---|---|---|---|
| S83L | WT | Sensitive | Resistant | 218 |
| D87N | WT | Sensitiveb | Resistant | 11 |
| S83L | S80R | Sensitive | Resistant | 10 |
| D87Y | WT | Sensitive | Resistant | 8 |
| D87G | WT | Sensitive | Resistant | 6 |
| S83L/D87N | WT | Resistant | Resistant | 4 |
| WT | S80R | Sensitive | Resistant | 2 |
| WT | WT | Sensitive | Resistant | 1 |
| S83L | E84K | Sensitive | Resistant | 1 |
| S83L | S80I | Sensitive | Resistant | 1 |
| S83L | S80C | Sensitive | Resistant | 1 |
| S83L | S80G | Sensitive | Resistant | 1 |
| D87E | WT | Sensitive | Resistant | 1 |
| S83A | WT | Sensitive | Resistant | 1 |
| S83L/D87N | S80I | Resistant | Resistant | 1 |
| S83L/D87G | WT | Sensitive | Resistant | 1 |
| WT | E84G | Sensitive | Resistant | 1 |
A, alanine; C, cysteine; D, aspartic acid; E, glutamic acid; G, glycine; I, isoleucine; K, lysine; L, leucine; N, asparagine; R, arginine; S, serine; Y, tyrosine; WT, wild type.
One isolate with this genotype was CIPr.
DISCUSSION
The use of antimicrobials in food animals, and their role in promoting resistance in food-borne bacteria, is an important public health issue. To measure the baseline resistance rates and the impact of different targeted interventions, an ongoing monitoring system is necessary. As part of the monitoring of commensal and food-borne bacteria, the U.S. NARMS program tracked antimicrobial resistance in E. coli isolates from retail meats from four states. We reported on the prevalence, antimicrobial susceptibility, and mechanisms of resistance in E. coli isolates collected during a 7-year sampling period.
E. coli was commonly present in all four retail meats, signifying a high overall rate of fecal contamination during slaughter and of postslaughter processing contaminations. The highest contamination was in poultry meats (>80%), followed by beef (68.9%) and pork (44%). Other surveys on the prevalence of E. coli in retail meats in the United States showed similar findings (18, 39). Johnson et al. (18) reported a survey study of 10 retail markets in the Minneapolis-St. Paul, MN, area during 2001 to 2003 and showed that the contamination rate was 92% in poultry and 69% in beef and pork. Various recovery rates are reported in other studies from the United States and abroad. All have demonstrated that E. coli frequently contaminates retail meat products (1, 9, 18, 27, 39).
In general, E. coli isolates were more frequently resistant to TET, STR, FIS, AMP, and GEN (19% to 50%) compared with resistance to the other tested agents (<1% to 8.4%). Similar findings of common resistance to TET, FIS, and AMP among E. coli isolates from food animals and derived meats were also reported by other investigators (18, 25, 27, 31). The level of resistance to different antimicrobials varied according to the source of the isolates. E. coli isolates from poultry meats had a higher resistance rate than those from beef and pork. The resistance of chicken isolates to ß-lactams AMC, FOX, AXO, and TIO was significantly greater than that of other meat isolates, whereas the turkey isolates were more resistant to KAN, AMP, FIS, CIP, and TET compared to other meat isolates (P ≤ 0.05). Similar results were observed with Salmonella isolates from retail poultry meat monitoring (40). Although, in general, the pork isolates had lower resistance than the poultry isolates, their resistance to KAN, STR, AMP, FIS, and TET was significantly higher than that of the beef isolates (P ≤ 0.05). These findings can be considered to reflect, at least in part, the selective pressures imposed by antimicrobial use in different food animal production and processing environments, despite the fact that we do not have antibiotic use histories to correlate with the susceptibility data. It is speculated that the higher levels of resistance in poultry may be partly due to modern production practices, in which antimicrobials for disease control and prevention are administered through water as well as feed. This imposes greater selection pressure for the development of antimicrobial resistance by exposing large numbers of animals in a treated group (10, 32, 38).
