Abstract
Propionibacterium freudenreichii is a bacterial species found in Swiss-type cheeses and is also considered for its health properties. The main claimed effect is the bifidogenic property. Some strains were shown recently to display other interesting probiotic potentialities such as anti-inflammatory properties. About 30% of strains were shown to produce a surface exopolysaccharide (EPS) composed of (1→3,1→2)-β-d-glucan due to a single gene named gtfF. We hypothesized that functional properties of P. freudenreichii strains, including their anti-inflammatory properties, could be linked to the presence of β-glucan. To evaluate this hypothesis, gtfF genes of three β-glucan-producing strains were disrupted. These knockout (KO) mutants were complemented with a plasmid harboring gtfF (KO-C mutants). The absence of β-glucan in KO mutants was verified by immunological detection and transmission electron microscopy. We observed by atomic force microscopy that the absence of β-glucan in the KO mutant dramatically changed the cell's topography. The capacity to adhere to polystyrene surface was increased for the KO mutants compared to wild-type (WT) strains. Anti-inflammatory properties of WT strains and mutants were analyzed by stimulation of human peripheral blood mononuclear cells (PBMCs). A significant increase of the anti-inflammatory interleukin-10 cytokine production by PBMCs was measured in the KO mutants compared to WT strains. For one strain, the role of β-glucan in mice gut persistence was assessed, and no significant difference was observed between the WT strain and its KO mutant. Thus, β-glucan appears to partly hide the anti-inflammatory properties of P. freudenreichii; which is an important result for the selection of probiotic strains.
INTRODUCTION
Bacteria are capable of producing a wide variety of extracellular polysaccharides. These exopolysaccharides (EPS) are known to exert important biological functions for the producing bacteria. For example, they are involved in processes such as biofilm formation (62) or protection against dehydration and bacteriophages (64). In lactic acid bacteria (LAB), many species have been shown to produce EPS molecules. The diversity of the produced molecules is huge in terms of monomer composition, molecular mass, degree of branching, and structure. The EPS can also occur in different forms. Some are capsular polysaccharides with polymers covalently bound to the cell wall. Others are loosely bound to the cell wall, while the remaining ones are secreted in the environment (slime polysaccharide) (7, 50). In fact, the wide diversity of such molecules is of great interest in the food industry. In dairy food, EPS have found extensive applications. For example, EPS-producing LAB are used to reduce syneresis of fermented milk, and they are also used as biological thickeners or stabilizers (for a review, see references 25 and 29).
Many probiotic bacteria (19) with various reported health effects have been shown to produce EPS, including the well-known Lactobacillus rhamnosus strain GG (16), which produces two types of molecules: one galactose-rich (the most abundant) (37) and the other glucose-rich (24). The described roles of EPS in probiotic actions are numerous (for a review, see reference 39), including prebiotic potential, possible involvement in the adhesion to the intestinal epithelium, and modulation of host immunity (38, 51, 65).
Dairy propionibacteria are widely used in the food industry, more particularly as starter in the production of Swiss-type cheeses such as Emmental and Maasdam. These bacteria are essential for the development of a nutty and sweet flavor (57). In Emmental cheese, dairy propionibacteria convert the lactate produced by the LAB into acetate, propionate, and CO2, the latter being responsible for the appearance of the typical cheese “holes.” These propionibacteria are ingested at high levels by consumers, i.e., per gram of Emmental, about 5 × 109 living cells of P. freudenreichii, the species most frequently used in cheese-making (13). This species has low nutritional requirements and showed a high adaptability and tolerance toward technological as well as digestive stresses, including acid (33) and bile salts (42). As a consequence of this hardiness, some strains of this species were shown to survive in the human gut at elevated levels (30). Thus, in addition to its traditional use in cheese, research has recently focused on health benefits related to P. freudenreichii strains displaying probiotic activities. Indeed, some probiotic potentialities of dairy propionibacteria are well documented in human (for a review, see reference 9). Their most studied property is the bifidogenic effect (5, 31, 32, 53). In a recent study (22), we investigated the immunomodulatory properties of 10 strains of dairy propionibacteria, based on the induction of regulatory anti-inflammatory cytokines in human peripheral blood mononuclear cells (PBMCs). All tested strains showed an anti-inflammatory potential, despite showing a strain-dependent efficacy.
Recent data indicate that P. freudenreichii produces a surface polysaccharide that is detected in nearly 30% of the strains. This polysaccharide is composed of (1→3,1→2)-β-d-glucan and is easily detected at the bacterial surface with a specific antiserum. Its biosynthesis is due to a single enzyme, encoded by the gtfF gene (12, 14). Here, our starting hypothesis is that the functional properties of P. freudenreichii and further impact on the host, such as anti-inflammatory properties, could be linked to EPS occurrence and type. In order to evaluate the role of the β-glucan polysaccharide and the physiological properties associated with this component, three P. freudenreichii strains known to produce β-glucan were selected. gtfF-negative mutants, as well as negative but complemented mutants, were constructed for the three strains. In addition to their morphological changes, the EPS knockout (KO) mutants, the wild type (WT), and complemented derivatives (KO-C) were investigated for their in vitro resistance to acid and bile salts stresses, their capacity to adhere to abiotic surfaces, and their survival inside the mouse gastrointestinal tract (GIT). The immunomodulatory properties of these isogenic mutants P. freudenreichii strains were also compared within the cytokine responses elicited after exposure to human PBMCs.
MATERIALS AND METHODS
Strains and medium.
The P. freudenreichii WT strains and their genetically modified derivatives are listed in Table 1. Strain CB1 was obtained from the collection of the Centre International de Resources Microbiennes–Bactéries d'Intérêt Alimentaire (CIRM-BIA; STLO, INRA Rennes, France). Strains LSP110 and LSP103 were obtained from Laboratoires Standa (Caen, France). All of the strains were grown at 30°C in YEL broth (45) in closed glass tubes without agitation. Such conditions are generally described as “microaerophilic” and are optimal for dairy propionibacteria. In some cases, specified in the text, YEL was supplemented with chloramphenicol (10 μg ml−1) or hygromycin B (750 μg ml−1 in YEL agar or 250 μg ml−1 in liquid YEL).
Table 1.
P. freudenreichii WT strains and their genetically modified derivatives used in this study
| Strain or plasmid | Relevant genotype and phenotype | Source or reference |
|---|---|---|
| Strains | ||
| CB1a (formerly TL34) | WT, carrying one genomic copy of gtfF and producing β-glucan | 17 |
| CB1-KO | Inactivation of gtfF gene | 14 |
| CB1-KO-C | CB1-KO complemented with gtfF and with reverted phenotype | This study |
| LSP110 (formerly TL162) | WT, carrying one genomic copy of gtfF and producing β-glucan | 12 |
| LSP110-KO | Inactivation of gtfF gene | This study |
| LSP110-KO-C | CB1-KO reverted to WT | This study |
| LSP103 (formerly TL176) | WT, carrying one genomic copy of gtfF and producing β-glucan | 12 |
| LSP103-KO | Inactivation of gtfF gene | This study |
| LSP103-KO-C | CB1-KO reverted to WT | This study |
| Plasmids | ||
| pUC:gtfF:CmR | pUC18 carrying a chloramphenicol resistance gene and a truncated fragment of gtfF | 14 |
| pPK705 | E. coli-P. freudenreichii shuttle vector, carrying an ampicillin resistance gene | 34 |
| pFB01:gtfF | pPK705 carrying gtfF gene under the control of the strong promoter Ptuf | This study |
CB, CIRM-BIA, Centre International de Ressources Microbiennes–Bactéries d'Intérêt Alimentaire, INRA, UMR 1253, Science et Technologie du Lait et de l'Œuf, Rennes, France. The TL collection does not exist anymore and is now included in the CB collection.
Some bacterial strains were used as reference strains for immune cell stimulation: Lactococcus lactis MG1363, Lactobacillus acidophilus NCFM, Lactobacillus salivarius Ls33, and Bifidobacterium longum BB536. They were prepared as previously described (23).
Construction of the genetically modified strains. (i) Insertional inactivation of the gtfF gene (KO mutants).
