Abstract
This article defines the term surrogate as an organism, particle, or substance used to study the fate of a pathogen in a specific environment. Pathogenic organisms, nonpathogenic organisms, and innocuous particles have been used as surrogates for a variety of purposes, including studies on survival and transport as well as for method development and as “indicators” of certain conditions. This article develops a qualitative surrogate attribute prioritization process and allows investigators to select a surrogate by systematically detailing the experimental process and prioritizing attributes. The results are described through the use of case studies of various laboratories that have used this process. This article also discusses the history of surrogate and microbial indicator use and outlines the method by which surrogates can be used when conducting a quantitative microbial risk assessment. The ultimate goal of selecting a sufficiently representative surrogate is to improve public health through a health-based risk assessment framework. Under- or overestimating the resistance, inactivation, or movement may negatively impact risk assessments that, in turn, will impact health assessments and estimated safety levels. Reducing uncertainty in a risk assessment is one of the objectives of using surrogates and the ultimate motive for any experiment investigating potential exposure of a pathogen.
INTRODUCTION
The term surrogate is used to indicate a substitute for an item of interest. In the context of environmental microbiology and health risk assessment, we have defined surrogates as organisms, particles, or substances used to study the fate of a pathogen in a specific environment. Early in the history of microbiology, it was realized that fecally contaminated water could transmit infectious disease and that some approach was needed to assess the safety of water (50). The discovery by Escherich of the common occurrence of coliform bacteria (Escherichia coli) in stools and of methods for their isolation from water led to early suggestions for its use as an indicator for waterborne pathogens and as a surrogate for the ability of treatment processes to remove them from drinking water (2). Both pathogenic and nonpathogenic organisms have been used as surrogates for a variety of purposes, including studies on survival (under environmental conditions and during disinfection) and transport as well as for method development and as “indicators” of certain conditions (Table 1). Safety is the major benefit of using nonpathogenic surrogate organisms. Another key to their use is the ability to easily cultivate the organisms, thus allowing the generation of large data sets.
Table 1.
Terms used to describe microbial indicators, index organisms, or other metrics to measure microbial contamination in the environment
| Term | Definition and/or purpose | Reference(s) |
|---|---|---|
| Process indicator | Used in food and water industries to demonstrate the efficacy of a process or if the process has been compromised | 4, 11 |
| Fecal indicator | An organism, such as Escherichia coli, that indicates the presence of fecal contamination | 2 |
| Model organism | Organism behaves in the same manner as a pathogen in a given environment or set of conditions; for example, the use of coliphages to model the behavior of human enteric viruses | 11, 53 |
| Tracer | Used in transport studies; examples include the use of coliphages or spores to trace groundwater movement or spore transport in aerosol | 3, 23, 42, 45 |
| Surrogate for assessment of environmental risk | An organism, particle, or substance used to study the fate of a pathogen in a specific environment | This article |
The pathogens associated with environmental transmission routes (water, food, soil, surfaces, and air) encompass hundreds of bacteria, protozoa, and viruses. Surrogates are essential for the progression of environmental microbiology as a science and for investigating bioterrorism, water quality, occupational health/infection control, food safety, aquatic microbiology, and industrial microbiology. The goals of this article are to (i) summarize the history of surrogate use, (ii) develop a conceptual decision framework to guide selection of appropriate surrogates, (iii) provide a process for prioritizing attributes of surrogates for intended uses, and (iv) provide case studies. Finally, the role that surrogates play in quantitative microbial risk assessment and public health protection will be discussed.
Inspired by previous disinfection work using anthrax spores (43), Harriet Chick used Bacillus anthracis and the nonsporulating Bacillus paratyphosus (now known as Salmonella enterica serovar Typhimurium) to quantify first-order disinfection kinetics using phenol (14). These organisms were used as surrogates to develop the principles for disinfection kinetics for groups of other pathogens. The concept of a fecal indicator was developed in 1885 by Theodor Escherich, a German pediatrician, to study how enteric pathogens spread through water and food. Escherich described motile, rod-shaped bacteria in the feces of newborn and suckling babies which he called Bacterium coli commune, later named Escherichia coli. These were again described in 1892 by Franz Schardinger (50) as characteristic components of the fecal flora whose presence in water could be taken as “an indicator of the presence of fecal pollution and therefore of the potential presence of enteric pathogens.”
The concept of fecal indicator organisms for water was operationally defined in 1901, when the coliform group was defined as Gram-negative, non-spore-forming facultative, anaerobic bacilli that ferment lactose, with production of acid and gas within 48 h (50). Later it was found that organisms defined as such are not always related to fecal contamination, and by 1904, C. Eijkman included a higher incubation temperature of 44°C to improve the specificity of the indicator (15). These fecal coliforms, or thermotolerant coliforms, are considered to be specific to fecal pollution. Table 1 describes other terms commonly used by various groups and agencies.
There is a heightened need for further definition of microbial surrogates because of the trend toward quantification of microbe concentrations for use in risk assessments. In studies of biothreat agents, data are needed to better document the effectiveness of decontamination methods and address the fate of biothreat agents under a wide variety of environmental scenarios. In most laboratories, the use of a specific biothreat agent is not possible because of the need for sophisticated containment and protection to minimize risk to the investigators and the public. Therefore, criteria for the selection and use of surrogates are needed to ensure that they reflect the behavior of the biothreat agents of concern and that safety measures are truly protective.
In the last 2 decades, quantitative microbial risk assessment has evolved as a useful tool for the guidance of water treatment goals for pathogen removal (31) and for assessing the risk from food and other environmental exposures (25). In the QMRA paradigm of hazard identification, exposure, dose response, and risk characterization, exposure assessment has the greatest amount of variability and uncertainty (77). The use of surrogates can help reduce the uncertainties associated with exposure assessment. For example, Tanner et al. (76) used the coliphage MS-2 to assess exposure via aerosols from human enteric pathogenic viruses to workers and nearby populations during the land application of wastewater biosolids under actual field conditions and to describe the distribution of viral inhalation over a specific time interval.
MATERIALS AND METHODS
Conceptual decision framework.