Some notable resistance trends were observed for E. coli isolates from certain meat types. Between 2002 and 2008, GT isolates showed a significant increase in resistance to AMP (P < 0.0001), TIO (P < 0.0001), AXO (P < 0.0001), FOX (P < 0.005), AMC (P < 0.0151), and TET (P < 0.0089) and decreased resistance to NAL (P < 0.0003). CB isolates showed a significant increase in resistance to GEN (P < 0.0034) and decreased resistance to STR (P < 0.0012) over the 7-year sampling time frame. Although CB isolates did not show a statistically significant increase in resistance to TIO and AXO, they maintained the highest resistance to these two compounds compared to isolates from other meats, ranging from 5.8% to 11.1% over the years of the study. Both GB and PC isolates were generally stable in resistance levels for all tested antimicrobials (data not shown).
Resistance to expanded-spectrum cephalosporins is a special concern, since these antimicrobials are a front-line therapeutic for treatment of numerous Gram-negative infections, including systemic and pediatric salmonellosis. In the last 10 years, this resistance phenotype has emerged in both humans and food animals in the United States (12, 40, 44). TIO is the only cephalosporin approved for systemic use in food animals in the United States. This drug was first approved in 1988 for treatment of acute bovine respiratory disease and subsequently for use in other food animals, including swine, sheep, chickens, and turkeys (5). It is also used extensively in an off-label manner in broiler chicks as a component of vaccinations. Because TIOr organisms also exhibit resistance to cephamycins and expanded-spectrum cephalosporins, the use of this antimicrobial in food animals as a selective agent responsible for the emergence and dissemination of AXOr enteric pathogens such as Salmonella has come under increasing scrutiny. The blaCMY gene encoding a cephamycinase (an extended-spectrum beta-lactamase) confers resistance to both TIO and AXO (44). Our study showed that all TIOr/AXOr isolates carried blaCMY but not blaSHV, blaOXA, or blaCTX-M. Similar findings were seen in our previous study on Salmonella isolates from NARMS retail meats (40). A major public health concern is that TIOr/AXOr isolates are also often MDR. Among the E. coli isolates examined here, we found that a very high proportion (30%) of TIOr/AXOr isolates were resistant to ≥9 antimicrobials and that 12% were MDR-AmpC. The blaCMY gene often resides in conjugative MDR plasmids, in some cases being present in multiple copies (36, 40). The poultry meat isolates have shown a steady increase in resistance to TIO and AXO over the years. This has prompted the U.S. Food and Drug Administration to propose limiting the extralabel use of extended-spectrum cephalosporins in order to preserve their effectiveness for treating human infections (13).
A small percentage (1.2% to 3.9%) of E. coli isolates from all meat types were resistant to CHL, a drug that was withdrawn from food animal use in the United States in the 1980s due to its toxicity (15, 29). However, CHL resistance has been persistently seen in E. coli and Salmonella isolates from food animals (3, 37, 40). Florfenicol, a closely related drug, is approved for veterinary use in food animals in the United States. Florfenicol resistance mediated by the flo gene confers nonenzymatic cross-resistance to chloramphenicol (3). This may contribute in part to the persistence of low CHL resistance in the food animal environment. Our data suggest that florfenicol coselection accounts for around 43% of CHL resistance, implying that other selecting factors play a role. In addition, CHL resistance is mediated by cmlA, catI, catII, and catIII, and these genes frequently reside on integrons and MDR plasmids (7, 16, 23) in enterics. These mobile elements often contain multiple resistance genes, such as sul, conferring resistance to SMX/FIS, and tet, conferring TET resistance. Over 90% of CHLr isolates in the present study had coresistance to TET and SUL/FIS. Therefore, coselection by these drug classes likely also plays an important role in the persistence of CHL resistance. Additionally, 42% and 12% of CHLr isolates had ACSSuT and MDR-AmpC resistance phenotypes, respectively. These phenotypes have emerged in Salmonella in the last 10 to 20 years. Genetic analysis of ACSSuT and MDR-AmpC phenotypes from Salmonella showed that cmlA, cat, and flo are often linked with numerous other resistance genes such as tet, sul, aadA, pse, qac, sugE, and blaCMY, which are resident on integrons and MDR plasmids (36, 41, 42). The qac and sugE genes encode resistance to disinfectants (quaternary ammonium compounds), including cetylpyridinium chloride (CPC). These loci are often found on MDR plasmids (36). CPC has been approved for use in chicken slaughterhouses for carcass decontamination. Thus, although on-farm antibiotic use is considered a major cause of antimicrobial resistance in retail meat isolates, consideration must be given to the role of other chemical treatments in slaughterhouses and in retail outlets that can select for strain types carrying CHL and other resistances. Some E. coli isolates harbored two or three of five CHL resistance genes, although no additive effect on MIC was found among isolates with multiple alleles (data not shown).
