Abstract
N-Acylated homoserine lactone (AHL) lactonases are capable of degrading signal molecules involved in bacterial quorum sensing and therefore represent a new approach to control bacterial infection. Here a gene responsible for the AHL lactonase activity of Bacillus sp. strain AI96, 753 bp in length, was cloned and then expressed in Escherichia coli. The deduced amino acid sequence of Bacillus sp. AI96 AiiA (AiiAAI96) is most similar to those of other Bacillus sp. AHL lactonases (∼80% sequence identity) and was consequently categorized as a member of the metallo-β-lactamase superfamily. AiiAAI96 maintains ∼100% of its activity at 10°C to 40°C at pH 8.0, and it is very stable at 70°C at pH 8.0 for at least 1 h; no other Bacillus AHL lactonase has been found to be stable under these conditions. AiiAAI96 resists digestion by proteases and carp intestinal juice, and it has broad-spectrum substrate specificity. The supplementation of AiiAAI96 into fish feed by oral administration significantly attenuated Aeromonas hydrophila infection in zebrafish. This is the first report of the oral administration of an AHL lactonase for the efficient control of A. hydrophila.
INTRODUCTION
Bacteria communicate with each other by quorum sensing, a mechanism that is dependent on their population density and which was first reported to be the trigger for the bioluminescence of the aquatic bacterium Vibrio fischeri (39). Quorum-sensing bacteria can release, detect, and respond to the accumulated small signal molecules and then regulate the expressions of target genes (21, 33). Quorum-sensing systems have been found in pathogenic bacteria of plants, animals, and humans (13, 53). Gram-negative bacteria use N-acylated homoserine lactones (AHLs) as signal molecules (13). AHLs vary in their acyl chain lengths (4 to 14 carbons) and/or contain an oxo or a hydroxyl substituent in the acyl chain (21, 33). Many of the infection-related phenotypes associated with quorum sensing are controlled by AHL/LuxR/LuxI systems: LuxR is the AHL receptor, and LuxI is the AHL synthase. The quorum-sensing systems can induce antibiotic production; plasmid conjugation; nodulation; biocorrosion; biofilm formation; and the expression of virulence factors, e.g., proteases, lytic enzymes, toxins, siderophores, and adhesion molecules (1, 8, 13, 33, 43, 53).
Aeromonas hydrophila is a primary, secondary, and opportunistic Gram-negative bacterial pathogen (2). It is found mainly in water and water-related environments and causes a wide variety of symptoms in fish, including tissue swelling, necrosis, ulceration, and hemorrhagic septicemia (2, 9). A. hydrophila has the typical AHL/LuxR/LuxI quorum-sensing system and the signal molecules N-butyryl-l-homoserine lactone (C4-HSL) (the major part) and N-hexanoyl-l-homoserine lactone (C6-HSL) (the minor part). Many pathogenesis-related factors, including the expression of virulence factors (such as hemolysin, protease, S-layer proteins, DNase, and amylase), biofilm maturation, and the type II, III, and VI secretion systems, have been reported to be under the regulation of quorum sensing in Aeromonas spp. (4, 25, 26, 48, 49, 51). A. hydrophila is strongly resistant to multiple antibiotics and consequently results in significant economic losses to freshwater and warm-water fish farming worldwide (11). Thus, quorum quenching may provide an alternative efficient strategy to control A. hydrophila infection (10–12, 22, 40). AhyI/AhyR interference and AHL-degrading enzymes might be used to inhibit quorum-sensing systems, of which AHL-degrading enzymes are the most readily available tools (10, 22).
The first AHL-degrading enzyme was identified in Bacillus sp. strain 240B1 (16). The expression of its gene in Erwinia carotovora SCG1, a plant soft-rot pathogen, substantially reduced the level of AHL and consequently decreased proteolysis regulated by AHL and attenuated E. carotovora pathogenicity in plants (16). To date, more than 20 AHL-degrading enzymes have been identified in bacteria, fungi, and mammals (10, 14, 15). According to their AHL cleavage sites, these enzymes are classified as AHL lactonases (EC 3.1.1.81) or AHL acylases (synonym, AHL amidases) (EC 3.5.1.97) (http://www.chem.qmul.ac.uk/iubmb/enzyme/). Paraoxonases (PONs) from mammalian sera also have lactonase-like activities in addition to their involvement in the hydrolysis of organophosphates (55). In contrast to AHL acylase and PONs, which have variable substrate spectra, as reported previously, AHL lactonase is by far the most specific AHL-degrading enzyme, with both short- and long-chain AHLs as substrates and no or little activity with other chemicals (18, 52).