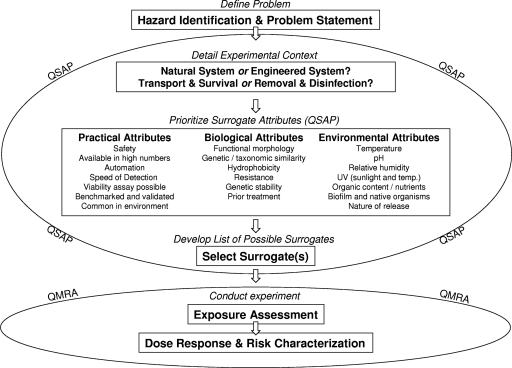
New microbial pathogens and threats and better microbial tools and mathematical approaches require that we establish criteria to better address the choices of surrogates for various evidence-based approaches. We propose a framework using a hypothesis-generating step to assist in selection: definition of the problem or the hazard identification step and the qualitative surrogate attribute prioritization (QSAP) process (Fig. 1). The QSAP process includes detailing the experimental context and prioritizing attributes. To detail the experimental context, a system is described as natural or engineered. The type of experiment is then planned as one of four broad groups: transport, survival, disinfection, and/or removal. The attributes are considered in three domains as practical attributes unique to the experimental setting, biological attributes unique to the organism, and environmental attributes unique to the extrinsic environment of the organism. The QSAP is designed to assist in choosing a surrogate, achieving a group consensus, and having the evidence to justify and support the decision. All attributes and definitions needed in this framework are obtained from previously published data or expert opinion. The case study section is designed to assess the conceptual framework as it applies to previously published selection strategies.
Fig 1.
Conceptual decision framework for selecting a surrogate.
Defining the problem.
Identification of the microbial pathogen/hazards is the first step in the surrogate selection process, followed by the question or hypothesis. Before a surrogate is selected, the pathogenic microbes in question, the types of diseases they are associated with, and their ability to be transmitted via the environment (air, water, food, fomites, etc.) must be identified.
Detailing the experimental context. (i) Describing the natural or engineered system.
After the pathogen(s) is identified, the environmental or engineered system should be described. Natural systems, such as recreational water contaminated with an enteric virus, elicit a much different QSAP then an engineered system, such as a drinking water distribution network contaminated with the same virus. Because there are different environmental attributes affecting both of these types of systems, the criteria for a surrogate may be very different.
(ii) Problem formulation.
The hypothesis should be clearly stated, as the type of surrogate to be selected depends on the type of questions to be answered and the information that is desired. For example, a particular surrogate may be useful for assessment of decontamination (disinfection) of an environment because of its extreme resistance, but it may not be useful to assess survival in a particular environment (e.g., water) because it may greatly overestimate the survival of the target pathogen. For this reason, it is necessary to first define the purpose or application of the study through the surrogate decision framework. Next, the practical, biological, and environmental attributes of the surrogate should be determined.
Assessment of environmental survival and transport. (i) Survival.
An understanding of the survival of organism populations in the natural or engineered system is one of the critical questions in this context. Survival—or the opposite term, decay—is described as a rate (loss of viability per unit of time), with the proportion of the number of live organisms over the total number of organisms at a given time. A number of factors, including temperature and moisture, influence this rate, and different microbes have different resistances to environmental stressors.
It is necessary to assess the survival of pathogens in the air, water, soil, fomites, food, or other environments to estimate exposure after release. Temperature is often the rate-controlling parameter for many chemical and physical processes (57) and is useful for modeling microbial decay rates of organism populations that cannot normally replicate outside a host. Relative humidity, water activity, and desiccation are additional environmental parameters that should be prioritized for survival studies in air, in food, and on fomites (7, 53). UV light and other factors are discussed as attributes to consider in the environmental attributes section (see below).
(ii) Transport.
The term “transport” assesses distance and spatial distribution in reference to the organism's concentration at a certain time after its release. It focuses on movement of an organism as distinct from viability. Therefore, when studying transport, inert particles, such as latex spheres, tracer solutes, or other inanimate substitutes, have been used as surrogates. In this approach, it is assumed that viability and transport are independent processes. For assessments involving ground and surface water transport, coliphages, enteric bacteria, and Bacillus subtilis spores (6, 42, 56) have often been proposed as live surrogates. Unique approaches, such as laboratory-constructed norovirus capsids, have been used to study virus transport through soil (59), while cauliflower mosaic virus was used as a surrogate to assess virus transport in day care centers (39, 55). The transport of an aerosol release of Bacillus anthracis has used aerial insecticide spray of Bacillus thuringiensis as a surrogate (46). Other studies have used indigenous bacteria, protozoa, and fluorescent latex spheres to investigate transport in indoor environments and ground water (33, 34).
These live tracers have several advantages over chemical tracers, including low detection limits, no toxic effects, and a finite lifetime (42). Perhaps the most commonly used surrogates are bacteriophages due to their ability to be produced in large numbers at low cost, their ease of detection, and their nonpathogenicity (72).
(iii) Disinfection.
The term “disinfection” describes the physical or chemical killing of microorganisms and does not necessarily imply complete destruction of all microorganisms (48). Common disinfectants, including chlorine, chlorine dioxide, ozone, UV, ammonia, and metals such as silver, copper, and zinc, are used for water and surfaces and for washing and laundering. Disinfection studies often evaluate the use of disinfectants through studies investigating the effectiveness of decontamination of surfaces, investigating the necessary applied dose of radiant energy (e.g., electron beam, gamma, or UV light), evaluating point-of-use microbiological water purifiers, and assessing wastewater or drinking water treatment plants. Disinfection efficacy is influenced by environmental (e.g., pH) and biological attributes of the organism. The organisms' resistance to disinfectants is often intrinsic. Compounds such as chlorine or other halogen oxidizers, quaternary ammonium compounds, UV, antimicrobials, and many others can stop metabolism, destroy membranes, damage DNA, lyse, desiccate, or have many other debilitating effects (5, 48, 82). The effectiveness of a disinfectant is often highly dependent on the nature of the medium and the environment, in which pH, time, temperature, and the presence of interfering substances (organics or particulates) can have an effect. The resistance of microorganisms to common chemical disinfectants generally proceeds in the following order, from most resistant to least resistant (48): bacterial spores → protozoan cysts → viruses → bacterial cells. This does not consider UV disinfection or physical processes.
Selecting attributes.
Using this paper's framework, it is possible to prioritize needs and select a surrogate. As detailed in Fig. 1, the first step is to identify the hazard. The second is to define and select the system in which the hazard exists. The third step defines the purpose of the study and hypothesis (i.e., removal, disinfection, transport, or survival). After the purpose of the study is defined, the attributes necessary for the study can be prioritized. Prioritizing attributes significantly helps narrow down the surrogate selection process. The prioritization assists in the decision of whether a single surrogate organism is sufficient or whether multiple organisms are necessary.
Once the data needs are defined, the practical, biological, and environmental sensitivities of surrogates can be selected and prioritized. Each application/purpose (disinfection, transport, and survival) will have certain dominant attributes. For example, the environmental attribute of relative humidity is appropriate for an aerosol survival study while not meaningful for a water disinfection study. Practical attributes include the safety, ease of use, and ability to benchmark (i.e., demonstrate similar behavior under the study conditions). The biological attributes include functional morphology, taxonomic and genetic similarity, the organism's resistance, hydrophobicity, life cycle stage, stability, and other considerations intrinsic to the microorganisms. The environmental sensitivities of a surrogate are the factors extrinsic to the organism, such as temperature, pH, relative humidity, UV, organic content and nutrients, biofilm and other native organisms, and the nature of the release.
