Abstract
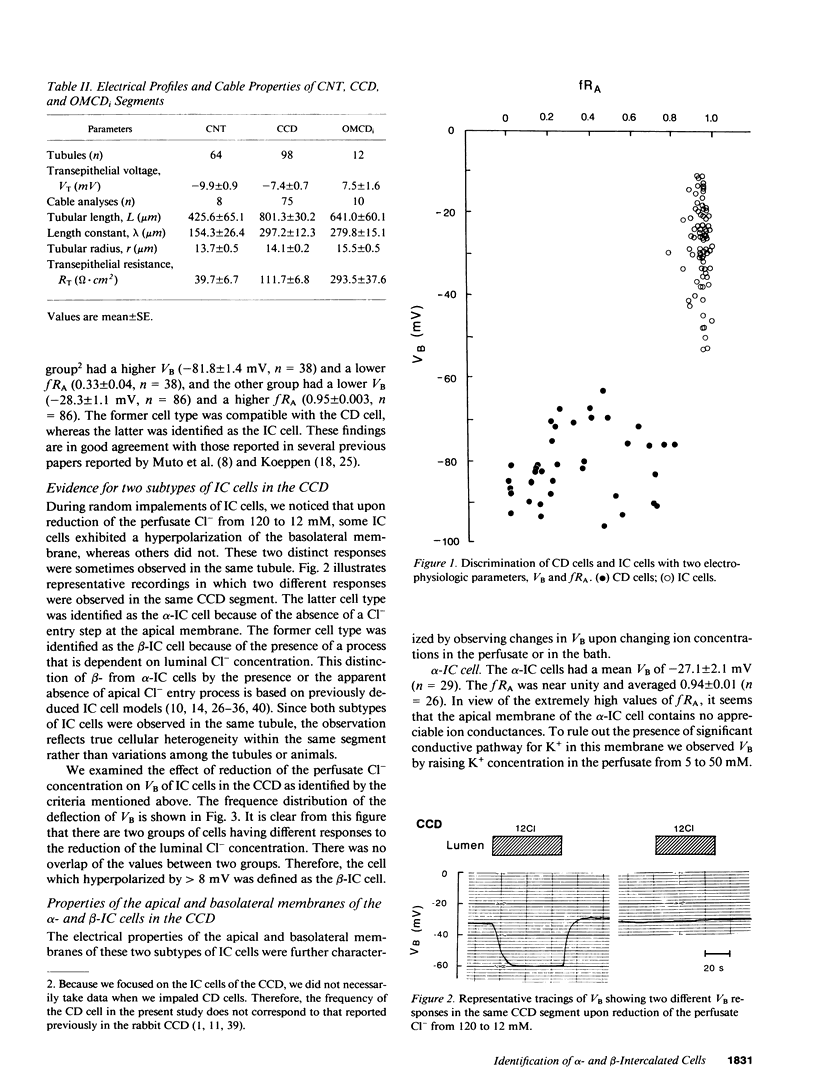
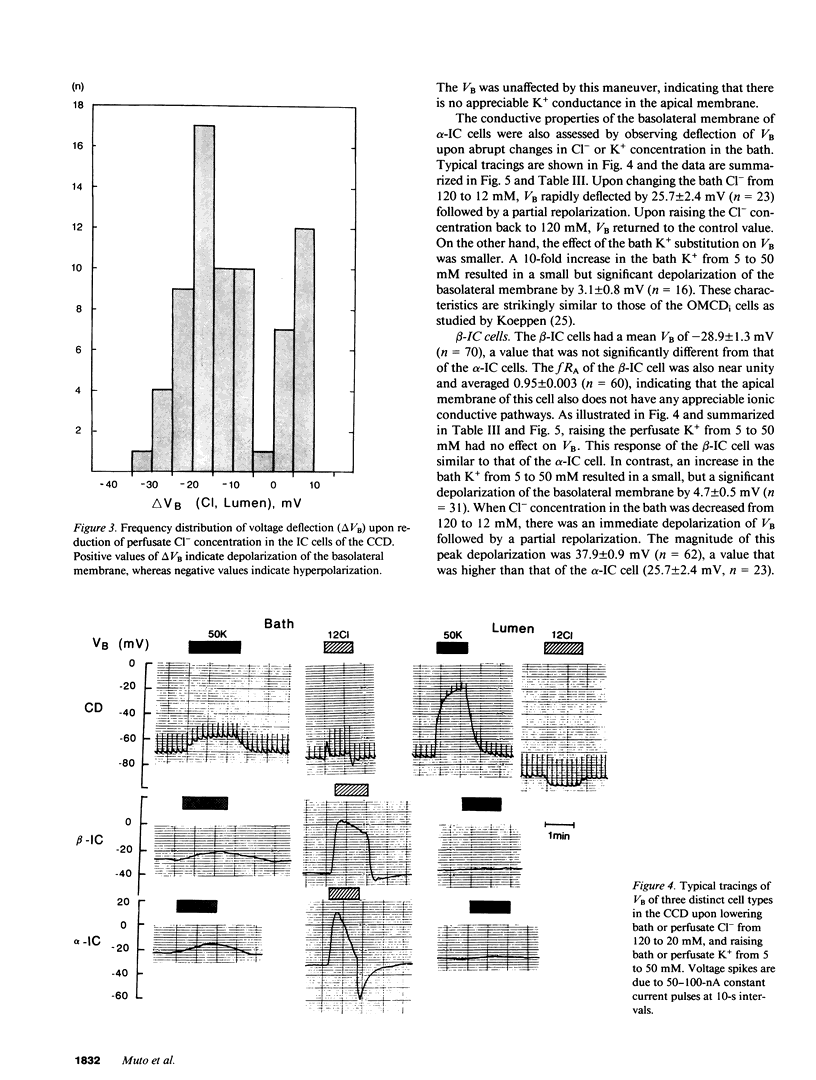
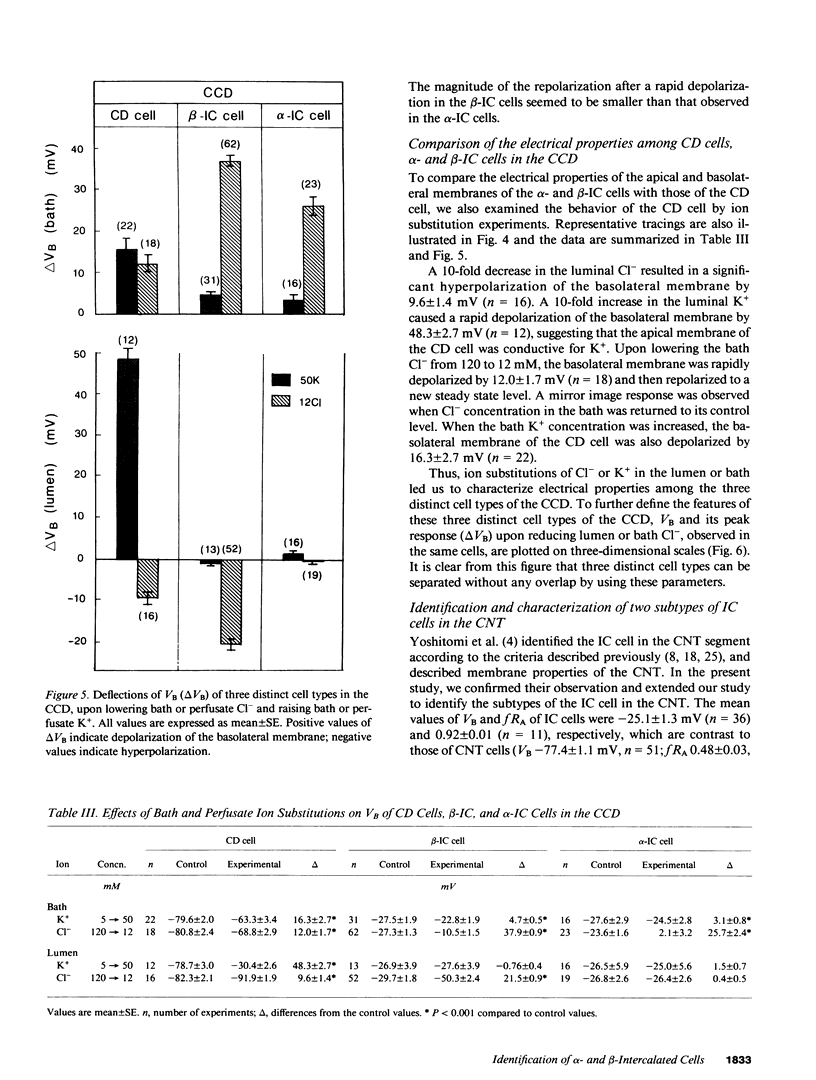
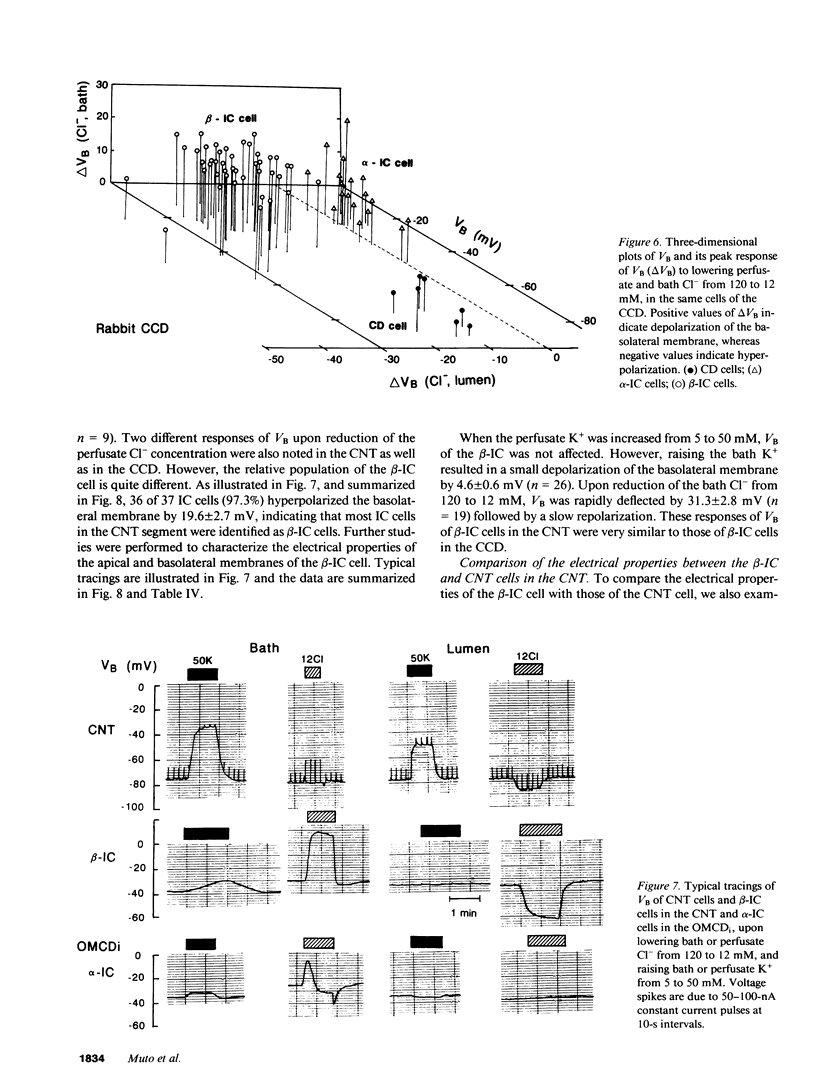
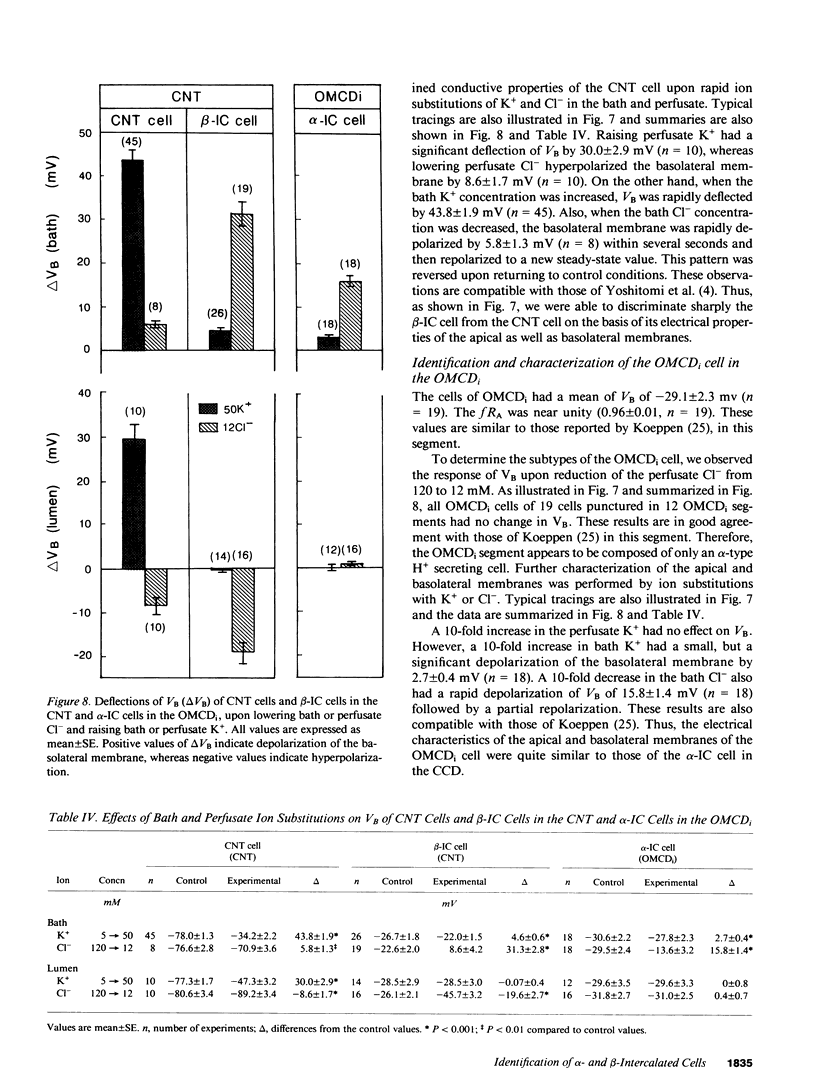
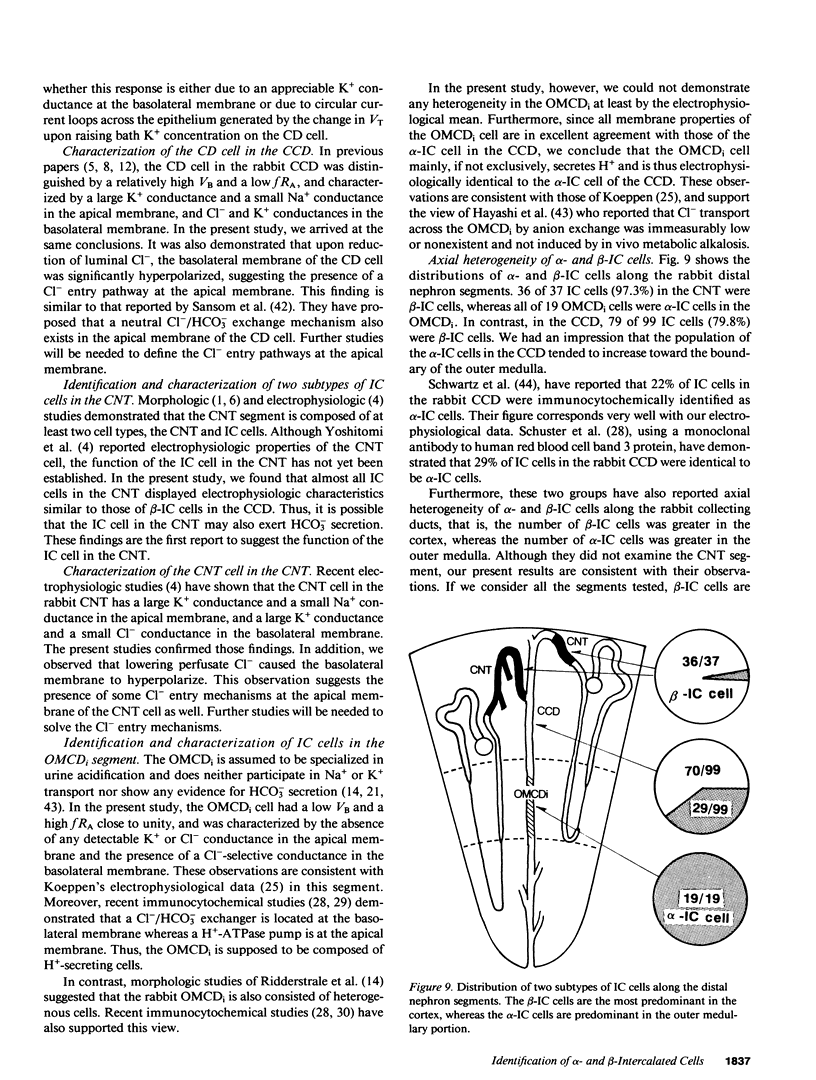
By cable analysis and intracellular microelectrode impalement in the in vitro perfused renal tubule, we identified alpha- and beta-intercalated (IC) cells along the rabbit distal nephron segments, including the connecting tubule (CNT), the cortical collecting duct (CCD), and the outer medullary collecting duct in the inner stripe (OMCDi). IC cells were distinguished from collecting duct (CD) cells by a relatively low basolateral membrane potential (VB), a higher fractional apical membrane resistance, and apparent high Cl- conductances of the basolateral membrane. Two functionally different subtypes of IC cells in the CCD were identified based on different responses of VB upon reduction of the perfusate Cl- from 120 to 12 mM: the basolateral membrane of beta-IC cells was hyperpolarized, whereas that of alpha-IC cells was unchanged. This is in accord with the hypothesis that the apical membrane of beta-IC cells contains some Cl(-)-dependent entry processes, possibly a Cl-/HCO3- exchanger. Further characterization of electrical properties of both subtypes of IC cells were performed upon lowering bath or perfusate Cl- from 120 to 12 mM, and raising bath or perfusate K+ from 5 to 50 mM. A 10-fold increase in the perfusate K+ had no effect on VB in both subtypes of IC cells. Upon abrupt changes in Cl- or K+ concentration in the bath, a large or a small depolarization of the basolateral membrane, respectively, was observed in both subtypes of IC cells. The electrical properties of alpha- and beta-IC cells were similar among the distal nephron segments, but