The insertional inactivation of gtfF was conducted as previously described (12, 14). Briefly, a suicide vector was constructed by inserting a chloramphenicol resistance gene in a pUC18 plasmid. In the resulting plasmid, an internal fragment of 551 bp of the gtfF open reading frame of the strain CB1 (accession number AM850120 [17]) was cloned, resulting in the vector pUC:gtfF:CmR. The strains of P. freudenreichii to be tested were transformed with this vector and transformants harboring inserted pUC:gtfF:CmR were selected on YEL agar medium with chloramphenicol. The stability of the insertion was checked in YEL without chloramphenicol.
(ii) Complementation of KO mutants with plasmid harboring gtfF (KO-C mutants).
The gtfF gene was cloned into pPK705 vector downstream the Ptuf promoter. Ptuf is the upstream region of the tuf gene (236 bp) of P. freudenreichii CB1 (accession number FN 806773) that encodes the translation elongation factor. This gene was shown to be strongly expressed during the growth of the strain in YEL (18). To perform the construction, Ptuf and gtfF were amplified by PCR from P. freudenreichii CB1 genomic DNA using the primers ptuf-bamH1-F/ptuf-xba1-R and gtfF-XbaI-F/gtfF-HindIII-R (see Table S1 in the supplemental material). For Ptuf amplification, PCR mixtures contained 2 mM MgCl2, 1 μM concentrations of each primer, 400 μM concentrations of each deoxynucleoside triphosphate, and 1.25 U of Taq polymerase (Fermentas International, Inc., Burlington, Ontario, Canada) in 1× buffer (20 mM Tris-HCl [pH 8.4], 50 mM KCl). PCR was performed under the following conditions: initial denaturation at 94°C for 2 min, followed by 20 cycles of 94°C for 45 s and 65°C for 45 s, with a decrease by 0.5°C at each cycle; 72°C for 45 s, followed by 10 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 45 s; and then a final step at 72°C for 5 min. High-fidelity PCR was required for cloning gtfF to ensure accurate and reliable PCR amplification. Platinum Taq high-fidelity polymerase (Invitrogen, Carlsbad, CA) was used, and PCR was performed under the same conditions as described above. The PCR mixture was then incubated at 72°C for 4 min in buffer, containing 100 μM ATP, 2 mM MgCl2, and 2 U of Taq polymerase (Fermentas) to perform adenylation and was then purified on QIAquick PCR purification kit (Qiagen). Each of the Ptuf and gtfF amplicons was cloned into a pGEM-T Easy vector (Promega Corp., Madison, WI) according to the manufacturer's instructions. pGEM-T plasmids were multiplied in Escherichia coli DH5α and extracted with Nucleospin Multi-8-Plasmid kit (Macherey-Nagel, Hoerdt, France) according to the manufacturer's instructions. pGEM-T:Ptuf and pGEM-T:gftF were double digested by BamHI-HindIII and XbaI-HindIII, respectively. Digestion products were subjected to 1.5% agarose gel electrophoresis. Agarose pieces containing Ptuf and gtfF were excised, and fragments were purified using a PCR cleanup gel electrophoresis kit (Qiagen) according to the manufacturer's instructions. Ptuf was introduced into a double-digested (XbaI-HindIII) pPK705 plasmid. Ligation was performed according to the manufacturer's instructions (Ligase; Invitrogen). The product was used to transform E. coli DH5α competent cells according to the method of Hanahan et al. (28), and transformants were selected on ampicillin. Plasmids were extracted, double digested with BamHI-HindIII, and ligated to fragments containing gtfF. The resulting ligation products were then used to transform CB1 competent cells as previously described (14). After electroporation, cells were regenerated by incubation for 3 h at 30°C and P. freudenreichii clones harboring the inserted vector were selected on YEL-agar containing 750 μg of hygromycin B/ml and incubated for 4 days at 30°C in anaerobic jars (Anaerocult A; Merck, Darmstadt, Germany).
Detection of polysaccharide: immunological methods and TEM.
Cultures were grown to an absorbance at 650 nm (A650) of 1. The presence of the β-glucan surface polysaccharide was detected by using an anti-S-pneumoniae serotype 37 antiserum purchased at the Statens Serum Institute (Hillerød, Denmark). This well-described antiserum was raised against (1→3,1→2)-β-d-glucan polysaccharide and was shown to be very specific for this structure (1, 44). The assays of β-glucan detection were performed as previously described (63). Whatever the tested strains, no agglutination was observed in the absence of antiserum (negative control). Optical microscopic observations were performed at a magnification of ×40. For transmission electron microscopy (TEM) observations, the cells were prepared as previously described (14).
Determination of zeta potential.
The measure of electrophoretic mobility (zeta potential) was performed according to the well-described protocol of Schär-Zammaretti and Ubbink (54). The cells from 5 ml of culture were harvested by centrifugation (6,000 × g, 10 min, room temperature) and washed twice with a 10 mM KH2PO4 solution (pH 7). The pellet was resuspended in a 10 mM KH2PO4 solution (pH 7.0). The approximate cell count of the final suspensions was 107 to 108 CFU/ml. The electrophoretic mobility was measured by using a ZetaSizer nanoZS (Malvern Instruments, Malvern, United Kingdom) and a glass capillary (Zetasizer Nanoseries DTS 1061) as the electrophoretic cell. Electrophoretic mobilities were converted to the ζ-potential using the Helmholtz-Schmoluchowski equation.
AFM analysis.
The strains LSP103 and LSP103-KO were analyzed by atomic force microscopy (AFM). The strains were grown in YEL for 48 h. Cultures (1 ml) were washed in a 2 mM HEPES buffer (pH 6.8) and resuspended in the same buffer. Then, 2 μl of this suspension was deposited onto a freshly cut disk of mica (1 cm in diameter) and allowed to dry for at least 30 min before imaging. All AFM work was performed in air, at a controlled temperature of 20°C, and using a MFP-3D-BIO microscope (Asylum Research, Santa Barbara, CA). Note that we chose to perform AFM imaging in air with such dried samples in order to emphasize the details of the bacterial surface morphology. Indeed, AFM imaging in liquid, besides being harder to achieve, is known to give images that are much less detailed and thus more difficult to interpret (4, 49). Images were acquired in tapping-mode using AC240TS cantilevers (Olympus, Tokyo, Japan), which are relatively sharp (∼9-nm tip radius) and soft (∼2-N/m spring constant). Both height and amplitude images were recorded. The height image is a measure of the sample topography, whereas the amplitude image, also known as the “error” image, does not provide any quantitative information but gives a good view of the edges of the surface features.
Sedimentation capacity.
The strains were grown in YEL medium at 30°C in the presence of hygromycin B for the three KO-C strains. After 48 h of incubation without agitation, the sedimentation rates of the cultures were recorded and compared.
PBMC isolation and cytokine release assays.
The cytokine induction pattern was evaluated as previously described (23) for the three WT strains of P. freudenreichii and their derivatives and for four reference strains. These four strains are known as covering the range of variations of secreted anti- and proinflammatory mediators (23). The P. freudenreichii strains were grown for 72 h at 30°C in YEL, supplemented with hygromycin when required, to reach the early stationary phase, washed twice in phosphate-buffered saline (PBS), and resuspended in PBS containing 20% glycerol using a portable photometer (Densimat; bioMérieux, Craponne, France). The cell density was adjusted to McFarland 3 (3). These standardized bacterial preparations, corresponding to approximately 2 × 108 CFU/ml, were stored at −80°C until further use.
Human PBMCs were obtained from three healthy donors and isolated as previously described (23). Briefly, after a Ficoll gradient centrifugation (Pharmacia, Uppsala, Sweden), mononuclear cells were collected, washed in RPMI 1640 medium (Life Technologies, Paisley, Scotland), and adjusted to 2 × 106 cells/ml in 1 ml of RPMI 1640 supplemented with gentamicin (150 μg/ml), l-glutamine (2 mM), and 10% fetal calf serum (Gibco-BRL). PBMCs (2 × 106 cells/ml) were seeded in 24-well tissue culture plates (Corning, Corning, NY). Either 2 or 10 μl of the thawed bacterial suspensions, prepared as described above, was added. This resulted in a bacterium/cell ratio of 2:1 or 10:1. PBS containing 20% glycerol was used as a negative (nonstimulated) control. After 24 h stimulation at 37°C in air with 5% CO2, culture supernatants were collected, clarified by centrifugation (6,000 × g, 10 min, 4°C), and stored at −20°C until cytokine analysis. Neither medium acidification (red phenol as pH marker) nor bacterial proliferation was observed. Cytokines were measured by enzyme-linked immunosorbent assay using BD Pharmingen antibody pairs (BD Biosciences, San Jose, CA) for interleukin-10 (IL-10) and IL-12 according to the manufacturer's recommendations.