Practical attributes. (i) Safety.
The foremost practical reason for selecting a surrogate is that it will not cause illness or infection in humans, animals, and plants. This allows flexibility with minimum laboratory safety needs and the potential to be used outside the laboratory. An example is the use of Bacillus thuringiensis as a surrogate for Bacillus anthracis. B. thuringiensis is not pathogenic, is a biosafety level 1 (BSL1) agent, and is easily obtained (62).
Although selecting a surrogate with no harmful effects is simple in principle, achieving this is sometimes difficult because many bacteria thought to be nonpathogenic, such as Lactobacillus spp., can cause opportunistic infections (37). An example is the 1949 U.S. military release of Serratia marcescens as a surrogate for a biological warfare agent in the San Francisco Bay (60) which resulted in an estimated 10 hospitalizations and an alleged fatality. A similarly cautious approach is considered when using live vaccines, which also have a possibility of adverse reactions in some individuals if they become infected. Safety considerations are necessary to avoid political, security, and public relation issues that may arise if the surrogate is to be used outside a controlled environment.
(ii) Ease of use.
Ease of use is a second practical attribute where “ease” refers to the organism's ability to be produced in high numbers, the speed and simplicity in detection, the availability of simple viability assays, the availability of an automated method for a large number of samples, and the lack of adverse effects for industrial process equipment (e.g., food processing equipment).
The ability to produce large numbers of surrogates at low cost is also important when it is necessary to determine large reductions in initial concentrations (e.g., disinfection studies) and because of dilution and dispersion that occur in transport studies, especially under field conditions (e.g., groundwater transport studies). Bacteriophages, such as MS-2, can be produced to concentrations as high as 1014 per ml, and a single phage can be detected in up to 100-ml volumes (26). This allows for practical detection at dilutions of up to 1016. Bacteria such as Escherichia coli can be practically produced in most laboratories in concentrations of 1012 CFU per milliliter.
Viability assays must be used for studies on survival, decontamination, or efficacy of antimicrobial products. This makes it necessary to use cultivation techniques for which the precision and accuracy of the assay depend on the method and the organism. A variety of methods which have very defined standard errors are readily available. The use of a flow cytometer, detection by PCR, and spectrophotometric methods does not provide information on viability, lacks sensitivity (e.g., only small volumes can be assayed), or is not specific to the surrogate. Despite the limitations, these assays are automated, offer the advantage of high-throughput processing of samples, and may be used in studies where viability may not be essential (e.g., tracing water or air circulation).
Surrogates are often released into the environment to evaluate decontamination or disinfection achieved within large-scale (water treatment plant) or industrial (food processing) processes. In these cases, the surrogate should not damage or contaminate the equipment or processes or at least the equipment should be able to be easily decontaminated. Surrogates that cause undesirable biofilm formation, taste, odor, physicochemical changes, or an opportunistic infection should be avoided.
In some cases, the surrogate should not have long-term persistence in the environment. It should be predictable, easily detected, and decontaminated without jeopardizing industrial equipment or natural environments (11). In these cases, nonviable surrogates, such as polyvinyl spheres, are useful because their persistence poses no concern for infection of laboratory personnel, environmental pollution, or mutation.
(iii) Benchmarking.
The process of benchmarking establishes a surrogate as a substitute for a standard target pathogen in a set of user-defined conditions. Performance of the surrogate is then measured as a benchmarked substitute for the standard target pathogen. Benchmarking does not require access to the target organism, while a true “validation” of the surrogate requires access to the target pathogen and the surrogate in the same experimental setting. Benchmarking and validation are described in Table 2 as functions of thorough investigations and experimental access to the target pathogen.
Table 2.
Possible types of surrogate benchmarking and validation experiments
| Type | Description |
|---|---|
| Benchmarking 1 | Conservative approach choosing the most resistant, persistent, and robust surrogate without exptl access to the organism |
| Benchmarking 2 | Through a literature review, demonstrating that the surrogate possesses the same exptl attributes as the target pathogen without access to the organism |
| Benchmarking 3 | Demonstrating and documenting the range of surrogate behaviors with confidence limits and then placing the target pathogens in the appropriate range on a full spectrum; does not require access to the target pathogen |
| Validation | Experimentally comparing the surrogate and the target pathogen under the same conditions in the same laboratory |
All three types of benchmarking should start with a review of the literature and then a prioritization of experimental attributes matched to the specific experiment. For example, if the survival of a target pathogen is being evaluated, benchmarked surrogates should have similar survival attributes, such as persistence. Conversely, if the performance of a chemical disinfectant is being evaluated, a more generic and conservative surrogate which survives longer than the target organism may be chosen.
Before any comparative efforts are initiated, it is necessary to show that the regression equation obeys assumptions of normality (30). It is useful to determine the best-fit predictions with confidence regions. As discussed in the disinfection analysis, the nonlinear least-squares method and the more robust maximum likelihood method are useful to assess the performance of the chosen kinetics. After this, a covariance analysis can be used to compare the two inactivation or survivability rates (62). Because covariance is a measure of how two variables change together, it is necessary to use the same inactivation model for the two compared organisms. A recent U.S. Environmental Protection Agency validation of surrogates used this method and superimposed inactivation curves of the surrogate onto the published UV inactivation curves of Bacillus subtilis from the European standard biodosimetry studies (62).
Biological attributes.
The biological attributes of interest for a surrogate include genetics and taxonomy, which can influence functional morphology and the surrogate's resistance or stability under a defined set of experimental conditions. Functional morphology includes virulence factors, receptors, and special proteins, all of which may be related to the organism's behavior in the environment. Functional morphology is defined here as its shape, size, surface properties, hydrophobicity, nucleic acid structure, and life cycle form (spores, vegetative, cysts, etc).
(i) Genetics and taxonomy.
The genetic code controls an organism's functional morphology, its persistence in stressful environments, its virulence, and many other traits. In most cases, there is not enough information about the genetic heterogeneity of many organisms, which could result in drastically different virulence or persistence levels. Only in a select few organisms is the genetic code known to a level where these differences can be characterized as phenotypic differences. For example, many members of the Bacillus group of bacteria, including Bacillus anthracis, B. thuringiensis, and Bacillus cereus organisms, have very similar genomes which have been distinguished only by genes carried on the plasmids. The characteristic lethality of B. anthracis, the insecticidal crystal-forming proteins of B. thuringiensis, and the characteristic opportunistic ability of B. cereus are all genetic traits unique to plasmids (36).