their distribution was different: in the CNT, which consists of IC cells and CNT cells, 97.3% (36/37) of IC cells were of the beta type. In the CCD, which consists of IC cells and CD cells, 79.8% (79/99) of IC cells were of the beta-type, whereas in the OMCDi 100% (19/19) were of the alpha type, suggesting that the beta type predominates in the earlier and the alpha type in the later segment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D., Hirsch S., Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature. 1988 Feb 18;331(6157):622–624. doi: 10.1038/331622a0. [DOI] [PubMed] [Google Scholar]
- Brown D., Hirsch S., Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest. 1988 Dec;82(6):2114–2126. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Burnatowska-Hledin M. A., Spielman W. S. Immunodissection of mitochondria-rich cells from rabbit outer medullary collecting tubule. Am J Physiol. 1988 Jun;254(6 Pt 2):F907–F911. doi: 10.1152/ajprenal.1988.254.6.F907. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Lucci M. S. Effect of carbonic anhydrase inhibition on superficial and deep nephron bicarbonate reabsorption in the rat. J Clin Invest. 1983 Jan;71(1):55–65. doi: 10.1172/JCI110751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer J. P., Laski M. E., Wesson D. E., Kurtzman N. A. Internephron heterogeneity for carbonic anhydrase-independent bicarbonate reabsorption in the rat. J Clin Invest. 1984 Apr;73(4):1034–1045. doi: 10.1172/JCI111288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Austt J., Good D. W., Burg M. B., Knepper M. A. Deoxycorticosterone-stimulated bicarbonate secretion in rabbit cortical collecting ducts: effects of luminal chloride removal and in vivo acid loading. Am J Physiol. 1985 Aug;249(2 Pt 2):F205–F212. doi: 10.1152/ajprenal.1985.249.2.F205. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Schuster V. L., Stokes J. B. Absence of transepithelial anion exchange by rabbit OMCD: evidence against reversal of cell polarity. Am J Physiol. 1988 Aug;255(2 Pt 2):F220–F228. doi: 10.1152/ajprenal.1988.255.2.F220. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Grantham J. J., Burg M. B. Effect of vasopressin on electrical resistance of renal cortical collecting tubules. Am J Physiol. 1971 Jun;220(6):1825–1832. doi: 10.1152/ajplegacy.1971.220.6.1825. [DOI] [PubMed] [Google Scholar]
- Helman S. I., O'Neil R. G. Model of active transepithelial Na and K transport of renal collecting tubules. Am J Physiol. 1977 Dec;233(6):F559–F571. doi: 10.1152/ajprenal.1977.233.6.F559. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Schulte B. A., Pasternack G., Siegel G. J., Spicer S. S. Three distinct cell populations in rat kidney collecting duct. Am J Physiol. 1987 Aug;253(2 Pt 1):C323–C328. doi: 10.1152/ajpcell.1987.253.2.C323. [DOI] [PubMed] [Google Scholar]
- Imai M. The connecting tubule: a functional subdivision of the rabbit distal nephron segments. Kidney Int. 1979 Apr;15(4):346–356. doi: 10.1038/ki.1979.46. [DOI] [PubMed] [Google Scholar]
- Ishibashi K., Sasaki S., Yoshiyama N., Shiigai T., Takeuchi J. Generation of pH gradient across the rabbit collecting duct segments perfused in vitro. Kidney Int. 1987 Apr;31(4):930–936. doi: 10.1038/ki.1987.88. [DOI] [PubMed] [Google Scholar]
- Kaissling B., Kriz W. Structural analysis of the rabbit kidney. Adv Anat Embryol Cell Biol. 1979;56:1–123. doi: 10.1007/978-3-642-67147-0. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Biagi B. A., Giebisch G. H. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol. 1983 Jan;244(1):F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M. Conductive properties of the rabbit outer medullary collecting duct: inner stripe. Am J Physiol. 