Many Bacillus AHL lactonase-like enzymes have been found, and they are closely related (∼90% sequence identity) (14, 28). They contain the conserved motif HXHXDH and a zinc binding motif, and they are classified as metallo-β-lactamases (MBLs) (16). The active sites of autoinducer inactivation (AiiA) AHL lactonases contain a dinuclear zinc binding center bridged by an aspartate and an oxygen species (29, 30, 35). The AHL lactonase AiiB from Agrobacterium tumefaciens also has the same active sites (31). Given their quorum-quenching abilities, AHL lactonases provide a new tool that may inhibit bacterial infection. For example, a plant engineered to express AHL lactonase had substantially enhanced resistance to E. carotovora infection (16, 17). AHL lactonase expression in the pathogens Erwinia amylovora, Pseudomonas aeruginosa PAO1, and Burkholderia cepacia reduced their virulence by degrading AHLs (34, 47, 54). In addition, AHL lactonases have also been expressed in Escherichia coli and Pichia pastoris (7, 52).
For the study reported here, we isolated a Bacillus strain from pond sediment in China and showed that it has AHL lactonase activity. The gene responsible for this activity, Bacillus sp. strain AI96 aiiA (aiiAAI96), was cloned and expressed in Escherichia coli. The physical properties that dictate the level of activity for the purified recombinant enzyme, AiiAAI96, suggest that it could be used as an aquatic food additive, and indeed, we found that it attenuated the virulence of Aeromonas hydrophila in zebrafish.
MATERIALS AND METHODS
Strains, plasmids, enzymes, and chemicals.
The AHL-sensing bacterium Agrobacterium tumefaciens KYC55(pJZ372)(pJZ384)(pJZ410), which has a β-galactosidase reporter gene and is most sensitive to N-(3-oxooctanoyl)-l-homoserine lactone (3-oxo-C8-HSL), was donated by Jun Zhu (Nanjing Agricultural University, Nanjing, People's Republic of China) (57). The biosensor Chromobacterium violaceum CV026, obtained from Shuishang Song (Hebei Academy of Sciences, Shijiazhuang, People's Republic of China), with the purple pigment violacein as a reporter, is most sensitive to C6-HSL and more sensitive to C4-HSL than strain KYC55 (32). A. hydrophila NJ-1, which had been isolated from a crucian carp (Carassius carassius), was donated by Yongjie Liu (Nanjing Agricultural University, Nanjing, People's Republic of China). E. coli Trans-I and the vector pEASY-T3 were used for gene cloning and sequencing (both from TransGen, Beijing, People's Republic of China). E. coli BL21(DE3) and the vector pET-28a(+) were used for gene expression (both from Novagen, Darmstadt, Germany). All strains were cultured in Luria-Bertani (LB) medium. Pfu Taq DNA polymerase and restriction endonucleases were obtained from TaKaRa (Otsu, Japan). T4 DNA ligase was obtained from Invitrogen (Carlsbad, CA). C4-HSL, C6-HSL, N-heptanoyl-dl-homoserine lactone (C7-HSL), N-octanoyl-l-homoserine lactone (C8-HSL), N-decanoyl-l-homoserine lactone (C10-HSL), N-dodecanoyl-l-homoserine lactone (C12-HSL), N-tetradecanoyl-l-homoserine lactone (C14-HSL), N-(3-oxohexanoyl)-d-homoserine lactone (3-oxo-C6-HSL), 3-oxo-C8-HSL, N-(3-oxodecanoyl)-l-homoserine lactone (3-oxo-C10-HSL), N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL), N-(3-oxotetradecanoyl)-l-homoserine lactone (3-oxo-C14-HSL), N-(3-hydroxydodecanoyl)-dl-homoserine lactone (3-hydroxy-C12-HSL), N-(3-hydroxytetradecanoyl)-dl-homoserine lactone (3-hydroxy-C14-HSL), and proteases (including trypsin, α-chymotrypsin, subtilisin A, collagenase, and proteinase K) were obtained from Sigma-Aldrich (St. Louis, MO). Isopropyl-β-d-1-thiogalactopyranoside (IPTG) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) were obtained from Promega (Madison, WI). The PageRuler prestained protein ladder was obtained from Fermentas, a subsidiary of Thermo Fisher Scientific (Waltham, MA). All other chemicals were analytical grade and commercially available.
AHL lactonase assay.