The genetic similarity of these organisms makes B. thuringiensis a useful surrogate for B. anthracis under some environmental situations; however, a surrogate's genetic relatedness and genetic distance should not be the only attributes used for a benchmark or validation justification to a target organism. Two examples are the two bacteriophages MS-2 and F2, which are genetically very similar (41) but which exhibit different resistances in various survival and disinfection settings (3). Also, Bacillus cereus has long filaments which make its transport in aerosols and water much different than that of the genetically similar B. thuringiensis or B. anthracis (10, 58, 61, 86). Because genetic relatedness does not always justify a good surrogate/pathogen match, surrogates should be selected based on a collection of other characteristics. The selected surrogate should be genetically stable, readily obtainable, and cultivatable. Genetics should not be completely ignored; propagation protocols should have limited risk of mutation, allowing for repeatable experimental conditions and results.
(ii) Functional morphology.
Functional morphology is defined as the study of the relationship between form and function of organisms and/or their parts (69). Considering this definition, a microbe's shape and size govern its ability to be transported independently or transported through its interaction and attachment to other particles in its environment. Cell mass is also important, as it affects settling in air and water. Major morphological differences can exist at the genus level. In the Bacillus group, for example, structures such as filamentous appendages, exosporia, size, and shape vary between spores of different species (32) and thereby affect survival, disinfection, and transport in different ways.
(iii) Hydrophobicity and isoelectric point.
Attraction to interfaces is governed, at least initially, by the isoelectric point and hydrophobicity. These two factors are also important in the transport of organisms through both air and water. As surrogates interact with interfaces, hydrophobicity and net surface charge become important (21). Generally, an organism with a lower isoelectric point and lower hydrophobicity travels greater distances because of fewer interactions with common environmental interfaces (16, 46). For example, most soil particles exhibit a negative charge and are hydrophobic (e.g., sand, organic particulates, most common clays). Some suggest that coliphages with a more hydrophobic viral capsid are attracted to air-water interfaces where denaturalization of the capsid proteins occurs (39). The more hydrophobic a virus is, the less likely it will be transported through unsaturated soils because of its retention at air-soil-water interfaces (22). Surface charges and molecular structure also play a role in hydrophobicity. Attractive forces may cause spores to clump or adhere to surfaces. As the organisms clump, their physical and biological behaviors change and may require a reevaluation of surrogate selection (47, 74, 83).
Studies with E. coli and Bacillus cereus spores show that the hydrophobicity of the organism, the surface, and the suspending medium can all interact to affect behavior toward inanimate surfaces (18, 19).
(iv) Preparation of organisms.
Treatment of organisms prior to use in survival studies is an important variable (67, 73). The preparation and handling of any particular surrogate can alter the measured response of the surrogate to the disinfectant agents, the environment, or its transport in a particular environment. Any benchmarking or comparison between organisms must address the potential differences between strains and consider parallel tests with identical conditions for growth, storage, sporulation and/or propagation, and purification (40, 62). If these differences are not appropriately addressed, the comparison can be invalid. Common mistakes occur because of a failure to describe methods, such as if the organism was centrifuged, the nature of the suspending medium, if the isolation medium is selective or specific for stressed organisms, and other similar factors. These can all influence viability, validity of a benchmarking effort, and the ratio of healthy to injured organisms.
Environmental attributes.
The final attribute to consider for selection of a surrogate is the environment which the pathogenic organism inhabits or the engineered or natural system under study. The physical and chemical composition of the surrounding liquid, air, or solid will always affect the survival of organisms. Because the list of environmental factors that affect organisms is large, we attempted to limit this list to the most common parameters that have the largest impact. These include the pH, temperature, relative humidity (desiccation and evaporation), UV (sunlight and lamp UV), organic matter, nutrients, water/air currents, biofilm, turbidity (particulates), the nature of the release, and the preparation of the organism.
(i) pH.
The pH of the surrounding environment affects the net charge of the organism's interface with the environment. This may lead to denaturing proteins and nucleic acids or affect the organisms' net charge and transport. Most organisms exhibit the greatest stability at near-neutral pH. Most enteric viruses are stable between pH 3 to 9, but in contrast, many respiratory viruses are not stable below pH 6.0 (51). A pH change can also affect other factors in the surrounding environment which may additionally damage the microorganism. This is often the case with some metals, which may have a more germicidal effect, once the pH drops below a certain point due to changes in the chemical speciation of the metal. One important consideration is that some pathogens, such as the Salmonella genus, can have stress response mechanisms when exposed to lethal acidic conditions. This is known as the cross-protection phenomenon, in which these acid-adapted cells become tolerant to multiple “heat, cold, osmotic and oxidative stresses” (84). Although there may be other pathogens and potential microbial surrogates that can exhibit this response, the consideration is not relevant to experimental contexts involving transport or removal.
(ii) Temperature.
Temperature stability is a dominating factor in all media. It controls most chemical and biological processes and can be described using the inactivation models previously mentioned. These inactivation models can predict the survival of pathogens in the environment for given temperatures (48). High temperatures denature proteins and nucleic acids, while only a few possibilities exist for resistant organisms, such as bacterial spores, parvovirus, and Bacillus stearothermophilus spores (11, 51). Additionally, the pH and cation/anion balance associated with the external environment can stabilize or destabilize proteins, a process which can therefore increase or decrease resistance to thermal inactivation (17).
(iii) Relative humidity.
Desiccation and relative humidity are critical controlling factors affecting survival in aerosols and on fomites. The desiccation rate on surfaces or in aerosols is determined principally by relative humidity and to some degree by the fluid in which the organisms were initially suspended. The loss of water causes a denaturation of proteins in which rapid aerosolization exposes the organism to damaging air-water interface effects (52). Generally, as the rate of evaporation increases, the loss of viability tends to increase. In the case of enteric and respiratory viruses, it appears that the greatest degree of inactivation on fomites occurs during this period (7). Some organisms survive in aerosols and on fomites within certain ranges of relative humidity. Thus, influenza virus survives best at relative humidity levels of 50% (68), while poliovirus survives best at a higher relative humidity of above 85%. Some viruses, such as smallpox, survive best at midrange relative humidity, and others survive only at high and low relative humidity (52, 70).
(iv) Mechanism of release.
The mechanism of organism release is also important, as a sneeze or other host organism efflux will have various moisture and other organic particles to propel the organism, while environmental characteristics, such as wind, settling, dilution, and currents, disperse the organism. A surrogate of pathogen risk in lakes was shown to be relevant only if it shares similar aggregation and settling characteristics with the pathogen of concern (9).
(v) UV light.
If exposure to sunlight is a factor, then the organism's resistance to UV light should be considered in engineered and natural systems (65). Exposure to UV-C and UV-B wavelengths causes cross-links among nucleotides (24, 64), while other UV-A light forms oxidized species in solution which can be lethal to microorganisms (38). UV was an important consideration in modeling virus transport in a natural river system using a bacteriophage as a surrogate (71). In engineered systems, MS-2 coliphage, E. coli, and Bacillus subtilis have been used as surrogates to measure the UV light dose for water disinfection (81).