RT-qPCR.
The experiments of reverse transcription-quantitative PCR (RT-qPCR) and the subsequent analysis of the data were performed according to previously described guidelines (6, 56).
Total RNA extraction.
P. freudenreichii strains were grown on YEL medium until an A650 of 1. Then, 4 ml of the culture was harvested (8,000 × g, 10 min, 4°C). The pellets were resuspended in 200 μl of lysis buffer (50 mM Tris-HCl, 1 mM EDTA [pH 8.0]) containing 20 mg of lysozyme ml−1, followed by incubation 15 min at 25°C. The suspensions were then transferred to 2-ml tubes containing 50 mg of zirconium beads (0.1 mm in diameter; BioSpec Products, Bartlesville, OK) and 100 μl of 10% sodium dodecyl sulfate (SDS). The tubes were shaken twice for 90 s at 30 Hz in a bead beater (MM301; Retsch, Haan, Germany) with chilling on ice for 2 min between each shaking step. Samples were then centrifuged twice to eliminate insoluble cell debris (8,000 × g, 10 min, 4°C). RNA extraction was then performed with the supernatants using the RNeasy minikit (Qiagen), according to the instructions of the manufacturer. RNA was suspended in 50 μl of RNase-free water and treated (twice) with DNase (DNA-free; Ambion, Cambridgeshire, United Kingdom), according to the instructions of the supplier. Quantification of RNA and its contamination by proteins was assessed spectrophotometrically using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Rockland, DE). RNA extractions were performed in triplicate. The absence of genomic DNA was confirmed by qPCR.
cDNA preparation by retrotranscription and qPCR.
RT reactions were carried out using an RT Bio-Rad iScript cDNA synthesis kit (Bio-Rad, Ivry-sur-Seine, France) according to the instructions of the manufacturer, with 3 μl of RNA sample. The cDNAs were stored at −80°C until use. The primer pairs used for the qPCR were F1 and R1 for the gene gtfF and GroL1-F and GroL1-R for the groL1 gene (see Table S1 in the supplemental material). qPCR was carried out on an Opticon2 (Bio-Rad) with the IQ SYBR green Supermix (Bio-Rad) under conditions previously described (14).
Data analysis.
The Cq values were analyzed with the geNorm application (58). groL1 was used as the normalization gene; its stability was verified with an application. The application (http://medgen.ugent.be/genorm/) was used to calculate the normalization factor (which was then applied to the Cq raw data) and to express the relative transcript ratios. Then, for each triplet of strains (WT, KO, and KO-C), the level of induction was expressed as the fold change with the level of expression in the WT strain as a reference.
Adhesion assays.
The ability to adhere to an abiotic surface was quantified by a method adapted from O'Toole and Kolter (48). Each bacterial strain was inoculated in the appropriate medium, and 16 × 200 μl of cell suspension was deposited in a 96-well polystyrene microtiter plate. After 7 days at 30°C, the wells were gently washed three times with 200 μl of 0.9% NaCl, dried in an inverted position, and stained with 1% crystal violet. The wells were rinsed again, and the crystal violet was solubilized in 100 μl of ethanol-acetone (80:20 [vol/vol]). The absorbance at 595 nm (A595) was determined using a microplate reader (Molecular Devices). The assay was performed in triplicate.
In vitro adhesion assay to Caco-2 human intestinal epithelial cell lines was monitored as described previously described for propionibacteria (41). Caco-2 cells were purchased from the American Type Culture Collection (Rockville, MD). Cells were routinely grown in Dulbecco modified Eagle medium (Gluta-MAX, high glucose; Gibco-Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (PAN; Dominique Dutscher, Brumath, France) and antibiotics (penicillin, 50 U/ml; streptomycin, 50 μg/ml), under conditions of 37°C, 5% CO2, and 90% relative humidity. For adhesion experiments, Caco-2 cells were plated at a density of 40,000 cells/cm2 in 12-well plates. Confluence was reached within 3 to 4 days after seeding, and monolayers of differentiated cells were used for the experiments 15 days after seeding. The adherence of P. freudenreichii (WT and mutants) to the epithelial cells was examined by adding 109 CFU of propionibacteria to cell culture medium. After incubation at 37°C for 60 min, epithelial cells were washed two times with prewarmed PBS. Subsequently, 100 μl of trypsin-EDTA (Invitrogen) was added to each well, followed by incubation for 5 min at 37°C. Finally, 900 μl of PBS was added and mixed, and serial dilutions were plated out. The plates were incubated at 30°C for 5 days. The percentage of adhesion was calculated as follows: 100 × (propionibacterium count after adhesion experiment/propionibacterium population added onto Caco-2 cells). Each adhesion assay was conducted in triplicate.
In vivo survival and counts of propionibacteria in fecal samples.
Conventional BALB/c mice (female, 7 weeks old) were obtained from Charles River (St. Germainsurl'Arbresle, France). Each strain of freshly (72 h) cultivated bacterial suspensions resuspended in PBS buffer (250 μl, containing 2.5 × 109 CFU) was administered intragastrically to groups of six mice for three consecutive days, whereas control mice received only the buffer. Feces were regularly collected and immediately processed during a 6-day period. Duplicate pooled samples of three mice were mechanically homogenized in sterile neutral and isotonic buffer at 50 mg of stool/ml. Serial dilutions were then plated onto selective Pal-Propiobac agar (Laboratoires Standa, Caen, France) supplemented with metronidazole (4 mg/liter), as described previously (30). No microorganism (bacteria or fungi) were detected in uninoculated mice using the selective media. This indicates that the medium is indeed selective and that there is no or negligible basal colonization of mice gastrointestinal tract by Propionibacterium species. The absence of propionibacteria in mouse feces upon arrival was also established by using specific multiplex pyrosequencing of hypervariable regions of 16SRNA, showing no actinobacteria at all in laboratory mice (unpublished data developed with DNAVision, Belgium).
Data and statistical analysis.
All analyses were performed by using the Student t test or nonparametric one-way analysis of variance Mann-Whitney where appropriate. The data are presented as means ± the standard deviation. Differences were judged to be statistically significant when the P value was <0.05.
RESULTS
Construction of P. freudenreichii mutants producing or not producing surface β-glucan: phenotypic consequences.
As previously described, strains CB1, LSP110, and LSP103 carry in their chromosomes one copy of the gtfF gene, responsible for the synthesis of a surface polysaccharide composed of (1→3,1→2)-β-d-glucan (14). The gtfF gene was disrupted in the three strains by chromosomal integration of a suicide vector. The corresponding KO mutants are named CB1-KO, LSP110-KO, and LSP103-KO. In order to revert the KO mutants to their original phenotype, they were transformed with a plasmid harboring a copy of the gtfF gene under the dependence of a strong promoter (Ptuf). The resulting mutants are named CB1-KO-C, LSP110-KO-C, and LSP103-KO-C. The accuracy of all of the constructions was ensured (i) by PCR (data not shown), (ii) by gtfF mRNA RT-qPCR analysis, and (iii) by immunoagglutination and TEM to detect the presence of the surface polysaccharide.