AHL lactonase activity was determined as described previously (46), with some modifications. Reaction mixtures containing 179 μl phosphate-buffered saline (PBS) (pH 8.0), 20 μl of AiiAAI96 solution, and 1 μl of 3-oxo-C8-HSL (l mg/ml) were incubated at 30°C for 30 min, and the reactions were then terminated by the addition of 10% SDS (50 μl) to the mixture. Agar (1.5% [wt/vol]) strips with dimensions of 6 by 8 mm were prepared, which contained AT minimal salt medium (50) and 20 μg/ml X-gal. After the termination of a reaction, 10 μl of each mixture was added into a punched circular area (r = 2 mm) at one end of the agar strip, and the AHL-sensing strain KYC55, on a toothpick, was placed onto the strip at 4-mm intervals away from the punched area. After the agar strip was cultivated at 30°C for 24 h, the colony point that turned blue was calculated to detect the 3-oxo-C8-HSL diffusion distance. For the standard curve (y = 6.52 × e0.35 × x/106; R2 = 0.995 [where y, in nmol, is the amount of 3-oxo-C8-HSL and x, in mm, is the distance of diffusion of 3-oxo-C8-HSL]), the initial concentrations of pure 3-oxo-C8-HSL were varied and added directly into the punched area of individual strips, and at the end of the assay, concentrations and the lengths over which the 3-oxo-C8-HSL samples had diffused were correlated. One unit of AHL lactonase activity was defined as the amount of enzyme that hydrolyzed 1 nmol 3-oxo-C8-HSL per minute.
Isolation and identification of Bacillus sp. AI96.
A sediment sample was collected from a pond in Wuqing, Tianjin, People's Republic of China. One gram of pond sediment was suspended in 10 ml of sterile water. The suspension was 10-fold serially diluted six times, and 100 μl from each dilution was individually cultivated on LB plates at 20°C for 5 days. Pure cultures were obtained by repeatedly streaking and cultivating the bacteria in LB medium at 20°C for 1 to 3 days. Cells were collected by centrifugation at 10,000 × g at 4°C for 5 min, suspended in PBS (pH 7.4), and sonicated in an ice bath. Cell lysates were centrifuged at 12,500 × g at 4°C for 15 min, and the supernatant were assessed for AHL lactonase activity by the agar strip assay. The genera of the strains with AHL lactonase activity were identified by the sequencing of their 16S rRNA genes (36). Bacillus sp. AI96 was chosen for further study for the greatest AHL lactonase activity.
Cloning of aiiAAI96.
Two primers, AI96-up (5′-CTGATATGAGAAGGTGGATA-3′) and AI96-down (5′-AACAGCATTATGATTTCCC-3′), with sequences derived from the upstream and downstream sequences of genes encoding known AiiA-like enzymes, and genomic DNA from Bacillus sp. AI96 were used to amplify the full-length aiiAAI96 gene. The PCR program was 95°C for 5 min, followed by 30 cycles of 94°C for 30 s, 45°C for 30 s, and 72°C for 1 min, with a final step of 72°C for 5 min. The PCR product was purified and ligated into pEASY-T3 for sequencing. The nucleotide sequence and open reading frame were determined by using Vector NTI 10 software and the NCBI open reading frame finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html), respectively. SignalP (http://www.cbs.dtu.dk/services/SignalP/) was used to search for a signal sequence. Sequence alignment was performed with BLAST at the NCBI website and with Vector NTI 10 software. The tertiary structure of AiiAAI96 was predicated by using SWISS-MODEL (http://swissmodel.expasy.org/) and Swiss-PdbViewer DeepView v4.0 software with the AHL lactonase from Bacillus thuringiensis (Protein Data Bank [PDB] accession number 2A7M) (79% sequence identity) as the template (30).
Expression and purification of AiiAAI96.
A gene encoding AiiAAI96 was amplified by using primers AI96aiia-EF (5′-CCGGAATTCATGACCGTTAAAAAACTTTAC-3′ [the EcoRI restriction site is underlined]) and AI96aiia-ER (5′-ATTGCGGCCGCTTATAAAAATTCAGGAAATG-3′ [the NotI restriction site is underlined]). The amplification program was 95°C for 5 min, followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, with a final step of 72°C for 10 min. The PCR product was gel purified, digested with EcoRI and NotI, and cloned into the EcoRI and NotI sites of pET-28a(+) using T4 DNA ligase. The recombinant plasmid, pET-aiiAAI96, was transformed by electroporation into E. coli BL21(DE3) competent cells that were then cultured overnight at 37°C in LB medium with 50 μg/ml kanamycin. Positive transformants were individually grown in LB medium containing 50 μg/ml kanamycin at 37°C for 2 to 3 h to an optical density at 600 nm (OD600) of ∼0.6. AHL lactonase expression was induced by the addition of 1 mM IPTG to the mixture, and the cells were cultured for an additional 12 h at 18°C with constant agitation at 180 rpm.