(vi) Organic content.
The organic content of liquid and soil matrices can affect microorganisms in various ways. If an organism is subject to disinfection in sewage, the high organic content will buffer the processes by combining with ammonia and organic substances, increasing the need for more chlorine (12). The high organic content can also supply nutrients for the organism or for active microbial communities, which may support the growth of microbial predators and grazers. The organic matter can also neutralize antagonistic substances (51).
(vii) Biofilms.
Natural microenvironments may develop in various media. The possibility of predator-prey relationships and biofilm formation should always be considered. Surface properties, such as porosity and roughness, can also affect these parameters when surrogates are selected for engineered water systems. Biofilm studies of water distribution systems often find microbial communities growing on the rough inner surfaces of municipal cast iron and galvanized pipes. These communities were shown to thrive in many types of water, including those with relatively low levels of organic content (44).
(viii) Turbidity and particulates.
In water systems, turbidity can protect organisms from UV light while necessitating a higher chlorine dose for chemical disinfection. Organisms can also become associated with suspended particulates and be transported with them in liquid or aerosol environments. Turbidity and particle size have also been used as part of a surrogate group to predict the presence of Cryptosporidium and other pathogens in lakes and reservoirs (9).
(ix) Interfaces.
Depending on the organism, there is usually greater stability at the solid-water interface and less stability at the air-water interface. This stability depends on the nature of the organism's outer surface and its resistance to damage or denaturation (78, 79).
There are three major factors affecting a surrogate's viability after introduction into an environment with existing indigenous organisms: competition for nutrients, predation, and production of antagonistic substances (48). Examples include regrowth of Salmonella in compost (85), bacterial ingestion by protozoa (29, 54), and antagonistic substances produced by marine bacteria against enteric viruses (20).
Inoculating surrogates into a complex natural system can result in many possible antagonistic, synergistic, or other consequences, and it is recommended to quantify the titer immediately after inoculation. Another consideration is the possibility of interactions and synergism, which occur in the natural environment.
RESULTS
Case studies.
Case studies of previously selected surrogates illustrate the decision process that was used to select surrogates. Case studies are presented below in terms of the problem statement, their experimental context, the attributes, and the selected surrogate. These case studies are summarized in Table 3.
Table 3.
Examples of surrogate selection
| Case study (reference[s]) | Type of expt | Medium | Target organism | Practical attributes | Biological attribute(s) | Environmental Attributes | Surrogate choice |
|---|---|---|---|---|---|---|---|
| Fomite survival of anthrax spores (26) | Environmental survival | Fomites | B. anthracis | Safety, cost, ease, benchmarked | Morphology, genetic relationship | Bacillus thuringiensis | |
| Standard guide and protocol for microbiological water purifiers (88) | Disinfection | Water | Coliform group | Common in environment, human health | Taxonomic similarity | pH, temp, organic content, turbidity | Klebsiella terrigena |
| Disinfection | Water | Virus group | Common in environment, human health | Taxonomic similarity | pH, temp, organic content, turbidity | Poliovirus, rotavirus | |
| Disinfection | Water | Cyst group | Common in environment, human health | Resistance, functional morphology | pH, UV, temp, organic content, turbidity | Cryptosporidium parvum | |
| Surrogates for disinfection—Department of Homeland Security (63, 68) | Disinfection | Water | B. anthracis | Safety, cost, benchmarked | Genetic/taxonomic similarity, resistance, morphology | Bacillus thuringiensis | |
| Surrogates for sterilization—Food and Drug Administration (91) | Disinfection | Food | Spoilage organisms | Safety, cost | Resistance, viable/cultivable | Geobacillus stearothermophilus |
Environmental persistence of Bacillus anthracis—surrogate selection for bioterrorism vulnerability.
With recent attention to our society's vulnerability to bioterrorism, much effort has been focused on characterizing the survival, transport, and decontamination options of biological agents of concern (BAC) in the environment (75). Currently, the BAC of the highest priority are categorized by the United States Centers for Disease Control and Prevention as category A select agents (66). Unfortunately, it is not possible to meet the personal safety precautions and expenses required to work with live pathogens because most BAC are biological safety level 3 (BSL3) (63). While this information is essential for risk management, studies are difficult because of potential exposures to the agent, the difficulty and cost in culturing the organism, and the efficacy, difficulty, and cost of performing experiments under BSL3 conditions.
As an example, a study was conducted with the objective to validate a surrogate for B. anthracis survival on common environmental surfaces, conducting parallel B. anthracis and B. thuringiensis (surrogate) studies in a BSL3 facility. An experiment was designed to investigate survival on multiple types of surfaces inside and outside a BSL3 laboratory. The experimental context involved investigating the survival of organisms on a natural fomite system with multiple environmental attributes. Decontamination and removal were also issues to be investigated.
An extensive literature search and previous experiments motivated the investigators to prioritize attributes focusing on genetic/taxonomic similarity and functional morphology (10, 13, 58, 61, 86). They concluded that Bacillus thuringiensis would make the best surrogate for B. anthracis because it is genetically very close and also expresses a very similar physical structure. It was thought that the two organisms would behave similarly in survival and transport experiments. B. thuringiensis also has the attributes of being safe to use, being available in high numbers, having the possibility of simple viability assays, and being common in the environment. A negative attribute to consider is that if experiments are not tightly controlled, the spores are easily aerosolized and can contaminate a large portion of the laboratory.
In a review of surrogate selection for Bacillus anthracis, one group (27) describes how Bacillus atrophaeus was initially chosen. This species is somewhat close phylogenetically, completely innocuous, and ubiquitous. However, B. atrophaeus is not the ideal candidate for all types of experiments. For example, B. atrophaeus has greater survivability with chlorine exposure and is therefore a more conservative surrogate for disinfection studies (8, 49). In contrast, B. atrophaeus cells are considerably smaller than those of B. anthracis and would not make an ideal surrogate during transport experiments.
Standard guide and protocol—surrogates for the registration of products or processes.
Portable and home water treatment devices (point-of-use treatment) have been developed to protect consumers from waterborne pathogens (1). Because there are more than 150 enteric pathogens that can be transmitted by drinking water, surrogates are a must to evaluate treatment approaches (80). To assess the registration of point-of-use microbiological purifiers, testing criteria were established based upon surrogates used to represent the different microbial classes of waterborne pathogens (i.e., viruses, bacteria, and protozoa). Before registration of such products, it must be demonstrated that such devices or treatments are capable of removing these surrogates to a level defined by the U.S. EPA (80). The experimental context involves conducting disinfection or removal tests of these engineered systems. The U.S. EPA developed guidelines so that these drinking water purifier technologies can comply with the Safe Drinking Water Act requirements (80) and be officially registered.