1985 Apr;248(4 Pt 2):F500–F506. doi: 10.1152/ajprenal.1985.248.4.F500. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M. Conductive properties of the rabbit outer medullary collecting duct: outer stripe. Am J Physiol. 1986 Jan;250(1 Pt 2):F70–F76. doi: 10.1152/ajprenal.1986.250.1.F70. [DOI] [PubMed] [Google Scholar]
- Laski M. E., Kurtzman N. A. Characterization of acidification in the cortical and medullary collecting tubule of the rabbit. J Clin Invest. 1983 Dec;72(6):2050–2059. doi: 10.1172/JCI111170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard W. E., Kokko J. P., Jacobson H. R. Bicarbonate transport in cortical and outer medullary collecting tubules. Am J Physiol. 1983 Mar;244(3):F289–F296. doi: 10.1152/ajprenal.1983.244.3.F289. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate absorption by rabbit cortical collecting tubules in vitro. Am J Physiol. 1978 Feb;234(2):F141–F145. doi: 10.1152/ajprenal.1978.234.2.F141. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate secretion by rabbit cortical collecting tubules in vitro. J Clin Invest. 1978 Jun;61(6):1421–1427. doi: 10.1172/JCI109061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Giebisch G., Sansom S. Effects of adrenalectomy on CCD: evidence for differential response of two cell types. Am J Physiol. 1987 Oct;253(4 Pt 2):F742–F752. doi: 10.1152/ajprenal.1987.253.4.F742. [DOI] [PubMed] [Google Scholar]
- Muto S., Sansom S., Giebisch G. Effects of a high potassium diet on electrical properties of cortical collecting ducts from adrenalectomized rabbits. J Clin Invest. 1988 Feb;81(2):376–380. doi: 10.1172/JCI113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil R. G., Hayhurst R. A. Functional differentiation of cell types of cortical collecting duct. Am J Physiol. 