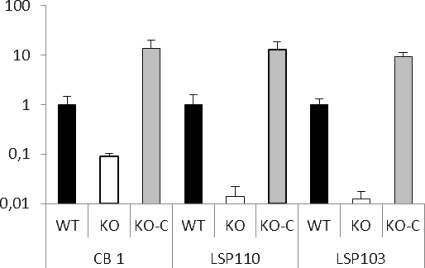
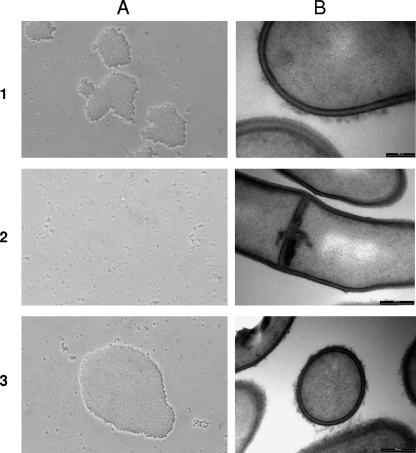
RT-qPCR analyses were performed to quantify the level of expression of the gtfF mRNA in the WT strains and in their derivatives (Fig. 1). The groL1 gene was used as the normalization gene, since it is known to be stable (14) (this was confirmed again here [data not shown]). For the three WT strains, the levels of gtfF mRNA expression were also comparable to those previously measured (data not shown). In the three KO mutants, gtfF mRNA was detected at low levels of expression: 11-, 72-, and 82-fold reductions compared to the WT strains CB1, LSP110, and LSP103, respectively (Fig. 1). Since in KO mutants the gene gtfF is not deleted but disrupted with the suicide vector, it is not surprising to detect mRNA. This could be due to the presence of a promoter-like sequence in the sequence of the suicide vector used. However, no intact copy of the gtfF gene exists. The presence of the surface polysaccharide was verified by immunoagglutination using a specific antiserum raised against the (1→3,1→2)-β-glucan. All three WT strains agglutinated in the presence of the antiserum, whereas none of the KO mutants did so under the same conditions, indicating the absence of the surface polysaccharide (Fig. 2A). The presence of the polysaccharide was also verified by TEM, and indeed a hairy layer was observed in the three WT strains, whereas it was absent in the KO mutants (Fig. 2B).
Fig 1.
Modulation of the expression levels of the gtfF gene, which is responsible for the production of the β-glucan surface polysaccharide in P. freudenreichii strains CB1, LSP110, and LSP103 and their corresponding mutants. Bars: ■, WT strains, producing surface β-glucan; □, KO mutants where gtfF has been inactivated; ▩, KO-C mutants (KO strains reverted to WT). gtfF gene expression is expressed as the induction rate compared to the expression measured in the WT strain. The data were normalized with the software geNorm using groL1 as normalization gene. These results are the average of three independent experiments. Error bars indicate the standard deviation.
Fig 2.
Inactivation of the gtfF gene led to the disappearance of the hairy surface. (A and B) Immunoagglutination test performed with a β-glucan-specific antiserum (A) and TEM observation (B) of P. freudenreichii WT strain CB1 (row 1), its abolished surface polysaccharide mutant CB1-KO (row 2), and the CB1-KO-C mutant (row 3), which was reverted to the original phenotype.
The gtfF expression level in the KO-C mutants was higher than in the WT strains, with induction levels of 9.4, 13.6, and 13.1 compared to their respective WT parent strains (Fig. 1). This result validates the fact that the promoter of the gene tuf used for these constructions is a “strong” promoter. Again, the presence of the surface polysaccharide was checked by immunoagglutination, and the agglutination capacity was successfully restored for the three strains. As an example, the results obtained for strain CB1 are shown in Fig. 2A3 and B3. As expected, when observed by TEM, the three strains presented a “hairy” surface as in the WT strains. Altogether, these results validate that the three KO-C mutants are efficiently complemented.
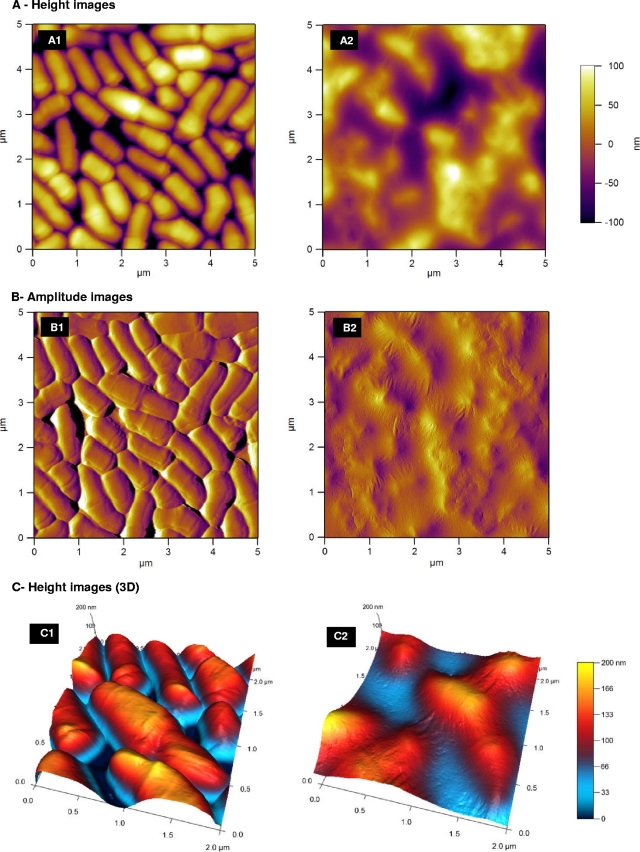
The presence of the surface polysaccharide dramatically changes the cell's topography (AFM) and sedimentation properties.
The strains P. freudenreichii LSP103 and its LSP103-KO variant were observed by AFM after depositing a small drop of culture onto a disk of mica. After drying, the mica surface was found to be heterogeneously (or partly) covered by a monolayer of cells in contact with each other. The images we provide are 5-by-5- and 2-by-2-μm images of the surface of this monolayer (Fig. 3). Such AFM images reveal obvious differences between the two strains. For LSP103-KO, the height picture, representing the topography of the bacterial surface, shows distinct cells with well-delimited contours (Fig. 3A1). Since the black zones between the cells correspond to the surface of the mica (meaning the cantilever is able to reach the mica between two consecutive cells), it is possible to estimate the height of the cells from the AFM results. It gives ∼200 nm, which is in good accordance with the sizes indicated in the literature (10) and by TEM (Fig. 2). In the exact same conditions of preparation and observation, the images obtained with LSP103 WT cells are fuzzy and much less distinct. The cells are indeed not clearly distinguishable, and the features are much less sharp than with the mutant (Fig. 3A2). These differences in sharpness between the two samples are also obvious when looking at the amplitude images (Fig. 3B1 and B2); these images are qualitatively representative of the relief of the sample. For LSP103-KO, the contours of the cells are still clearly distinguishable (Fig. 3B1). In contrast, the amplitude image for LSP103 shows cells that are covered by an elastic layer, which is presumably the EPS layer. The three-dimensional views of the height images (Fig. 3C1 and C2) give another view of this, with the LSP103 cells that are swamped in the (probable) EPS layer, whereas the LSP103-KO cells sit on the surface as individual and distinctive entities.
Fig 3.
P. freudenreichii LSP103 and its surface polysaccharide-abolished mutant. AFM two-dimensional height (A), three-dimensional height (C), and amplitude (B) images were recorded with cells recorded at stationary growth phase and resuspended in HEPES buffered solution. (A2, B2, and C2) LSP103 strain, which produces a β-glucan surface polysaccharide; (A1, B1, and C1) its derivative strain LSP103-KO, wherein the gene responsible for the production of the surface polysaccharide has been inactivated.
The spontaneous sedimentation propensity of the strains WT/KO/KO-C was studied after 2 days of growth. For the strains CB1 and its mutants CB1-KO and CB1-KO-C, no obvious difference of sedimentation was observed after 48 h of growth. The strain LSP110 WT moderately sedimented within 48 h: a small pellet of cells was observed at the bottom of the tube, and the supernatant was cloudy. On the contrary, for LSP110-KO mutant a strong sedimentation was observed: all of the cells were pelleted, and the supernatant was clear (see Fig. S1 in the supplemental material). The LSP103 WT culture was not sedimented, whereas its KO derivative was fully sedimented. For both the LSP110-KO-C and the LSP103-KO-C mutants, an intermediary state was observed: the cells were partially sedimented, and the supernatants were slightly cloudy. Interestingly, strain LSP103 WT is difficult to centrifuge under standard conditions (6,000 × g, 10 min), and the pellet obtained has an unstable form. Its KO derivative can easily be centrifuged, and the pellet is stable (data not shown).
The surface properties of the P. freudenreichii WT strains and their mutants have been investigated by measuring their zeta (ζ) potentials. At pH 7, the three WT strains presented a ζ potential close to −3 mV. The ζ potentials of the KO and KO-C mutants were not significantly different, and all of the values were comprised between −3 and −4.6 mV (data not shown).