To qualify as a microbiological water purifier, the units must achieve a 6-log reduction in bacteria, 3-log reduction in viruses, and 2.5-log reduction in cysts (80). The tests require that the selected surrogates represent practical, biological, and environmental attributes that are very similar to those of the pathogens. The resistance of the organism and its functional morphology are discussed as the biological attributes, while the majority of the document focuses on environmental attributes, such as pH, temperature, organic matter, and turbidity of the test water.
When selecting surrogates for the U.S. EPA standard guideline, the following surrogates were chosen for the challenge test setup: Klebsiella terrigena was chosen for the coliform group, Cryptosporidium parvum was chosen for the cyst group, and poliovirus or rotavirus was chosen for the virus group. A coliform bacterium was selected to represent waterborne bacteria, and Cryptosporidium (a protozoan) was selected to represent a waterborne parasite. Originally, Giardia was used, but the surrogate was later changed to Cryptosporidium as its cells are smaller and more resistant to chemical disinfectants. It was stated that enteric viruses are more resistant to common treatment methods (i.e., filtration, disinfection, heat, and UV light) than enteric bacteria and protozoa, so two surrogate enteric viruses were used, i.e., a poliovirus type 1 vaccine strain and a simian rotavirus (nonhuman pathogen). They were selected because of differences in size, nucleic acid composition, and capsid composition.
DISCUSSION
The ultimate goal of selecting a sufficiently representative surrogate is to improve public health through a health-based risk assessment framework. Under- or overestimating the resistance, inactivation, or movement may negatively impact risk assessments that, in turn, will impact health assessments and estimated safety levels. Reducing uncertainty in a risk assessment is one of the objectives of using surrogates and the ultimate motive for any experiment investigating potential exposure of a pathogen.
Quantitative data are needed to develop and validate models for agents by routes or environments. This becomes an issue, in particular, for potential bioterrorist agents for which data are nonexistent (28). This is problematic because exposure to these organisms may occur by nonnatural routes of transmission, such as aerosol exposure by yellow fever virus (35). The use of surrogates in these scenarios can allow quantification of the degree of exposure and assist in the development of appropriate control strategies (i.e., water treatment, fomite decontamination, or food processing methods).
ACKNOWLEDGMENTS
This study was supported by the Center for Advancing Microbial Risk Assessment, funded by the U.S. Environmental Protection Agency Science to Achieve Results and U.S. Department of Homeland Security University Programs grant number R3236201. Ryan G. Sinclair was supported through the National Research Council's Research Associate Program with funding from the U.S. Department of Homeland Security.
Footnotes
Published ahead of print 13 January 2012
REFERENCES
- 1. Abbaszadegan M, et al. 1997. The disinfection efficacy of a point-of-use water treatment system against bacterial, viral and protozoan waterborne pathogens. Water Res. 31:574–582 [Google Scholar]
- 2. Ashbolt N, Grabow W, Snozzi M. 2001. Indicators of microbial water quality, p 289–316 In Fewtrell L, Bartram J. (ed), Water quality: guidelines, standards and health. IWA Publishing, London, United Kingdom [Google Scholar]
- 3. Bales RC, Gerba CP, Grondin GH, Jensen SL. 1989. Bacteriophage transport in sandy soil and fractured tuff. Appl. Environ. Microbiol. 55:2061–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banks JG, Board RG. 1983. The incidence and level of contamination of British fresh sausages and ingredients with salmonellas. J. Hyg. (Lond.) 90:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bitton G. 2005. Wastewater microbiology, 3rd ed, p 23 Wiley-Liss, John Wiley & Sons, Hoboken, NJ [Google Scholar]
- 6. Blanford WJ, Brusseau ML, Yeh TCJ, Gerba CP, Harvey R. 2005. Influence of water chemistry and travel distance on bacteriophage PRD-1 transport in a sandy aquifer. Water Res. 39:2345–2357 [DOI] [PubMed] [Google Scholar]
- 7. Boone SA, Gerba CP. 2007. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 73:1687–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brazis AR, Leslie JE, Kabler PW, Woodward RL. 1958. The inactivation of spores of Bacillus globigii and Bacillus anthracis by free available chlorine. Appl. Microbiol. 6:338–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brookes JD, et al. 2005. Relative value of surrogate indicators for detecting pathogens in lakes and reservoirs. Environ. Sci. Technol. 39:8614–8621 [DOI] [PubMed] [Google Scholar]
- 10. Bulla LA, St Julian G, Rhodes RA, Hesseltine CW. 1969. Scanning electron and phase-contrast microscopy of bacterial spores. Appl. Microbiol. 18:490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Busta FF, et al. 2003. The use of indicators and surrogate microorganisms for the evaluation of pathogens in fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2:179–185 [Google Scholar]
- 12. Carpenter C, Fayer R, Trout J, Beach MJ. 1999. Chlorine disinfection of recreational water for Cryptosporidium parvum. Emerg. Infect. Dis. 5:579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carrera M, Zandomeni RO, Sagripanti JL. 2007. Difference between the spore sizes of Bacillus anthracis and other Bacillus species. J. Appl. Microbiol. 102:303. [DOI] [PubMed] [Google Scholar]
- 14. Chick H. 1908. An investigation of the laws of disinfection. J. Hyg. 8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clegg L, Sherwood H. 1939. Incubation at 44°C as a test for faecal coli. J. Hyg. 39:361–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cox CS. 1995. Physical aspects of bioaerosols, p 15–25 In Cox CS, Wathes CM. (ed), Bioaerosols handbook. Lewis Publishers, Boca Raton, FL [Google Scholar]
- 17. Estes MK, Graham DY, Smith EM, Gerba CP. 1979. Rotavirus stability and inactivation. J. Gen. Virol. 43:403–409 [DOI] [PubMed] [Google Scholar]
- 18. Faille C, et al. 2002. Adhesion of Bacillus spores and Escherichia coli cells to inert surfaces: role of surface hydrophobicity. Can. J. Microbiol. 48:728–738 [DOI] [PubMed] [Google Scholar]