1985 Mar;248(3 Pt 2):F449–F453. doi: 10.1152/ajprenal.1985.248.3.F449. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Helman S. I. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol. 1977 Dec;233(6):F544–F558. doi: 10.1152/ajprenal.1977.233.6.F544. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Sansom S. C. Electrophysiological properties of cellular and paracellular conductive pathways of the rabbit cortical collecting duct. J Membr Biol. 1984;82(3):281–295. doi: 10.1007/BF01871637. [DOI] [PubMed] [Google Scholar]
- Ridderstrale Y., Kashgarian M., Koeppen B., Giebisch G., Stetson D., Ardito T., Stanton B. Morphological heterogeneity of the rabbit collecting duct. Kidney Int. 1988 Nov;34(5):655–670. doi: 10.1038/ki.1988.230. [DOI] [PubMed] [Google Scholar]
- Sansom S. C., Weinman E. J., O'Neil R. G. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol. 1984 Aug;247(2 Pt 2):F291–F302. doi: 10.1152/ajprenal.1984.247.2.F291. [DOI] [PubMed] [Google Scholar]
- Satlin L. M., Schwartz G. J. Cellular remodeling of HCO3(-)-secreting cells in rabbit renal collecting duct in response to an acidic environment. J Cell Biol. 1989 Sep;109(3):1279–1288. doi: 10.1083/jcb.109.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatter E., Schafer J. A. Electrophysiological studies in principal cells of rat cortical collecting tubules. ADH increases the apical membrane Na+-conductance. Pflugers Arch. 1987 Jun;409(1-2):81–92. doi: 10.1007/BF00584753. [DOI] [PubMed] [Google Scholar]
- Schuster V. L., Bonsib S. M., Jennings M. L. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol. 1986 Sep;251(3 Pt 1):C347–C355. doi: 10.1152/ajpcell.1986.251.3.C347. [DOI] [PubMed] [Google Scholar]