Surface β-glucan modifies adhesion properties.
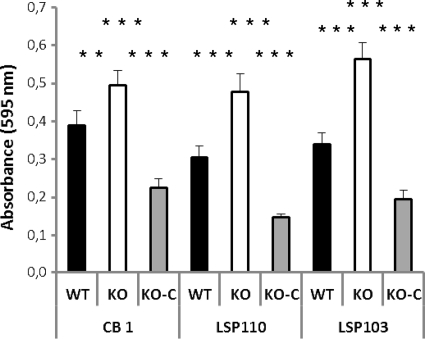
We investigated the role of surface β-glucan in the adhesion of the bacteria to human epithelial cells and abiotic surfaces. The level of adhesion of the strains to polystyrene surface was assessed (Fig. 4). The inactivation of the gtfF gene had the same effect for the three WT strains studied: a significant increase in the adhesion capacity was observed. Restoration of the production of the surface β-glucan polysaccharide led to the diminishing of the adhesion capacity, with absorbance levels lower than those measured for the WT strains. The capacity to adhere to eukaryotic Caco-2 cells was tested for the three WT strains and their KO derivatives. In contrast to biofilm formation on plastic microplates, none of the three WT strains studied here adhered under the conditions used (<1% of the initial value). In this context, EPS inactivation had no effect (data not shown).
Fig 4.
Inactivation of the gtfF gene led to a higher adhesion of P. freudenreichii to abiotic surface. The adherence on polystyrene surface of strains CB1, LSP110, LSP103, and their mutants was evaluated. Bars: ■, WT strains, producing surface β-glucan; □, KO mutants, inactivated in the gtfF gene, responsible for the biosynthesis of the β-glucan polysaccharide; ▩, KO-C mutants (KO strains reverted to the WT). The values are the means for three independent experiments; the significance of the results was checked by using the Mann-Whitney test (
 , P ≤ 0.01;
, P ≤ 0.01; 

 , P ≤ 0.001).
, P ≤ 0.001).
In vitro immunomodulatory evaluation of cells with or without the surface β-glucan.
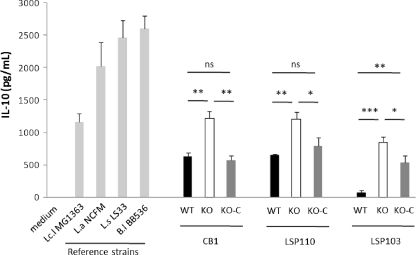
By using a well-described in vitro assay of cytokine release by human PBMCs, the cytokine induction pattern of the three triplets of strains was evaluated. The strains were tested for the induction of the anti-inflammatory IL-10 and the proinflammatory IL-12. The three WT strains producing surface β-glucan presented various anti-inflammatory properties, with LSP110 and CB1 being the highest IL-10-inducing strains (651 and 631 pg/ml, respectively) and LSP103 being a weak inducer of IL-10 (74 pg/ml) (Fig. 5). For these three strains, the levels of IL-10 induced were low and below those observed with the reference strain L. lactis MG1363, which is generally considered as a medium to low IL-10 inducer. An increase in IL-10 cytokine induction was observed for the three KO variants compared to their respective parent strains: 92%, 85%, and >10-fold induction (P < 0.05) for CB1-KO, LSP110-KO, and LSP103-KO, respectively. In the three β-glucan restored mutants, the levels of IL-10 induction tended to reach the values measured for the respective WT parent strains with 52.5, 46, and 37% (P < 0.05) reduction of the IL-10 levels measured between the KO and KO-C mutants. Interestingly, only slight but nonsignificant variations of IL-10 stimulation were observed between the WT and restored LSP110 and CB1 strains (8.6 and 21.5%, respectively).
Fig 5.
Anti-inflammatory cytokine responses of human PBMCs for P. freudenreichii strains producing or not producing a surface β-glucan polysaccharide. Bars: black, WT strains that produce the surface β-glucan polysaccharide; white, KO mutant strains wherein gtfF, the gene responsible for synthesis of the β-glucan polysaccharide has been inactivated; dark gray, KO-C strains, KO strains reverted to WT, producing β-glucan; light gray, reference strains (Lactococcus lactis MG1363, Lactobacillus acidophilus NCFM, Lactobacillus salivarius LS33, and Bifidobacterium longum BB536). The data are expressed in pg/ml as means ± the standard error of the mean, with n = 3 distinct healthy blood donors.  , P ≤ 0.05;
, P ≤ 0.05; 
 , P ≤ 0.01;
, P ≤ 0.01; 

 , P ≤ 0.001.
, P ≤ 0.001.
In addition, IL-12 and IFN-γ were not detectable in any of the supernatants when stimulated by the WT, mutants, or complemented strains. The higher level of induction of IL-10 due to the lack of EPS is not counteracted by these two proinflammatory signals, which generally antagonize the anti-inflammatory IL-10 cytokine (23).
Role of β-glucan surface polysaccharide in the gastrointestinal persistence of strains in the mice.
First, the WT strains and mutants were evaluated for their in vitro resistance to acid stress (33) (pH 2.5, 37°C, 1 h) and bile salt stress (1 g/liter, 37°C, 1 h) (42). For both types of stresses, when the cells were challenged during the exponential growth phase, they exhibited pronounced stress susceptibility, with survival rates of <0.1%. Cells challenged during the stationary growth phase (72 h of growth) were much more resistant to both stresses. Survival rates between 15 and 60% for the acidic challenge and 40 and 100% for the bile salt stress were measured. No significant difference in stress tolerance was observed between the three WT strains and their KO mutants (data not shown).
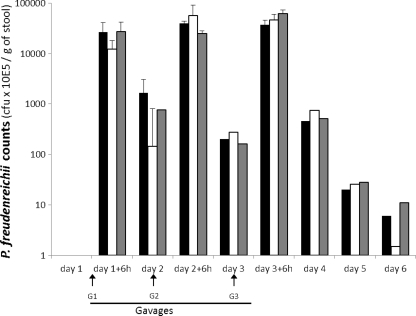
In order to know whether the β-glucan surface polysaccharide plays a role in the persistence of P. freudenreichii in the GIT, mice received by gavage the WT strain LSP103 or its mutants LSP103-KO and LSP103-KO-C. Each animal received a daily dose comprised between 2.27 × 109 and 2.42 × 109 CFU for three consecutive days. The persistence was evaluated by enumeration of viable propionibacteria on selective medium at different times during the gavage period and for 3 days after the last gavage. Before the ingestion of P. freudenreichii bacteria, no propionibacteria were detected (detection level of <1 × 102 CFU/g of stool) in the feces of animals (Fig. 6; see also Materials and Methods). Whatever the variants of P. freudenreichii LSP103 (WT, KO, and KO-C), the counts were comprised between 1.2 and 2.7 × 109 CFU/g of feces 6 h after the last gavage of the animals. After the last gavage (G3), the three P. freudenreichii variants tested could be detected in the mice feces for up to 72 h with a progressive decrease in counts. No significant longer or shorter persistence was observed according to the variant tested, since LSP103 WT, LSP103-KO, and LSP103-KO-C were all undetectable on the same time (day 7).
Fig 6.
In vivo persistence of P. freudenreichii strains that produce or do not produce β-glucan surface polysaccharide. Mice were fed 3 days consecutively (G1, G2, and G3) either with the β-glucan-producing WT strain LSP103 (■), its non-β-glucan-producing mutant LSP103-KO (□), or LSP103-KO-C (LSP103-KO mutant reverted to WT phenotype) (▩). Viable counts of P. freudenreichii strains were measured on selective medium in feces 6 h and 24 h after the gavages and 48 and 72 h after the last gavage. ND, not detectable (<1E+02 CFU/g of stool).
Altogether, these in vitro and in vivo results suggested that the β-glucan surface polysaccharide is not involved in the P. freudenreichii tolerance toward diverse digestive stresses or in gut persistence.