- 19. Faille C, Membre JM, Kubaczka M, Gavini F. 2002. Altered ability of Bacillus cereus spores to grow under unfavorable conditions (presence of nisin, low temperature, acidic pH, presence of NaCl) following heat treatment during sporulation. J. Food. Prot. 65:1930–1936 [DOI] [PubMed] [Google Scholar]
- 20. Fujioka RS, Loh PC, Lau L. 1980. Survival of human enteroviruses in the Hawaiian ocean environment: evidence for virus-inactivating microorganisms. Appl. Environ. Microbiol. 39:1105–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gassilloud B, Gantzer C. 2005. Adhesion-aggregation and inactivation of poliovirus 1 in groundwater stored in a hydrophobic container. Appl. Environ. Microbiol. 71:912–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerba CP. 1984. Applied and theoretical aspects of virus adsorption to surfaces. Adv. Appl. Microbiol. 30:133–168 [DOI] [PubMed] [Google Scholar]
- 23. Gerba CP. 1983. Virus survival and transport in groundwater. Dev. Ind. Microbiol. 24:247–251 [Google Scholar]
- 24. Gerba CP, Gramos DM, Nwachuku N. 2002. Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Appl. Environ. Microbiol. 68:5167–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gibson LL, Rose JB, Haas CN, Gerba CP, Rusin PA. 2002. Quantitative assessment of risk reduction from hand washing with antibacterial soaps. J. Appl. Microbiol. 92:136S–143S [PubMed] [Google Scholar]
- 26. Grabow W, Coubrough P. 1986. Practical direct plaque assay for coliphages in 100-ml samples of drinking water. Appl. Environ. Microbiol. 52:430–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenberg DL, Busch JD, Keim P, Wagner DM. 2010. Identifying experimental surrogates for Bacillus anthracis spores: a review. Invest. Genet. 1:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haas CN, Rose J, Gerba CP. 1999. Quantitative microbial risk assessment, p 137 Wiley, New York, NY [Google Scholar]
- 29. Haas CN. 1981. Application of predator-prey models to disinfection. J. Water Pollut. Control Fed. 53:378–386 [Google Scholar]
- 30. Haas CN, Hornberger J, Anmangandla U, Heath M, Jacangelo J. 1993. Experimental methodologies for the determination of disinfection effectiveness. American Water Works Association, Denver, CO [Google Scholar]
- 31. Haas CN, Rose JB, Gerba C, Regli S. 1993. Risk assessment of virus in drinking water. Risk Anal. 13:545–552 [DOI] [PubMed] [Google Scholar]
- 32. Hachisuka Y, Kozuka S, Tsujikawa M. 1984. Exosporia and appendages of spores of Bacillus species. Microbiol. Immunol. 28:619–624 [DOI] [PubMed] [Google Scholar]
- 33. Harvey R. 1997. Microorganisms as tracers in groundwater injection and recovery experiments: a review. FEMS Microbiol. Rev. 20:461–472 [DOI] [PubMed] [Google Scholar]
- 34. Harvey RW, Kinner NE, Bunn A, Macdonald D, Metge D. 1995. Transport behavior of groundwater protozoa and protozoan-sized microspheres in sandy aquifer sediments. Appl. Environ. Microbiol. 61:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hearn HJ, Chappell WA, Demchak P, Kominik JW. 1966. Attenuation of aerosolized yellow fever virus after passage in cell culture. Bacteriol. Rev. 30:615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Helgason E, et al. 2000. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl. Environ. Microbiol. 66:2627–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Husni RN, Gordon SM, Washington JA, Longworth DL. 1997. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin. Infect. Dis. 25:1048–1055 [DOI] [PubMed] [Google Scholar]
- 38. Jagger J. 1967. Introduction to research in ultraviolet photobiology, p 100–105 Prentice-Hall, Inc., Englewood Cliffs, NJ [Google Scholar]
- 39. Jiang X, et al. 1998. Pathogen transmission in child care settings studied by using a cauliflower virus DNA as a surrogate marker. J. Infect. Dis. 177:881–888 [DOI] [PubMed] [Google Scholar]
- 40. Johns DE, Rose JB. 2005. Review of factors affecting microbial survival in groundwater. Environ. Sci. Technol. 39:7345–7346 [DOI] [PubMed] [Google Scholar]
- 41. Jou WM, Fiers W. 1976. Studies on the bacteriophage MS2: XXXIII. Comparison of the nucleotide sequences in related bacteriophage RNAs. J. Mol. Biol. 106:1047–1060 [DOI] [PubMed] [Google Scholar]
- 42. Keswick BH, Gerba CP, DuPont HL, Rose JB. 1984. Detection of enteric viruses in treated drinking water. Appl. Environ. Microbiol. 47:1290–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kronig B, Paul TL. 1897. The chemical foundations of the study of disinfection and of the action of poisons. Z. Hyg. 25:1–112 [Google Scholar]
- 44. LeChevallier MW, Welch NJ, Smith DB. 1996. Full-scale studies of factors related to coliform regrowth in drinking water. Appl. Environ. Microbiol. 62:2201–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Levin DB, Valadares de Amorim G. 2003. Potential for aerosol dissemination of biological weapons: lessons from biological control of insects. Biosecur. Bioterror. 1:37–42 [DOI] [PubMed] [Google Scholar]
- 46. Lighthart B. 1994. Physics of microbial bioaerosols, p 5–27 In Lighthart B, Mohr AJ. (ed), Atmosphere microbial aerosols. Chapman and Hall, New York, NY [Google Scholar]
- 47. Lytle DA, Johnson CH, Rice EW. 2002. A systematic comparison of the electrokinetic properties of environmentally important microorganisms in water. Colloids Surf. B Biointerfaces 24:91–101 [Google Scholar]
- 48. Maier RM, Pepper IL, Gerba CP. 2009. Environmental microbiology, p 539–551 Academic Press, San Diego, CA [Google Scholar]
- 49. Majcher MR, Bernard KA, Sattar SA. 2008. Identification by quantitative carrier test of surrogate spore-forming bacteria to assess sporicidal chemicals for use against Bacillus anthracis. Appl. Environ. Microbiol. 74:676–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Medema G, et al. 2003. Safe drinking water: an ongoing challenge, p 1–20 World Health Organization, Geneva, Switzerland: http://www.who.int/water_sanitation_health/dwq/9241546301_chap1.pdf [Google Scholar]
- 51. Melnick JL, Gerba CP. 1980. The ecology of enteroviruses in natural-waters. Crit. Rev. Environ. Control 10:65–93 [Google Scholar]
- 52. Mohr AJ. 2002. Fate and transport of microorganisms in air, p 827–837 In Hurst CJ, Crawford RL, Knudsen GR, McInerney MJ, Stetzenbach LD. (ed), Manual of environmental microbiology. ASM Press, Washington, DC [Google Scholar]
- 53. Mossel DA. 1995. Essentials of the microbiology of foods: a textbook for advanced studies, p 71 and 288 J. Wiley, New York, NY [Google Scholar]
- 54. Nwachuku N, Gerba CP. 2004. Health effects of Acanthamoeba spp. and its potential for waterborne transmission. Rev. Environ. Contam. Toxicol. 180:93–131 [DOI] [PubMed] [Google Scholar]