To purify AiiAAI96, cells were harvested by centrifugation, washed with Ni-nitrilotriacetic acid (NTA) elution buffer (20 mM Tris-HCl [pH 7.6], 500 mM NaCl, 10% [wt/vol] glycerol), and resuspended in the same buffer. The cells, on ice, were disrupted by sonication, and the cell lysate was collected by centrifugation at 12,500 × g for 15 min. The cell lysate (5 ml) was loaded onto Ni-NTA resin (1 ml; Qiagen, Hilden, Germany) that had been equilibrated with elution buffer. The protein was eluted with a linear gradient of 20 to 300 mM imidazole in elution buffer. Fractions with AHL lactonase activity were pooled and concentrated with a Nanosep centrifugal device (10 kDa; Pall, East Hills, NY).
The apparent molecular mass of the purified protein was determined by SDS-PAGE (12% [wt/vol]) after staining it and molecular mass standards with Coomassie brilliant blue R-250. After isolation from the gel, AiiAAI96 was sequenced by liquid chromatography-electrospray ionization-tandem mass spectroscopy (LC-ESI-MS/MS) at Tianjin Biochip Corporation Co. Ltd. (Tianjin, People's Republic of China). The peptide sequences determined by tandem mass spectrometry were compared with those found in the deduced AiiAAI96 sequence. Protein concentrations were routinely determined by the Bradford method (5).
Physical parameters that affect AiiAAI96 activity and measurement of catalytic constants.
3-Oxo-C8-HSL was routinely used as the substrate in all assays except for the substrate specificity assay. The activity-pH profile was determined at 30°C with buffers with pH values that ranged from 4.0 to 11.0. To determine the stability of the enzyme at various pH values, it was incubated at 30°C in various buffers for 30 min without the substrate, and the residual activity was then measured under standard conditions (pH 8.0 at 30°C for 30 min). The buffers used were McIlvaine buffer (pH 3.0 to 6.0), PBS (pH 6.0 to 8.0), 0.05 M Tris-HCl (pH 8.0 to 9.0), and 0.1 M glycine-NaOH (pH 9.0 to 12.0).
The activity-temperature profile was determined at pH 8.0 at temperatures between 0°C and 70°C. The thermal stability of AiiAAI96 was determined by measuring its residual activity under standard conditions after incubating the enzyme at 70°C or 80°C for 2, 5, 10, 15, 20, 30, and 60 min and at 90°C for 1, 3, and 5 min. The effects of metal ions and chemical reagents on activity were determined at concentrations of 1 or 10 mM. A sample without an additive served as the control.
Values of the kinetic constants Km and Vmax were determined by using assay systems that consisted of mixtures of PBS (pH 8.0) and 0.017 to 16.578 nM 3-oxo-C8-HSL and an incubation time of 5 min at 30°C. To determine the kinetic values, the data were plotted as a Lineweaver-Burk curve.
To determine its resistance to proteolysis, AiiAAI96 was individually incubated with trypsin, α-chymotrypsin, proteinase K, subtilisin A, or collagenase at a protease-to-protein (wt/wt) ratio of 1:10 (20) at 30°C for 30 and 60 min, respectively. The residual activities were detected under standard conditions with no protease-treated enzyme as the control.
The resistance of AiiAAI96 to digestion with intestinal juice was also evaluated. Intestinal juice was prepared as described previously (44). In brief, the full-length intestine of a carp (Cyprinus carpio) that had been anesthetized with the anesthetic MS-222 (tricaine methanesulfonate) 1 h after feeding was gently washed three times with PBS (pH 7.4) and then dissected with a scalpel. The intestinal mucus was washed out with PBS (pH 7.4) and centrifuged at 8,000 × g for 20 min at 4°C. The supernatant (carp intestinal juice) was retained and was incubated with AiiAAI96 at a ratio of 1:10 (vol/vol) for 30 and 60 min at 30°C. Residual activity was determined under standard conditions.
