Abstract
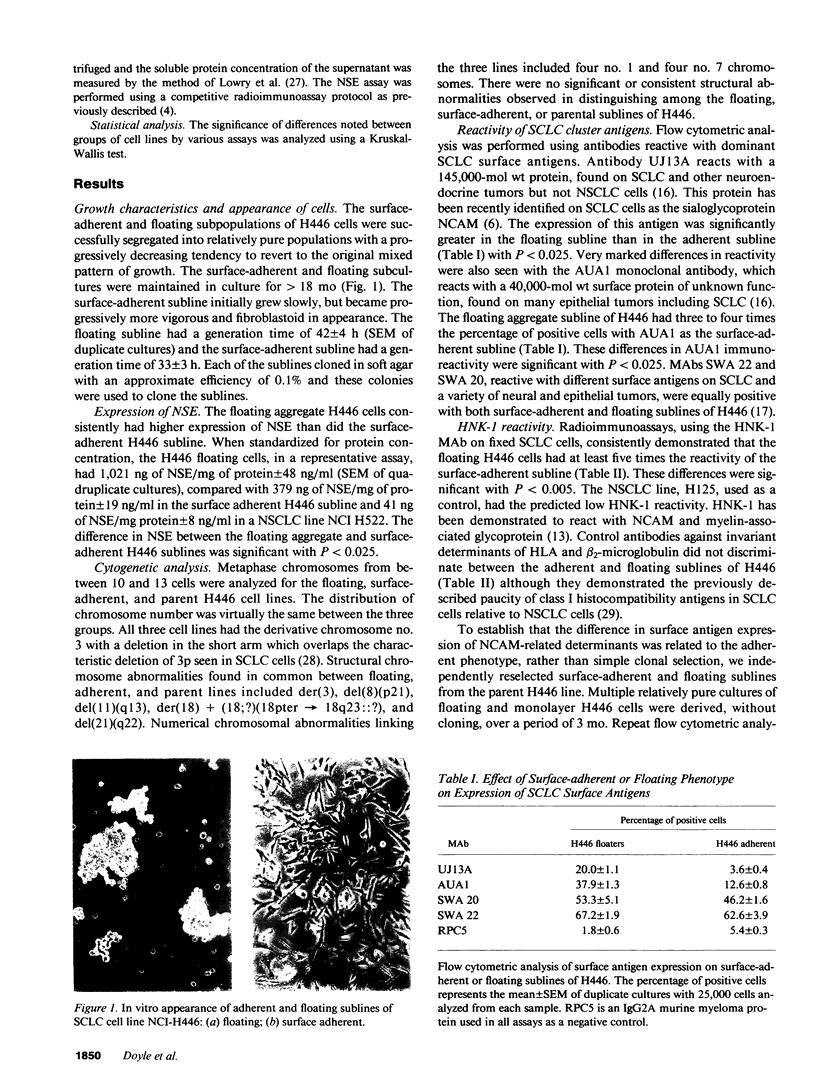
Small-cell lung cancer (SCLC) lines are distinguished from non-small-cell lung cancer (NSCLC) lines by their growth in floating aggregates, in contrast to the adherent monolayers formed by NSCLC cells in culture. Of 50 well-characterized SCLC lines recently described by the National Cancer Institute (NCI)-Navy Medical Oncology Branch, only four variant cell lines (SCLC-v) grew as adherent monolayers. One line, NCI-H446, was unique in growing long-term with coexisting floating and surface adherent subpopulations. We have physically segregated these two populations over many passages in vitro to enrich for relatively pure cultures of floating and adherent cells. No differences in c-myc expression, keratin pattern, or cytogenetic appearance were found between the adherent and floating sublines. However, expression of the neuroendocrine marker neuron-specific enolase in the floating cells was three times that found in the adherent cells. The floating subline also had much greater surface expression of neuroendocrine tumor antigens detected by monoclonal antibodies UJ13A and HNK-1, which have been recently shown to detect the neural cell adhesion molecule (NCAM) on SCLC cells. Two other adherent SCLC-v lines were also found to be unreactive with UJ13A and HNK-1, generalizing the association between NCAM expression and the growth of most SCLC cultures as floating aggregates. In conclusion, we have an interesting model to study expression of NCAM as related to the adhesive properties of SCLC cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Baylin S. B., Abeloff M. D., Goodwin G., Carney D. N., Gazdar A. F. Activities of L-dopa decarboxylase and diamine oxidase (histaminase) in human lung cancers and decarboxylase as a marker for small (oat) cell cancer in cell culture. Cancer Res. 1980 Jun;40(6):1990–1994. [PubMed] [Google Scholar]
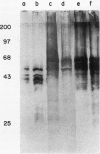
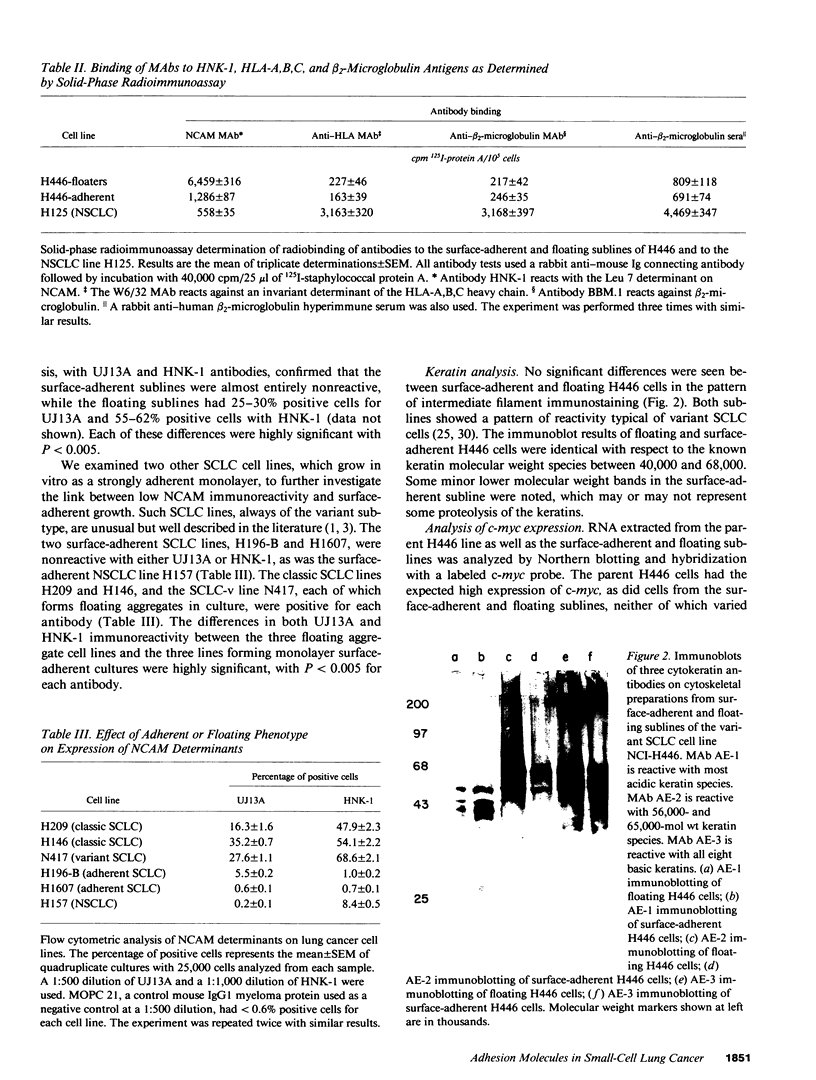