- 55. Oelberg DG, et al. 2000. Detection of pathogen transmission in neonatal nurseries using DNA markers as surrogate indicators. Pediatrics 105:311–315 [DOI] [PubMed] [Google Scholar]
- 56. Pang L, Close M, Goltz M, Noonan M, Sinton L. 2005. Filtration and transport of Bacillus subtilis spores and the F-RNA phage MS2 in a coarse alluvial gravel aquifer: implications in the estimation of setback distances. J. Contam. Hydrol. 77:165–194 [DOI] [PubMed] [Google Scholar]
- 57. Pepper IL, Gerba CP, Brusseau ML. 2006. Environmental and pollution science, p 472 Academic Press, San Diego, CA [Google Scholar]
- 58. Plomp M, Leighton TJ, Wheeler KE, Malkin AJ. 2005. Architecture and high-resolution structure of Bacillus thuringiensis and Bacillus cereus spore coat surfaces. Langmuir 21:7892–7898 [DOI] [PubMed] [Google Scholar]
- 59. Redman JA, Grant SB, Olson TM, Estes MK. 2001. Pathogen filtration, heterogeneity, and the potable reuse of wastewater. Environ. Sci. Technol. 35:1798–1805 [DOI] [PubMed] [Google Scholar]
- 60. Regis E. 1999. The biology of doom: the history of America's secret germ warfare project. Henry Holt, New York, NY [Google Scholar]
- 61. Rety S, et al. 2005. The crystal structure of the Bacillus anthracis spore surface protein BclA shows remarkable similarity to mammalian proteins. J. Biol. Chem. 280:43073–43078 [DOI] [PubMed] [Google Scholar]
- 62. Rice EW, Adcock NJ, Sivaganesan M, Rose LJ. 2005. Inactivation of spores of Bacillus anthracis Sterne, Bacillus cereus, and Bacillus thuringiensis subsp. israelensis by chlorination. Appl. Environ. Microbiol. 71:5587–5589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Richardson JH, Barkley WE. 1989. Biosafety in microbiological and biomedical laboratories, p 38 U.S. Centers for Disease Control, Atlanta, GA [Google Scholar]
- 64. Rijal GK, Fujioka RS. 2001. Synergistic effect of solar radiation and solar heating to disinfect drinking water sources. Water Sci. Technol. 43:155–162 [PubMed] [Google Scholar]
- 65. Rijal GK, Fujioka RS. 2003. Use of reflectors to enhance the synergistic effects of solar heating and solar wavelengths to disinfect drinking water sources. Water Sci. Technol. 48:481–488 [PubMed] [Google Scholar]
- 66. Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. 2002. Public health assessment of potential biological terrorism agents. Emerg. Infect. Dis. 8:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Royalty-Hann W. 2007. Solutions for biological indicator problems from a quality assurance viewpoint. Biocontrol Sci. 12:77–81 [DOI] [PubMed] [Google Scholar]
- 68. Sattar SA, et al. 2001. Transfer of bacteria from fabrics to hands and other fabrics: development and application of a quantitative method using Staphylococcus aureus as a model. J. Appl. Microbiol. 90:962–970 [DOI] [PubMed] [Google Scholar]
- 69. Savazzi E. 1999. Introduction to functional morphology, p 4–13 In Savazzi E. (ed), Functional morphology of the invertebrate skeleton. J. Wiley, New York, NY [Google Scholar]
- 70. Schaffer FL, Soergel ME, Straube DC. 1976. Survival of airborne influenza virus: effects of propagating host, relative humidity, and composition of spray fluids. Arch. Virol. 51:263–273 [DOI] [PubMed] [Google Scholar]
- 71. Shen C, et al. 2008. Evaluating bacteriophage P22 as a tracer in a complex surface water system: the Grand River, Michigan. Environ. Sci. Technol. 42:2426–2431 [DOI] [PubMed] [Google Scholar]
- 72. Shields PA, Farrah SR. 2002. Characterization of virus adsorption by using DEAE-sepharose and octyl-sepharose. Appl. Environ. Microbiol. 68:3965–3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shintani H. 1996. Factors in the preparation of biological indicators that affect the decimal reduction time. Biomed. Instrum. Technol. 30:449–453 [PubMed] [Google Scholar]
- 74. Stenstrom TA. 1989. Bacterial hydrophobicity, an overall parameter for the measurement of adhesion potential to soil particles. Appl. Environ. Microbiol. 55:142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stuart AL, Wilkening DA. 2005. Degradation of biological weapons agents in the environment: implications for terrorism response. Environ. Sci. Technol. 39:2736–2743 [DOI] [PubMed] [Google Scholar]
- 76. Tanner BD, Brooks JP, Haas CN, Gerba CP, Pepper IL. 2005. Bioaerosol emission rate and plume characteristics during land application of liquid class B biosolids. Environ. Sci. Technol. 39:1584–1590 [DOI] [PubMed] [Google Scholar]
- 77. Teunis P, Medema G, Kruidenier L, Havelaar A. 1997. Assessment of the risk of infection by Cryptosporidium or Giardia in drinking water from a surface water source. Water Res. 31:1333–1346 [Google Scholar]
- 78. Thompson SS, Yates MV. 1999. Bacteriophage inactivation at the air-water-solid interface in dynamic batch systems. Appl. Environ. Microbiol. 65:1186–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tiwari A, Patnayak DP, Chander Y, Parsad M, Goyal SM. 2006. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 50:284–287 [DOI] [PubMed] [Google Scholar]
- 80. US Environmental Protection Agency 1987. Guide standard and protocol for testing microbiological water purifiers. Registration Division Office of Pesticide Programs Criteria and Standards Division, Office of Water, United States Environmental Protection Agency, Washington, DC [Google Scholar]
- 81. US Environmental Protection Agency 2003. Ultraviolet disinfection guidance manual. Document number EPA 815-D-03-007 Office of Water, United States Environmental Protection Agency, Washington, DC [Google Scholar]
- 82. Watson HE. 1908. A note on the variation of the rate of disinfection with change in the concentration of the disinfectant. J. Hyg. 8:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wiencek KM, Klapes NA, Foegeding PM. 1990. Hydrophobicity of Bacillus and Clostridium spores. Appl. Environ. Microbiol. 56:2600–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xu H, Lee H, Ahn J. 2010. Growth and virulence properties of biofilm-forming Salmonella enterica serovar Typhimurium under different acidic conditions. Appl. Environ. Microbiol. 76:7910–7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zaleski KJ, Josephson KL, Gerba CP, Pepper IL. 2005. Potential regrowth and recolonization of salmonellae and indicators in biosolids and biosolid-amended soil. Appl. Environ. Microbiol. 71:3701–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zolock RA, Li G, Bleckmann C, Burggraf L, Fuller DC. 2006. Atomic force microscopy of Bacillus spore surface morphology. Micron 37:363–369 [DOI] [PubMed] [Google Scholar]