The protease sensitivity experiment was carried out as described above, with casein as the positive control, and the degree of degradation of casein was determined by the Folin-phenol method (27). The stability of proteases and intestinal juice in the absence of the target protein was measured. After being treated by using the same protocol, residual protease activities were estimated by the Folin-phenol method (27).
Substrate specificity assays.
The ability of AiiAAI96 to hydrolyze C4-HSL, C6-HSL, C7-HSL, C8-HSL, C10-HSL, C12-HSL, C14-HSL, 3-oxo-C6-HSL, 3-oxo-C10-HSL, 3-oxo-C12-HSL, 3-oxo-C14-HSL, 3-hydroxy-C8-HSL, and 3-hydroxy-C14-HSL, which were individually reacted in PBS (pH 8.0), was measured as described above for 3-oxo-C8-HSL under otherwise standard assay conditions, with A. tumefaciens KYC55 or C. violaceum CV026 as the biosensor. All hydrolytic reactions were carried out for 30 min under conditions (pH 8.0 at 30°C) in which A. hydrophila is apt to grow.
Preparation of A. hydrophila NJ-1 cells and fish diet.
After cultivation at 30°C for 12 h, A. hydrophila NJ-1 cells were collected by centrifugation at 3,000 × g for 15 min, washed three times with sterile PBS (pH 7.4), and suspended in sterile distilled water at an OD600 of ∼0.15. This bacterial suspension was used to grow fish. To ensure the validity of pathogens, a fresh A. hydrophila NJ-1 cell suspension was prepared and exchanged every 2 days. The CK diet (control diet) was supplied by Zhejiang Xinxin Feed Co. Ltd., Jiaxing, People's Republic of China, and contained 47% fishmeal, 24% soybean meal, 24% wheat flour, 2% soybean oil, 3% premix, and no antibiotics (42.0% crude protein and 7.29% crude lipid).
Effect of AiiAAI96 on A. hydrophila NJ-1 infection.
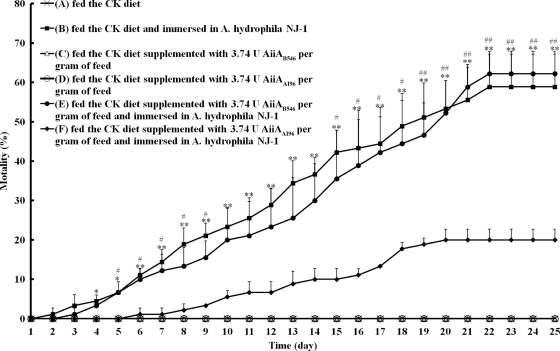
Wild-type zebrafish (4 months of age, with an average weight of ∼200 mg and an average length of ∼2.5 cm) were randomly divided into six test groups: group A, which was fed the CK diet and immersed in clean water; group B, which was fed the CK diet and immersed in A. hydrophila NJ-1-containing water; group C, which was fed the CK diet supplemented with 3.74 U AiiAB546 per gram of feed and immersed in clean water (7); group D, which was fed the CK diet supplemented with 3.74 U AiiAAI96 per gram of feed and immersed in clean water; group E, which was fed the CK diet supplemented with 3.74 U AiiAB546 per gram of feed and immersed in A. hydrophila NJ-1-containing water; and group F, which was fed the CK diet supplemented with 3.74 U AiiAAI96 per gram of feed and immersed in A. hydrophila NJ-1-containing water. Each group had four replicate tanks, and each tank had 30 fish. The zebrafish were allowed free access to food (see below) at 09:30 and 16:30. Mortality during the 25-day experimental period was recorded each day. Dead fish of groups B, E, and F were removed daily and examined for bacteriological contamination, as was the water containing A. hydrophila NJ-1. The fish and water of groups A, C, and D, which served as controls, were also examined at 25 days. To test bacterial contamination, the fish body was sterilized with 75% ethanol, and the body fluid was extracted with a syringe under sterile conditions and streaked onto an ampicillin blood agar plate (24). The water sample was streaked onto the same plate directly.