- Broers J. L., Carney D. N., de Ley L., Vooijs G. P., Ramaekers F. C. Differential expression of intermediate filament proteins distinguishes classic from variant small-cell lung cancer cell lines. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4409–4413. doi: 10.1073/pnas.82.13.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn P. A., Jr, Linnoila I., Minna J. D., Carney D., Gazdar A. F. Small cell lung cancer, endocrine cells of the fetal bronchus, and other neuroendocrine cells express the Leu-7 antigenic determinant present on natural killer cells. Blood. 1985 Mar;65(3):764–768. [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Minna J. D. Positive correlation between histological tumor involvement and generation of tumor cell colonies in agarose in specimens taken directly from patients with small-cell carcinoma of the lung. Cancer Res. 1980 Jun;40(6):1820–1823. [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Rosen S., Gazdar A. F., Minna J. D. Monoclonal antibodies that demonstrate specificity for several types of human lung cancer. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4591–4595. doi: 10.1073/pnas.78.7.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
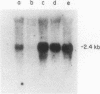
- Dalla-Favera R., Martinotti S., Gallo R. C., Erikson J., Croce C. M. Translocation and rearrangements of the c-myc oncogene locus in human undifferentiated B-cell lymphomas. Science. 1983 Feb 25;219(4587):963–967. doi: 10.1126/science.6401867. [DOI] [PubMed] [Google Scholar]
- Doyle A., Martin W. J., Funa K., Gazdar A., Carney D., Martin S. E., Linnoila I., Cuttitta F., Mulshine J., Bunn P. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985 May 1;161(5):1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Elias A. D., Cohen B. F., Bernal S. D. Keratin subtypes of small cell lung cancer. Cancer Res. 1988 May 15;48(10):2724–2729. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Nau M. M., Minna J. D. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985 Jun;45(6):2924–2930. [PubMed] [Google Scholar]
- Gazdar A. F., Zweig M. H., Carney D. N., Van Steirteghen A. C., Baylin S. B., Minna J. D. Levels of creatine kinase and its BB isoenzyme in lung cancer specimens and cultures. Cancer Res. 1981 Jul;41(7):2773–2777. [PubMed] [Google Scholar]
- Graziano S. L., Mazid R., Newman N., Tatum A., Oler A., Mortimer J. A., Gullo J. J., DiFino S. M., Scalzo A. J. The use of neuroendocrine immunoperoxidase markers to predict chemotherapy response in patients with non-small-cell lung cancer. J Clin Oncol. 1989 Oct;7(10):1398–1406. doi: 10.1200/JCO.1989.7.10.1398. [DOI] [PubMed] [Google Scholar]