DISCUSSION
Surface EPS may highly impact some functional and probiotic properties of lactic acid and other food bacteria (25, 39). However, it is not possible to make general statements on the consequences that EPS may have, since the EPS molecule's nature is highly diverse. P. freudenreichii is known as a probiotic bacterium mainly for its bifidogenic properties demonstrated in humans (31, 53). Other health-promoting properties were described for the species (for a review, see reference 9). Recently, dairy propionibacteria were shown to display promising anti-inflammatory potentials that are strain dependent (22). On the other hand, 30% of P. freudenreichii strains produce a surface polysaccharide composed of (1→3,1→2)-β-d-glucan (12, 14). The same β-d-glucan polysaccharide is present on the surface of cider pediococci strains, which also display probiotic properties (20). In the present study, we investigated different properties associated with the presence of this molecule, particularly those that are considered as typical selection criteria for probiotic assessment: adhesion, stress resistance, immunomodulation, and survival in the GIT. For that purpose, we selected three wild P. freudenreichii strains producing the β-glucan surface polysaccharide and constructed KO and “KO and complemented” (KO-C) mutants regarding the gtfF gene associated with β-glucan synthesis. All of these constructions were validated by different methods. As expected, the inactivation of gtfF led to (i) a strong decrease in the gtfF expression and (ii) the loss of the anti-type 37 immunoagglutination capacities bound to the presence of the surface β-glucan polysaccharide. High gtfF expression was observed in the three KO-C strains, in conjunction with the restitution of the agglutination phenotype. This result confirms that the Ptuf promoter is a strong promoter for the efficient construction of recombinant strains in P. freudenreichii.
The presence of the (1→3,1→2)-β-d-glucan polysaccharide is quite scarce among bacteria, and it has been described for only a few food-related species: Pediococcus sp. (15, 60), Oenococcus oeni (8), and Lactobacillus suebicus (26), as well as in the nonpathogenic Streptococcus pneumoniae type 37 (44). The associated properties are not well known. In the present study we observed that β-glucan presence modifies the surface properties of the strains. It seems that the presence of β-glucan lowers the sedimentation of the cells in liquid culture and decreased adhesion to the abiotic surface. Indeed, the β-glucan KO mutants LSP103 and LSP110 showed a markedly high capacity for sedimentation compared to the WT strains. To our surprise, the presence of β-glucan did not lead to increased adhesion to abiotic surface, as was observed previously for Pediococcus parvulus (15). The presence of β-glucan was also reported to increase adhesion to epithelial cell lines (2); however, due to the very low basal in vitro adherence of P. freudenreichii strains to Caco-2 cells, we have not been able to demonstrate any difference between WT and mutants. Lebeer et al. (40) also observed in Lactobacillus rhamnosus an increase in the adhesion capacity for an isogenic mutant of the GG strain, inactivated in the production of the galactose-rich polysaccharide. The mutant still produced glucose-rich EPS, meaning that the nature of EPS is a determinant for the adhesion capacities. In any case, it is clear that EPS-producing species are distinctly charged, which may modify the interactions with their environment. This was demonstrated by the use of purified EPS fractions, which can either limit or promote the adherence of various bacteria (probiotics and commensals, as well as pathogens) in a specific and dose-dependent way (51). Our results demonstrate that the role of EPS in the context of adhesion clearly requires further study and, as with many other probiotic characteristics, requires a case-by-case analysis. In our study, the ζ potential of the three WT strains and their mutants was measured in order to know whether the surface charge could explain the differences of sedimentation and adhesion observed between the WT strains and their KO mutants. All strains presented a ζ potential at pH 7 close to −3.5 mV. The ζ potentials of 23 other strains of P. freudenreichii were measured at pH 7, and the potentials varied between −15 and −3 mV (G. Jan, unpublished data). These results indicate that the presence of a β-glucan surface polysaccharide renders the surface charge slightly negative and that the differences in sedimentation that we observed are probably not due to charge repulsion. The presence of a β-glucan could therefore be considered in selecting a strain for industrial processes in which rheological performance is important. When cells of LSP103 and LSP103-KO are observed using AFM, the structural differences found between the two strains are due to changes in the rigidity or in the hydration state of the cell wall, linked to the presence or absence of the polysaccharide layer. The atomic forces associated with the presence of the polysaccharide are currently under further study, since the resolution of the images obtained here does not permit us to determine whether the polymer is organized as a network or not. Marieta et al. used AFM analysis to show that in aqueous solution the polymer formed a network (46). Altogether, these results indicate that the surface properties of the strains investigated are affected by the loss of β-glucan, albeit not in the same way for all strains. Again, careful screening is necessary to select strains with the desired characteristics.
We also studied some properties that are used as selection criteria for possible probiotic strains: tolerance toward acidic stress and bile salts (52), adhesion, and persistence in the GIT. Tolerance toward digestive stresses was shown in vitro to be highly strain dependent in P. freudenreichii strains (36). Compared to their corresponding WT strains, the three KO mutants showed different survival rates under acidic and bile salt stresses, although these differences did not reach significance. This result strongly suggests that the surface β-glucan does not play a major role in the tolerance of P. freudenreichii toward digestive stresses. This result confirms those previously obtained with another β-glucan-producing organism, Pediococcus parvulus (20). Interestingly, the in situ heterologous production of β-glucan in Lactobacillus paracasei NCFB 338 led to a significant increase in tolerance to both simulated gastric juice and HCl acid stresses (55). Since previous studies reported either a positive (15, 20) or a negative (35) link between EPS production and stress tolerance, it is clear that no general statement can currently be made on the impact of EPS on stress resistance for lactobacilli, bifidobacteria, or any other genus or species, such as P. freudenreichii. However, it may depend on the other multiple strategies that each bacterium has developed to resist to stresses, e.g., propionibacteria, which are intrinsically very tolerant to various stresses independent of EPS occurrence.
In our animal trial, we found no evidence of a significant role for EPS in the survival and persistence of the propionibacteria in the murine GIT. Again, this result is in contrast to data obtained with an EPS-KO mutant of Lactobacillus rhamnosus GG (38). In Lactobacillus johnsonii a slightly increased gut persistence time with the EPS-KO mutant, compared to that with the WT strain, was also observed (11). Hence, the nature of the EPS may be a determinant.
In a previous work, we observed that P. freudenreichii presented interesting anti-inflammatory properties. This was based on results from a human PBMC screening, in which high levels of the anti-inflammatory cytokine IL-10 were found (22). The specific compounds associated with these anti-inflammatory properties are still unknown. Many studies reported immunomodulatory effects for β-glucans, especially in yeast. The activity of β-glucans strongly depends on the nature of the molecules, and the most active forms are β-glucans containing a β(1,3) backbone branched with β(1,6)-linked side chains (43, 61). The properties of (1→3,1→2)-β-d-glucans are much less studied. Previously, we observed that strains of P. freudenreichii producing a β-glucan surface polysaccharide were generally weaker inducers of the anti-inflammatory cytokine IL-10 than the non-β-glucan-producing ones (ca. 70% of the P. freudenreichii strains) (12, 14). Many publications suggested that the anti-inflammatory properties of food bacteria could be due to the presence of surface compounds: the s-layer in Lactobacillus acidophilus (35, 59), lipoteichoic acid (47), peptidoglycan (21), or specific outer membrane proteins (27). Here, we observed that the surface β-glucan produced by P. freudenreichii cells does not have intrinsic proinflammatory properties but rather contributes to either lower or at least neutralize the intrinsic immunomodulating properties of the bacterial cell wall. That β-glucan cannot be proinflammatory “by itself” is in agreement with recent observations reported for a strain of P. parvulus producing a similar β-glucan structure. In this case, the presence of EPS is associated with less proinflammatory signals (tumor necrosis factor alpha and IL-8) in M1-activated macrophages compared to the nonproducing EPS mutant (20); the intrinsic cell wall of the pediococcus shown here, in contrast, was immunoenhancing, as well as proinflammatory. The polysaccharide layer would then mask components that could be of immunological interest (either pro- or anti-inflammatory) depending on the type, strain, and/or species of bacteria.
Nevertheless, in P. freudenreichii strains, the β-glucan obviously affects the anti-inflammatory properties of the strains, since its inactivation systematically led to an increase in the anti-inflammatory response. We report here that for three distinct strains of P. freudenreichii the presence of a hairy surface due to the production of the polysaccharide layer could mask components that could be of immunological interest. The restored low levels of cytokine induction after complementation of the KO gene confirmed these observations.