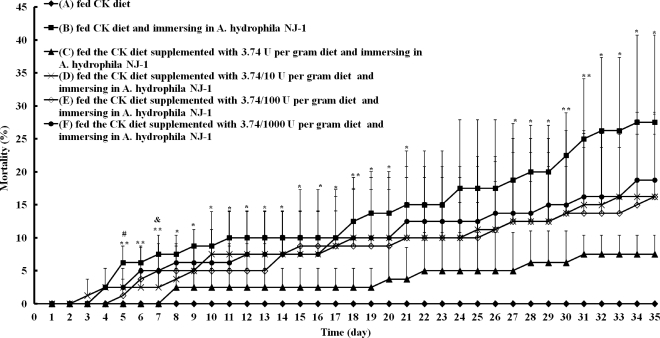
The amount of AiiAAI96 needed to protect the fish against A. hydrophila was assessed by adding various amounts of AiiAAI96 to the feed. The zebrafish were divided into six groups: group A, which was fed the CK diet (the regular diet) and immersed in clean water; group B, which was fed the CK diet and immersed in A. hydrophila NJ-1-containing water; and groups C, D, E, and F, which were fed the CK diet supplemented with 3.74, 3.74 × 10−1, 3.74 × 10−2, and 3.74 × 10−3 U AiiAAI96 per gram of feed, respectively, and immersed in A. hydrophila NJ-1-containing water. Each group had four replicate tanks, and each tank had 30 fish. Mortality during the 35-day experimental period was recorded each day.
The stability of AiiAAI96 in the experimental diet was assessed by storing AiiAAI96-supplemented feed at 25°C for 2, 4, and 8 weeks and then assaying the feed for activity with 3-oxo-C8-HSL as the substrate.
Nucleotide sequence accession numbers.
The aiiAAI96 and the 16S rRNA gene nucleotide sequences of Bacillus sp. AI96 have been deposited in the GenBank database under accession numbers HM750247 and HQ680867, respectively.
RESULTS
Identification of Bacillus sp. AI96.
Significant AHL lactonase activity was detected only in the intracellular protein of strain AI96, and this strain was classified as a Bacillus strain based on its 16S rRNA gene sequence, which was identical to those of Bacillus pseudomycoides J14 (GenBank accession number GU826151.1) (100% identity) and B. mycoides (accession number GU171377.1) (100% identity). A sample of Bacillus sp. AI96 was deposited in the China General Microbiological Culture Collection Center (Beijing, People's Republic of China) under registration number CGMCC 4164.
Cloning and sequence analysis of aiiAAI96.
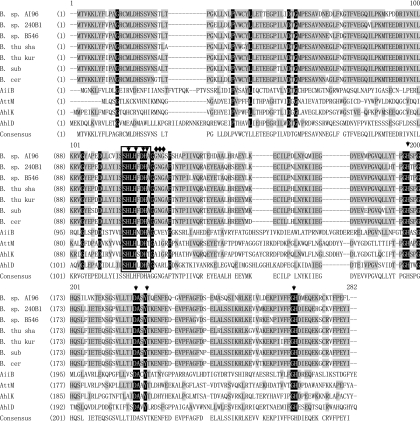
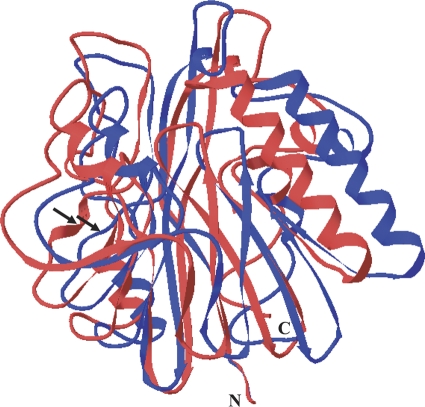
An aiiAAI96 genomic gene fragment of ∼900 bp was amplified by using AI96 genomic DNA and primers AI96-up and AI96-down. The aiiAAI96 open reading frame is 753 bp and encodes a protein of 250 residues and a stop codon, with a calculated molecular mass of 28.0 kDa and a pI of 5.08. No signal peptide was identified by using SignalP. The deduced AiiAAI96 sequence is most similar to a hypothetical protein of B. pseudomycoides DSM 12442 (GenBank accession number ZP_04152085) (98% sequence identity), is ∼80% identical to the sequences of AHL lactonases from other Bacillus spp., and is ∼32 to 43% identical to those from other sources (Fig. 1). Homology modeling revealed that AiiAAI96 has a putative structure typical of metallo-β-lactamase (MBL) family proteins. Compared to the AHL lactonase from B. thuringiensis (PDB accession number 2A7M), AiiAAI96 tended to have variation in the location of the helical or loop-rich regions (Fig. 2).
Fig 1.