- Hoffman S., Edelman G. M. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5762–5766. doi: 10.1073/pnas.80.18.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbelaar R. E., Moolenaar C. E., Michalides R. J., Bitter-Suermann D., Addis B. J., Mooi W. J. Expression of the embryonal neural cell adhesion molecule N-CAM in lung carcinoma. Diagnostic usefulness of monoclonal antibody 735 for the distinction between small cell lung cancer and non-small cell lung cancer. J Pathol. 1989 Sep;159(1):23–28. doi: 10.1002/path.1711590108. [DOI] [PubMed] [Google Scholar]
- Kruse J., Mailhammer R., Wernecke H., Faissner A., Sommer I., Goridis C., Schachner M. Neural cell adhesion molecules and myelin-associated glycoprotein share a common carbohydrate moiety recognized by monoclonal antibodies L2 and HNK-1. Nature. 1984 Sep 13;311(5982):153–155. doi: 10.1038/311153a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Anchorage dependency effects on difluoromethylornithine cytotoxicity in human lung carcinoma cells. Cancer Res. 1986 Apr;46(4 Pt 1):1844–1848. [PubMed] [Google Scholar]
- Marangos P. J., Gazdar A. F., Carney D. N. Neuron specific enolase in human small cell carcinoma cultures. Cancer Lett. 1982 Jan;15(1):67–71. doi: 10.1016/0304-3835(82)90077-5. [DOI] [PubMed] [Google Scholar]
- Moolenaar C. E., Muller E. J., Schol D. J., Figdor C. G., Bock E., Bitter-Suermann D., Michalides R. J. Expression of neural cell adhesion molecule-related sialoglycoprotein in small cell lung cancer and neuroblastoma cell lines H69 and CHP-212. Cancer Res. 1990 Feb 15;50(4):1102–1106. [PubMed] [Google Scholar]
- Noronha A. B., Harper J. R., Ilyas A. A., Reisfeld R. A., Quarles R. H. Myelin-associated glycoprotein shares an antigenic determinant with a glycoprotein of human melanoma cells. J Neurochem. 1986 Nov;47(5):1558–1565. doi: 10.1111/j.1471-4159.1986.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Pettengill O. S., Sorenson G. D., Wurster-Hill D. H., Curphey T. J., Noll W. W., Cate C. C., Maurer L. H. Isolation and growth characteristics of continuous cell lines from small-cell carcinoma of the lung. Cancer. 1980 Mar 1;45(5):906–918. doi: 10.1002/1097-0142(19800301)45:5<906::aid-cncr2820450513>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Rosenblatt H. M., Imai K., Ferrone S. Common antigenic determinants on human melanoma, glioma, neuroblastoma, and sarcoma cells defined with monoclonal antibodies. Cancer Res. 1981 Jul;41(7):2714–2717. [PubMed] [Google Scholar]