The development of resistance to FQs is also a particular concern, since these antimicrobials are critically important for treating many human infections. Only 6 CIPr isolates were found among 269 NALr isolates, and all 6 were isolated from GT. The NALr isolates had decreased susceptibility to CIP, with MICs ranging from 0.125 to 2 μg/ml. All had QRDA mutations in gyrA or parC or both. Because of rising quinolone resistance in Campylobacter, approval of fluoroquinolones for use in poultry was withdrawn from the market in September 2005. Our data show that the proportion of NALr E. coli strains isolated from chicken breast dropped from 6.6% in 2005 to 2.9% in 2009. Among ground turkey isolates, NAL resistance declined from 10.4% in 2005 to 2.6% in 2009.
In summary, the present report demonstrates a high prevalence of E. coli in retail meats, indicating that fecal contamination at slaughter and processing is very common and highlighting the importance of consumer awareness of safe food handling and cooking. The relative levels and types of resistance in E. coli (including MDR) from different sources represent a useful indicator of selection pressure imposed by antimicrobials used in different food animals and perhaps processing plants. Since retail meats represent a point of exposure close to the consumer, monitoring the prevalence of antimicrobial resistance among food-borne pathogenic and commensal organisms from such commodities is necessary to identify emerging resistance problems in the food supply. The integration of antimicrobial susceptibility and genetic analysis provides important information on the dissemination, linkage, and recombination of genes underlying MDR in food-borne bacteria in the animal production environment, retail foods, and humans.
ACKNOWLEDGMENTS
We thank CDC and all FoodNet sites for their contributions to the NARMS retail meats program. We also thank Mark Rasmussen and Jeffrey Ward for critical review of the manuscript.
Footnotes
Published ahead of print 13 January 2012
REFERENCES
- 1. Ahmed AM, Shimabukuro H, Shimamoto T. 2009. Isolation and molecular characterization of multidrug-resistant strains of Escherichia coli and Salmonella from retail chicken meat in Japan. J. Food Sci. 74:M405–M410 [DOI] [PubMed] [Google Scholar]
- 2. Alhaj N, Mariana N, Raha A, Ishak Z. 2007. Prevalence of antimicrobial resistance among Escherichia coli from different sources in Malaysia. Int. J. Poult. Sci. 6:293–297 [Google Scholar]
- 3. Bischoff KM, et al. 2002. Characterization of chloramphenicol resistance in beta-hemolytic Escherichia coli associated with diarrhea in neonatal swine. J. Clin. Microbiol. 40:389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolton LF, Kelley LC, Lee MD, Fedorka-Cray PJ, Maurer JJ. 1999. Detection of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 based on a gene which confers cross-resistance to florfenicol and chloramphenicol. J. Clin. Microbiol. 37:1348–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bradford PA, Petersen PJ, Fingerman IM, White DG. 1999. Characterization of expanded-spectrum cephalosporin resistance in E. coli isolates associated with bovine calf diarrhoeal disease. J. Antimicrob. Chemother. 44:607–610 [DOI] [PubMed] [Google Scholar]