These results obtained with parental recombinant strains are consistent with our previous observations (22), wherein immunomodulatory screening showed an intraspecific variability in the cytokine responses for various strains of P. freudenreichii. Indeed, we tested all of these strains for the production of the surface β-glucan polysaccharide, and none of the highest IL-10-inducing strains had a detectable β-glucan, whereas most of the weak IL-10 inducers effectively expressed it, confirming the “hiding” role of the β-glucan.
Conclusion.
In the present study, we demonstrated that the presence of surface β-glucan polysaccharide in P. freudenreichii gives bacteria some specific properties: reducing the capacity to adhere to the abiotic surface (but not epithelial cells) and partly hiding anti-inflammatory moieties, although no effect on stress survival or persistence in GIT was observed. Altogether, these results confirm our interest in studying the relationships between surface compounds of the bacteria and the anti-/proinflammatory balance. Here, we demonstrated that the production of a surface polysaccharide has to be considered in the selection of P. freudenreichii strains with anti-inflammatory potentialities. Considering both the food and probiotic applications, the selection of strains based on the occurrence of β-glucan EPS types may depend on the technological and health benefit to be reached. As an example, an anti-inflammatory strain (“β-glucan−”) will be preferable in an inflammatory bowel disease (IBD) context, whereas a more immunoenhancing bacteria (“β-glucan+”) will be more appropriate as an adjuvant in a vaccine context. Moreover, since we demonstrated that the removal of the β-glucan does not affect the stress tolerance abilities of the strains, it offers reliable and convenient tools to modulate functional parameters without bacterial counts concerns. As a consequence, we recommend taking this into account in the future selection of strains for specific applications.
Supplementary Material
ACKNOWLEDGMENTS
We thank Bruno Pot (Institut Pasteur de Lille) for critical reading of the manuscript. We thank Nathalie Roland from Laboratoires Standa for kindly providing the LSP strains of P. freudenreichii and for help in their selection. We also thank Y. Murooka for providing the pPK705 cloning vector, Christine Longin (INRA, Jouy-en-Josas, France), who performed the TEM observations, and Arnaud Waeghe (Institut Pasteur de Lille) for photographs.
This study has been partially financially supported by the ANR (Agence Nationale pour la Recherche) through the SURFING project (Starter SURFace against INFlammation of the Gut, ANR-10-ALIA-016). The purchase of the MFP-3D AFM by UMR1253 Science et Technologie du Lait et de l'Œuf has been partly funded by the European Union through the European Regional Development Fund (ERDF) project Compétitivité Régionale et Emploi coordinated by the Region Bretagne.
Footnotes
Published ahead of print 13 January 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Allen PZ, Bowen WH. 1988. Immunochemical studies on pneumococcal type 37 capsular polysaccharide. Mol. Immunol. 25:1011–1017 [DOI] [PubMed] [Google Scholar]
- 2. Alp G, Aslim B, Suludere Z, Akca G. 2010. The role of hemagglutination and effect of exopolysaccharide production on bifidobacteria adhesion to Caco-2 cells in vitro. Microbiol. Immunol. 54:658–665 [DOI] [PubMed] [Google Scholar]
- 3. Araujo R, Rodrigues AG, Pina-Vaz C. 2004. A fast, practical and reproducible procedure for the standardization of the cell density of an Aspergillus suspension. J. Med. Microbiol. 53:783–786 [DOI] [PubMed] [Google Scholar]
- 4. Bolshakova AV, et al. 2001. Comparative studies of bacteria with an atomic force microscopy operating in different modes. Ultramicroscopy 86:121–128 [DOI] [PubMed] [Google Scholar]
- 5. Bouglé D, Roland N, Lebeurrier F, Arhan P. 1999. Effect of propionibacteria supplementation on fecal bifidobacteria and segmental colonic transit time in healthy human subjects. Scand. J. Gastroenterol. 34:144–148 [DOI] [PubMed] [Google Scholar]
- 6. Bustin SA, et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611–622 [DOI] [PubMed] [Google Scholar]
- 7. Cerning J. 1995. Production of exopolysaccharides by lactic acid bacteria and dairy propionibacteria. Lait 75:463–472 [Google Scholar]
- 8. Ciezack G, et al. 2010. Evidence for exopolysaccharide production by Oenococcus oeni strains isolated from non-ropy wines. J. Appl. Microbiol. 108:499–509 [DOI] [PubMed] [Google Scholar]
- 9. Cousin FJ, Mater DDG, Foligné B, Jan G. 2011. Dairy propionibacteria as human probiotics: a review of recent evidence. Dairy Sci. Technol. 91:1–26 [Google Scholar]
- 10. Cummins C, Johnson J. 1992. The genus Propionibacterium, p 834–849 In Balows A, et al. (ed), The prokaryotes, 2nd ed Springer-Verlag, Inc., New York, NY [Google Scholar]
- 11. Denou E, et al. 2008. Identification of genes associated with the long-gut-persistence phenotype of the probiotic Lactobacillus johnsonii strain NCC533 using a combination of genomics and transcriptome analysis. J. Bacteriol. 190:3161–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deutsch S-M, Falentin H, Dols-Lafargue M, LaPointe G, Roy D. 2008. Capsular exopolysaccharide biosynthesis gene of Propionibacterium freudenreichii subsp. shermanii. Int. J. Food Microbiol. 125:252–258 [DOI] [PubMed] [Google Scholar]
- 13. Deutsch S-M, Ferain T, Delcour J, Lortal S. 2002. Lysis of lysogenic strains of Lactobacillus helveticus in Swiss cheeses and first evidence of concomitant Streptococcus thermophilus lysis. Int. Dairy J. 12:591–600 [Google Scholar]
- 14. Deutsch S-M, et al. 2010. Correlation of the capsular phenotype in Propionibacterium freudenreichii with the level of expression of gtf, a unique polysaccharide synthase-encoding gene. Appl. Environ. Microbiol. 76:2740–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dols-Lafargue M, et al. 2008. Characterization of gtf, a glucosyltransferase gene in the genomes of Pediococcus parvulus and Oenococcus oeni, two bacterial species commonly found in wine. Appl. Environ. Microbiol. 74:4079–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doron S, Snydman DR, Gorbach SL. 2005. Lactobacillus GG: bacteriology and clinical applications. Gastroenterol. Clin. N. Am. 34:483–498 [DOI] [PubMed] [Google Scholar]
- 17. Falentin H, et al. 2010. The complete genome of Propionibacterium freudenreichii CIRM-BIA1, a hardy actinobacterium with food and probiotic applications. PLoS One 5:e11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falentin H, et al. 2010. Specific metabolic activity of ripening bacteria quantified by real-time reverse transcription PCR throughout Emmental cheese manufacture. Int. J. Food Microbiol. 144:10–19 [DOI] [PubMed] [Google Scholar]
- 19. FAO-WHO 2002. Report of a joint FAO/WHO expert consultation on guidelines for the evaluation of probiotics in food. Canada World Health Organization/Food and Agriculture Organization of the United Nations, London, Ontario, Canada [Google Scholar]
- 20. Fernández de Palencia P, et al. 2009. Probiotic properties of the 2-substituted (1,3)-β-d-glucan-producing bacterium Pediococcus parvulus 2.6. Appl. Environ. Microbiol. 75:4887–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernandez EM, et al. 2011. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 60:1050–1059 [DOI] [PubMed] [Google Scholar]
- 22. Foligné B, et al. 2010. Promising immunomodulatory effects of selected strains of dairy propionibacteria evidenced in vitro and in vivo. Appl. Environ. Microbiol. 76:8259–8264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Foligné B, et al. 2007. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J. Gastroenterol. 13:236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Francius G, et al. 2008. Detection, localization, and conformational analysis of single polysaccharide molecules on live bacteria. ACS Nano. 2:1921–1929 [DOI] [PubMed] [Google Scholar]
- 25. Freitas F, Alves VD, Reis MA. 2011. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 29:388–398 [DOI] [PubMed] [Google Scholar]