- Simms E., Gazdar A. F., Abrams P. G., Minna J. D. Growth of human small cell (oat cell) carcinoma of the lung in serum-free growth factor-supplemented medium. Cancer Res. 1980 Dec;40(12):4356–4363. [PubMed] [Google Scholar]
- Souhami R. L., Beverley P. C., Bobrow L. G. Antigens of small-cell lung cancer. First International Workshop. Lancet. 1987 Aug 8;2(8554):325–326. doi: 10.1016/s0140-6736(87)90904-4. [DOI] [PubMed] [Google Scholar]
- Strnad J., Hamilton A. E., Beavers L. S., Gamboa G. C., Apelgren L. D., Taber L. D., Sportsman J. R., Bumol T. F., Sharp J. D., Gadski R. A. Molecular cloning and characterization of a human adenocarcinoma/epithelial cell surface antigen complementary DNA. Cancer Res. 1989 Jan 15;49(2):314–317. [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa J. R., Misawa S., Oguma N., Van Sloten K., Wiernik P. H. Chromosomal alterations in acute leukemia patients studied with improved culture methods. Cancer Res. 1985 Jan;45(1):430–434. [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Waibel R., O'Hara C. J., Smith A., Stahel R. A. Tumor-associated membrane sialoglycoprotein on human small cell lung carcinoma identified by the IgG2a monoclonal antibody SWA20. Cancer Res. 1988 Aug 1;48(15):4318–4323. [PubMed] [Google Scholar]
- Whang-Peng J., Kao-Shan C. S., Lee E. C., Bunn P. A., Carney D. N., Gazdar A. F., Minna J. D. Specific chromosome defect associated with human small-cell lung cancer; deletion 3p(14-23). Science. 1982 Jan 8;215(4529):181–182. doi: 10.1126/science.6274023. [DOI] [PubMed] [Google Scholar]
- Willison H. J., Minna J. D., Brady R. O., Quarles R. H. Glycoconjugates in nervous tissue and small cell lung cancer share immunologically cross-reactive carbohydrate determinants. J Neuroimmunol. 1986 Feb;10(4):353–365. doi: 10.1016/S0165-5728(86)90018-4. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Rheinwald J. G. A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell. 1981 Sep;25(3):627–635. doi: 10.1016/0092-8674(81)90170-7. [DOI] [PubMed] [Google Scholar]