Amino acid sequence alignment of AiiAAI96 with Bacillus AiiA-like proteins and with AHL lactonases from other species. The sequences are those of Bacillus sp. AI96 CGMCC 4164 (GenBank accession number HM750247) (B. sp. AI96), Bacillus sp. strain 240B1 (accession number AAF62398) (B. sp. 240B1), Bacillus sp. strain B546 (accession number FJ816104) (B. sp. B546), Bacillus thuringiensis serovar shandongiensis (accession number AAR85481) (B. thu sha) (referred to as AiiA SS10 in this paper), Bacillus thuringiensis serovar kurstaki (accession number AAM92140) (B. thu kur) (referred to as PDB accession number 2A7M in Fig. 2), Bacillus cereus (accession number ACR46836) (B. cer), Bacillus subtilis (accession number AAY51611) (B. sub), Arthrobacter sp. strain IBN110 (accession number AAP57766) (AhlD), Klebsiella pneumoniae (accession number AAO47340) (AhlK), Agrobacterium tumefaciens C58 (accession number AAK91031) (AiiB), and Agrobacterium tumefaciens C58 (accession number AAD43990) (AttM). The similar and identical residues are shaded in gray and black, respectively. The conserved motif HXHXDH is boxed. The active-site amino acids are indicated with arrows, and the amino acids involved in the modification of a helix to a random coil (Fig. 2) are indicated with diamonds.
Fig 2.
Putative tertiary structure of AiiAAI96 modeled using the crystal structure of B. thuringiensis serovar kurstaki AiiA (GenBank accession number AAM92140 and PDB accession number 2A7M) (30) as the template. The AiiAAI96 and AiiA (PDB accession number 2A7M) ribbon diagrams are shown in blue and red, respectively.
Expression and purification of AiiAAI96.
In the expression study, AHL lactonase activity (2.57 U/ml) was found in the cell lysate at the end of the culture period. No activity was detected in the cell lysate supernatants of uninduced transformants or of induced transformants that harbored an empty plasmid.
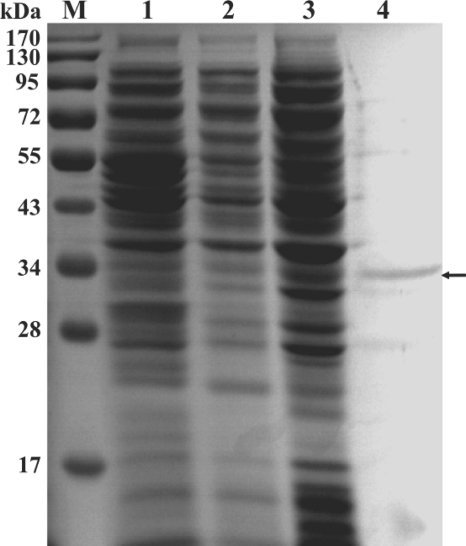
AiiAAI96 in the cell lysate was purified to electrophoretic homogeneity by Ni-NTA chromatography. In SDS-PAGE gels, the purified protein migrated as a single band with an apparent molecular mass of ∼31 kDa (including part of the His tag) (Fig. 3). The predicted and experimental molecular masses were nearly the same. Four interval peptides sequenced by LC-ESI-MS/MS (LYFLPAGR, GTFVEGQILPK, TEHDAALHR, and SGSVLLTIDASYTQENFEQGVPFAGFDSEMASQSINR) have the same sequences as those deduced from the aiiAAI96 nucleotide sequence. The specific activity of purified AiiAAI96 was 9.13 U/mg.
Fig 3.
SDS-PAGE gel of recombinant AiiAAI96 from E. coli BL21(DE3). Lanes: M, protein molecular mass markers; 1, cell extract of E. coli BL21(DE3) that harbored an empty pET28a(+) vector; 2, cell extract of E. coli BL21(DE3) that harbored pET28a(+) carrying aiiAAI96 and that was not treated with IPTG; 3, cell extract of E. coli BL21(DE3) that harbored pET28a(+) carrying aiiAAI96 and that was treated with IPTG; 4, purified AiiAAI96.
Physical parameters that affect AiiAAI96 activity and kinetic constants.