- 26. Gaizka GI, et al. 2010. Naturally occurring 2-substituted (1,3)-β-d-glucan-producing Lactobacillus suebicus and Pediococcus parvulus strains with potential utility in the production of functional foods. Bioresource Technol. 101:9254–9263 [DOI] [PubMed] [Google Scholar]
- 27. Guglielmetti S, et al. 2008. Implication of an outer surface lipoprotein in adhesion of Bifidobacterium bifidum to Caco-2 cells. Appl. Environ. Microbiol. 74:4695–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580 [DOI] [PubMed] [Google Scholar]
- 29. Hassan AN. 2008. ADSA Foundation Scholar Award: possibilities and challenges of exopolysaccharide-producing lactic cultures in dairy foods. J. Dairy Sci. 91:1282–1298 [DOI] [PubMed] [Google Scholar]
- 30. Hervé C, Fondrevez M, Chéron A, Barloy-Hubler F, Jan G. 2007. Transcarboxylase mRNA: a marker which evidences P. freudenreichii survival and metabolic activity during its transit in the human gut. Int. J. Food Microbiol. 113:303–314 [DOI] [PubMed] [Google Scholar]
- 31. Hojo K, et al. 2002. Effect of ingested culture of Propionibacterium freudenreichii ET-3 on fecal microflora and stool frequency in healthy females. Biosci. Microfl. 21:115–120 [Google Scholar]
- 32. Isawa K, et al. 2002. Isolation and identification of a new bifidogenic growth stimulator produced by Propionibacterium freudenreichii ET-3. Biosci. Biotech. Biochem. 66:679–681 [DOI] [PubMed] [Google Scholar]
- 33. Jan G, Leverrier P, Pichereau V, Boyaval P. 2001. Changes in protein synthesis and morphology during acid adaptation of Propionibacterium freudenreichii. Appl. Environ. Microbiol. 67:2029–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kiatpapan P, et al. 2000. Characterization of pRGO1, a plasmid from Propionibacterium acidipropionici, and its use for development of a host-vector system in propionibacteria. Appl. Environ. Microbiol. 66:4688–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Konstantinov SR, et al. 2008. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. U. S. A. 105:19474–19478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lan A, et al. 2007. Survival and metabolic activity of selected strains of Propionibacterium freudenreichii in the gastrointestinal tract of human microbiota-associated rats. Br. J. Nutr. 97:714–724 [DOI] [PubMed] [Google Scholar]
- 37. Landersjo C, Yang ZN, Huttunen E, Widmalm G. 2002. Structural studies of the exopolysaccharide produced by Lactobacillus rhamnosus strain GG (ATCC 53103). Biomacromolecules 3:880–884 [DOI] [PubMed] [Google Scholar]
- 38. Lebeer S, Claes IJ, Verhoeven TL, Vanderleyden J, De Keersmaecker SC. 2010. Exopolysaccharides of Lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine. Microb. Biotechnol. 4:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lebeer S, Vanderleyden J, De Keersmaecker SCJ. 2008. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 72:728–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lebeer S, et al. 2009. Identification of a gene cluster for the biosynthesis of a long, galactose-rich exopolysaccharide in Lactobacillus rhamnosus GG and functional analysis of the priming glycosyltransferase. Appl. Environ. Microbiol. 75:3554–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lehto EM, Salminen S. 1997. Adhesion of two Lactobacillus strains, one Lactococcus and one Propionibacterium strain to cultured human intestinal Caco-2 cell line. Biosci. Microfl. 16:13–17 [Google Scholar]
- 42. Leverrier P, et al. 2003. Susceptibility and adaptive response to bile salts in Propionibacterium freudenreichii: physiological and proteomic analysis. Appl. Environ. Microbiol. 69:3809–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu J, Gunn L, Hansen R, Yan J. 2009. Combined yeast-derived beta-glucan with anti-tumor monoclonal antibody for cancer immunotherapy. Exp. Mol. Pathol. 86:208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Llull D, García E, López R. 2001. Tts, a processive β-glucosyltransferase of Streptococcus pneumoniae, directs the synthesis of the branched type 37 capsular polysaccharide in pneumococcus and other gram-positive species. J. Biol. Chem. 276:21053–21061 [DOI] [PubMed] [Google Scholar]
- 45. Malik AC, Reinbold GW, Vedamuthu ER. 1968. An evaluation of the taxonomy of Propionibacterium. Can. J. Microbiol. 14:1185–1191 [DOI] [PubMed] [Google Scholar]
- 46. Marieta C, Ibarburu I, Duenas M, Irastorza A. 2009. Supramolecular structure and conformation of a (1→3)(1→2)-β-d-glucan from Lactobacillus suebicus CUPV221 as observed by tapping mode atomic force microscopy. J. Agric. Food Chem. 57:6183–6188 [DOI] [PubMed] [Google Scholar]
- 47. Mohamadzadeh M, et al. 2011. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc. Natl. Acad. Sci. U. S. A. 108:4623–4630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 28:449–461 [DOI] [PubMed] [Google Scholar]
- 49. Robichon D, Girard J-C, Cenatiempo Y, Cavellier J-F. 1999. Atomic force microscopy imaging of dried or living bacteria. C.R. Acad. Sci. III 322:687–693 [DOI] [PubMed] [Google Scholar]
- 50. Ruas-Madiedo P, Abraham A, Mozzi F, de los Reyes-Gavilán GC. 2008. Functionality of exopolysaccharides produced by lactic acid bacteria, p 137–166 In Mayo B, Lopez P, Perez-Martinez G. (ed), Molecular aspects of lactic acid bacteria for traditional and new applications. Research Signpost, Trivandrum, India [Google Scholar]
- 51. Ruas-Madiedo P, Gueimonde M, Margolles A, de los Reyes-Gavilán GC, Salminen S. 2006. Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J. Food Prot. 69:2011–2015 [DOI] [PubMed] [Google Scholar]
- 52. Sanders ME. 2008. Probiotics: definition, sources, selection, and uses. Clin. Infect. Dis. 46:S58–S61 [DOI] [PubMed] [Google Scholar]
- 53. Satomi K, Kurihara H, Isawa K, Mori H, Kaneko T. 1999. Effects of culture-powder of Propionibacterium freudenreichii ET-3 on fecal microflora of normal adults. Biosci. Microfl. 18:27–30 [Google Scholar]
- 54. Schär-Zammaretti P, Ubbink J. 2003. The cell wall of lactic acid bacteria: surface constituents and macromolecular conformations. Biophys. J. 85:4076–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stack HM, Kearney N, Stanton C, Fitzgerald G, Ross R. 2010. Association of beta-glucan endogenous production with increased stress tolerance of intestinal lactobacilli. Appl. Environ. Microbiol. 76:500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. 2010. A practical approach to RT-qPCR-publishing data that conform to the MIQE guidelines. Methods 50:S1–S5 [DOI] [PubMed] [Google Scholar]
- 57. Thierry A, et al. 2011. New insights into physiology and metabolism of Propionibacterium freudenreichii. Int. J. Food Microbiol. 149:19–27 [DOI] [PubMed] [Google Scholar]
- 58. Vandesompele J, et al. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:0034.1–0034.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Hemert S, et al. 2010. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 10:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Velasco SE, et al. 2009. Chemical and rheological properties of the beta-glucan produced by Pediococcus parvulus 2.6. J. Agric. Food Chem. 57:1827–1834 [DOI] [PubMed] [Google Scholar]
- 61. Vetvicka V. 2011. Glucan-immunostimulant, adjuvant, potential drug. World J. Clin. Oncol. 2:115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vu B, Chen M, Crawford RJ, Ivanova EP. 2009. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14:2535–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Walling E, Gindreau E, Lonvaud-Funel A. 2005. A putative glucan synthase gene dps detected in exopolysaccharide-producing Pediococcus damnosus and Oenococcus oeni strains isolated from wine and cider. Int. J. Food Microbiol. 98:53–62 [DOI] [PubMed] [Google Scholar]
- 64. Weiner R, Langille S, Quintero E. 1995. Structure, function and immunochemistry of bacterial exopolysaccharides. J. Industrial Microbiology 15:339–346 [DOI] [PubMed] [Google Scholar]
- 65. Yasuda E, Serata M, Sako T. 2008. Suppressive effect on activation of macrophages by Lactobacillus casei strain Shirota genes determining the synthesis of cell wall-associated polysaccharides. Appl. Environ. Microbiol. 74:4746–4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.