- Schuster V. L., Stokes J. B. Chloride transport by the cortical and outer medullary collecting duct. Am J Physiol. 1987 Aug;253(2 Pt 2):F203–F212. doi: 10.1152/ajprenal.1987.253.2.F203. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Al-Awqati Q. Carbon dioxide causes exocytosis of vesicles containing H+ pumps in isolated perfused proximal and collecting tubules. J Clin Invest. 1985 May;75(5):1638–1644. doi: 10.1172/JCI111871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J., Al-Awqati Q. Regulation of transepithelial H+ transport by exocytosis and endocytosis. Annu Rev Physiol. 1986;48:153–161. doi: 10.1146/annurev.ph.48.030186.001101. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Satlin L. M., Bergmann J. E. Fluorescent characterization of collecting duct cells: a second H+-secreting type. Am J Physiol. 1988 Nov;255(5 Pt 2):F1003–F1014. doi: 10.1152/ajprenal.1988.255.5.F1003. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Yoshitomi K., Nakamura M., Imai M. Site and mechanism of action of trichlormethiazide in rabbit distal nephron segments perfused in vitro. J Clin Invest. 1988 Aug;82(2):721–730. doi: 10.1172/JCI113653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton B. A., Biemesderfer D., Wade J. B., Giebisch G. Structural and functional study of the rat distal nephron: effects of potassium adaptation and depletion. Kidney Int. 1981 Jan;19(1):36–48. doi: 10.1038/ki.1981.5. [DOI] [PubMed] [Google Scholar]
- Star R. A., Burg M. B., Knepper M. A. Bicarbonate secretion and chloride absorption by rabbit cortical collecting ducts. Role of chloride/bicarbonate exchange. J Clin Invest. 1985 Sep;76(3):1123–1130. doi: 10.1172/JCI112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson D. L., Steinmetz P. R. Alpha and beta types of carbonic anhydrase-rich cells in turtle bladder. Am J Physiol. 1985 Oct;249(4 Pt 2):F553–F565. doi: 10.1152/ajprenal.1985.249.4.F553. [DOI] [PubMed] [Google Scholar]