- 6. Briñas L, Zarazaga M, Saenz Y, Ruiz-Larrea F, Torres C. 2002. Beta-lactamases in ampicillin-resistant Escherichia coli isolates from foods, humans, and healthy animals. Antimicrob. Agents Chemother. 46:3156–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen S, et al. 2004. Characterization of multiple-antimicrobial-resistant Salmonella serovars isolated from retail meats. Appl. Environ. Microbiol. 70:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; 15th informational supplement (M100–S20). Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Cook A, et al. 2009. Antimicrobial resistance in Campylobacter, Salmonella, and Escherichia coli isolated from retail turkey meat from southern Ontario, Canada. J. Food Prot. 72:473–481 [DOI] [PubMed] [Google Scholar]
- 10. Engberg J, Aarestrup FM, Taylor DE, Gerner-Smidt P, Nachamkin I. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Everett MJ, Jin YF, Ricci V, Piddock LJ. 1996. Contributions of individual mechanisms to fluoroquinolone resistance in 36 Escherichia coli strains isolated from humans and animals. Antimicrob. Agents Chemother. 40:2380–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Folster JP, et al. 2010. Characterization of extended-spectrum cephalosporin-resistant Salmonella enterica serovar Heidelberg isolated from humans in the United States. Foodborne Pathog. Dis. 7:181–187 [DOI] [PubMed] [Google Scholar]
- 13. Food and Drug Administration 2008. An order prohibiting the extralabel use of cephalosporin antimicrobial drugs in food-producing animals. Fed. Regist. 73:38110–38113 [Google Scholar]
- 14. Gay K, et al. 2006. Plasmid-mediated quinolone resistance in non-Typhi serotypes of Salmonella enterica. Clin. Infect. Dis. 43:297–304 [DOI] [PubMed] [Google Scholar]
- 15. Gilmore A. 1986. Chloramphenicol and the politics of health. CMAJ 134:423, 426,–428, 433–435 [PMC free article] [PubMed] [Google Scholar]
- 16. Hall RM, et al. 1999. Mobile gene cassettes and integrons in evolution. Ann. N. Y. Acad. Sci. 870:68–80 [DOI] [PubMed] [Google Scholar]
- 17. Hammerum AM, Heuer OE. 2009. Human health hazards from antimicrobial-resistant Escherichia coli of animal origin. Clin. Infect. Dis. 48:916–921 [DOI] [PubMed] [Google Scholar]
- 18. Johnson JR, Kuskowski MA, Smith K, O'Bryan TT, Tatini S. 2005. Antimicrobial-resistant and extraintestinal pathogenic Escherichia coli in retail foods. J. Infect. Dis. 191:1040–1049 [DOI] [PubMed] [Google Scholar]
- 19. Johnson JR, et al. 2009. Molecular analysis of Escherichia coli from retail meats (2002–2004) from the United States National Antimicrobial Resistance Monitoring System. Clin. Infect. Dis. 49:195–201 [DOI] [PubMed] [Google Scholar]
- 20. Johnson JR, et al. 2007. Antimicrobial drug-resistant Escherichia coli from humans and poultry products, Minnesota and Wisconsin, 2002–2004. Emerg. Infect. Dis. 13:838–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keyes K, et al. 2000. Detection of florfenicol resistance genes in Escherichia coli isolated from sick chickens. Antimicrob. Agents Chemother. 44:421–424 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kikuvi GM, Schwarz S, Ombui JN, Mitema ES, Kehrenberg C. 2007. Streptomycin and chloramphenicol resistance genes in Escherichia coli isolates from cattle, pigs, and chicken in Kenya. Microb. Drug Resist. 13:62–68 [DOI] [PubMed] [Google Scholar]
- 23. Recchia GD, Hall RM. 1997. Origins of the mobile gene cassettes found in integrons. Trends Microbiol. 5:389–394 [DOI] [PubMed] [Google Scholar]
- 24. Robicsek A, et al. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat. Med. 12:83–88 [DOI] [PubMed] [Google Scholar]
- 25. Sáenz Y, et al. 2001. Antibiotic resistance in Escherichia coli isolates obtained from animals, foods and humans in Spain. Int. J. Antimicrob. Agents 18:353–358 [DOI] [PubMed] [Google Scholar]
- 26. Saladin M, et al. 2002. Diversity of CTX-M beta-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol. Lett. 209:161–168 [DOI] [PubMed] [Google Scholar]