- Stokes J. B., Ingram M. J., Williams A. D., Ingram D. Heterogeneity of the rabbit collecting tubule: localization of mineralocorticoid hormone action to the cortical portion. Kidney Int. 1981 Sep;20(3):340–347. doi: 10.1038/ki.1981.144. [DOI] [PubMed] [Google Scholar]
- Stokes J. B. Ion transport by the cortical and outer medullary collecting tubule. Kidney Int. 1982 Nov;22(5):473–484. doi: 10.1038/ki.1982.200. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Anion dependence of rabbit medullary collecting duct acidification. J Clin Invest. 1983 May;71(5):1505–1508. doi: 10.1172/JCI110905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Mineralocorticoid modulation of rabbit medullary collecting duct acidification. A sodium-independent effect. J Clin Invest. 1983 Jul;72(1):77–83. doi: 10.1172/JCI110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlander J. W., Madsen K. M., Low P. S., Allen D. P., Tisher C. C. Immunocytochemical localization of band 3 protein in the rat collecting duct. Am J Physiol. 1988 Jul;255(1 Pt 2):F115–F125. doi: 10.1152/ajprenal.1988.255.1.F115. [DOI] [PubMed] [Google Scholar]
- Wade J. B., O'Neil R. G., Pryor J. L., Boulpaep E. L. Modulation of cell membrane area in renal collecting tubules by corticosteroid hormones. J Cell Biol. 1979 May;81(2):439–445. doi: 10.1083/jcb.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner I. D., Hamm L. L. Regulation of intracellular pH in the rabbit cortical collecting tubule. J Clin Invest. 1990 Jan;85(1):274–281. doi: 10.1172/JCI114423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner I. D., Hamm L. L. Use of fluorescent dye BCECF to measure intracellular pH in cortical collecting tubule. Am J Physiol. 1989 May;256(5 Pt 2):F957–F964. doi: 10.1152/ajprenal.1989.256.5.F957. [DOI] [PubMed] [Google Scholar]