- 27. Schroeder CM, et al. 2003. Isolation of antimicrobial-resistant Escherichia coli from retail meats purchased in greater Washington, DC, USA. Int. J. Food Microbiol. 85:197–202 [DOI] [PubMed] [Google Scholar]
- 28. Scott HM, et al. 2005. Patterns of antimicrobial resistance among commensal Escherichia coli isolated from integrated multi-site housing and worker cohorts of humans and swine. Foodborne Pathog. Dis. 2:24–37 [DOI] [PubMed] [Google Scholar]
- 29. Settepani JA. 1984. The hazard of using chloramphenicol in food animals. J. Am. Vet. Med. Assoc. 184:930–931 [PubMed] [Google Scholar]
- 30. Sørum H, Sunde M. 2001. Resistance to antibiotics in the normal flora of animals. Vet. Res. 32:227–241 [DOI] [PubMed] [Google Scholar]
- 31. Teshager T, et al. 2000. Surveillance of antimicrobial resistance in Escherichia coli strains isolated from pigs at Spanish slaughterhouses. Int. J. Antimicrob. Agents 15:137–142 [DOI] [PubMed] [Google Scholar]
- 32. Tollefson L, Miller MA. 2000. Antibiotic use in food animals: controlling the human health impact. J. AOAC Int. 83:245–254 [PubMed] [Google Scholar]
- 33. Vassort-Bruneau C, Lesage-Descauses MC, Martel JL, Lafont JP, Chaslus-Dancla E. 1996. CAT III chloramphenicol resistance in Pasteurella haemolytica and Pasteurella multocida isolated from calves. J. Antimicrob. Chemother. 38:205–213 [DOI] [PubMed] [Google Scholar]
- 34. Vieira AR, et al. 2011. Association between antimicrobial resistance in Escherichia coli isolates from food animals and blood stream isolates from humans in Europe: an ecological study. Foodborne Pathog. Dis. 8:1295–1301 doi:10.1089/fpd.2011.0950 [DOI] [PubMed] [Google Scholar]
- 35. von Baum H, Marre R. 2005. Antimicrobial resistance of Escherichia coli and therapeutic implications. Int. J. Med. Microbiol. 295:503–511 [DOI] [PubMed] [Google Scholar]
- 36. Welch TJ, et al. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One 2:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White DG, et al. 2003. Characterization of integron mediated antimicrobial resistance in Salmonella isolated from diseased swine. Can. J. Vet. Res. 67:39–47 [PMC free article] [PubMed] [Google Scholar]
- 38. Witte W. 2000. Selective pressure by antibiotic use in livestock. Int. J. Antimicrob. Agents 16(Suppl. 1):S19–S24 [DOI] [PubMed] [Google Scholar]
- 39. Zhao C, et al. 2001. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the greater Washington, D.C., area. Appl. Environ. Microbiol. 67:5431–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao S, et al. 2009. β-Lactam resistance in Salmonella strains isolated from retail meats in the United States by the National Antimicrobial Resistance Monitoring System between 2002 and 2006. Appl. Environ. Microbiol. 75:7624–7630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao S, et al. 2005. Characterization of Salmonella Typhimurium of animal origin obtained from the National Antimicrobial Resistance Monitoring System. Foodborne Pathog. Dis. 2:169–181 [DOI] [PubMed] [Google Scholar]
- 42. Zhao S, et al. 2007. Characterization of multidrug resistant Salmonella recovered from diseased animals. Vet. Microbiol. 123:122–132 [DOI] [PubMed] [Google Scholar]
- 43. Zhao S, et al. 2003. Characterization of Salmonella enterica serotype Newport isolated from humans and food animals. J. Clin. Microbiol. 41:5366–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao S, et al. 2001. Identification and expression of cephamycinase blaCMY genes in Escherichia coli and Salmonella isolates from food animals and ground meat. Antimicrob. Agents Chemother. 45:3647–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]