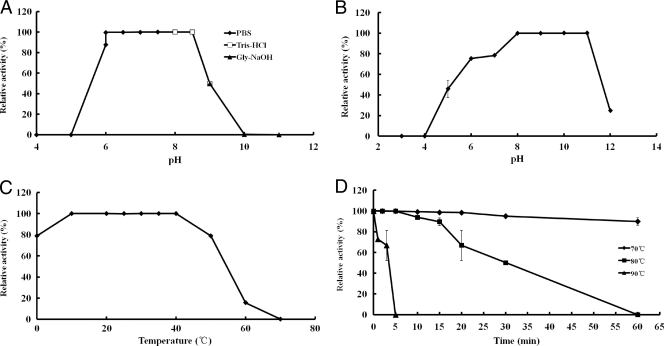
AiiAAI96 was fully active against 3-oxo-C8-HSL at pH 8.0 when the temperature was 30°C, and it retained >95% of the maximal activity between pH 6.0 and 8.5 (Fig. 4A). The protein was stable between pH 6.0 and 11.0 at 30°C, as assessed by its complete retention of activity after preincubation in buffers of differing pHs (Fig. 4B). The optimal temperature at which AiiAAI96 retained higher levels of activity at pH 8.0 was ∼10°C to 40°C (Fig. 4C). AiiAAI96 retained >90% of its activity after incubation in PBS (pH 8.0) at 70°C for 60 min, and it retained ∼60% of its activity after incubation for 20 min at 80°C or for 3 min at 90°C (Fig. 4D).
Fig 4.
Physical parameters that affect the enzymatic activity of AiiAAI96. (A) Effect of pH on AiiAAI96 activity at 30°C. (B) pH stability assay. (C) Effect of temperature on AiiAAI96 activity at pH 8.0. (D) Thermostability of AiiAAI96. Each value is the mean ± standard deviation (SD) (n = 3).
The effects of various chemicals on AiiAAI96 activity were determined under standard conditions (Table 1). None of the chemicals (1 mM) stimulated activity. At 10 mM, Co2+, Cr3+, Pb2+, Fe2+, and Mn2+ partially inactivated the enzyme. Ag+ and SDS at both 1 mM and 10 mM inhibited AiiAAI96 activity.
Table 1.
Effects of metal ions and chemicals on AiiAAI96 activity
| Chemical | Mean relative activity (%) ± SD at concn (mM) ofa: |
|
|---|---|---|
| 1 | 10 | |
| None | 100.00 | 100.00 |
| Na+ | 98.65 ± 0.22 | 99.52 ± 0.00 |
| K+ | 99.33 ± 0.00 | 99.52 ± 0.00 |
| Li+ | 99.33 ± 0.00 | 99.52 ± 0.00 |
| Ca2+ | 99.59 ± 0.11 | 99.52 ± 0.00 |
| Mg2+ | 99.02 ± 0.22 | 83.58 ± 0.00 |
| Co2+ | 99.33 ± 0.00 | 167.03 ± 0.22 |
| Cr3+ | 99.02 ± 0.22 | 118.12 ± 0.22 |
| Cu2+ | 99.33 ± 0.00 | 99.52 ± 0.00 |
| Ni2+ | 99.33 ± 0.00 | 99.52 ± 0.00 |
| Zn2+ | 99.33 ± 0.00 | 99.78 ± 0.11 |
| Pb3+ | 99.33 ± 0.00 | 118.12 ± 0.22 |
| Fe2+ | 99.81 ± 0.11 | 118.12 ± 0.22 |
| Mn2+ | 99.02 ± 0.22 | 118.43 ± 0.00 |
| Ag+ | 21.86 ± 1.10 | 21.90 ± 0.53 |
| SDS | 83.67 ± 0.00 | 40.61 ± 0.00 |
| β-Mercaptoethanol | 99.33 ± 0.00 | 99.52 ± 0.00 |
| EDTA | 99.59 ± 0.10 | 99.52 ± 0.00 |
Each value is the mean ± standard deviation (n = 3) and was normalized to the control value.
The Km and Vmax values were 8.87 mM and 1.65 mM/min, respectively, with 3-oxo-C8-HSL as the substrate.
Resistance of AiiAAI96 to proteases and intestinal juice.
AiiAAI96 was very resistant to all proteases tested, retaining ∼100% of its activity after 30- and 60-min incubations with proteases. AiiAAI96 was also resistant to carp intestinal juice and retained ∼90 and 80% of its activity after 30 and 60 min, respectively. In the protease sensitivity experiment, casein (the positive control) was almost completely degraded by all proteases used and intestinal juice (data not shown). The residual activities of trypsin, α-chymotrypsin, proteinase K, subtilisin A, collagenase, and intestinal juice were 98, 96, 97, 99, 106, and 98%, respectively, at the end of the protocol period. The results indicated that proteases and intestinal juice are highly active under the tested conditions.
Hydrolytic activities of AiiAAI96 toward AHLs.
The hydrolytic activities of AiiAAI96 toward various AHL substrates are shown in Table 2. AiiAAI96 had a broad substrate spectrum, including C4-HSL, C6-HSL, C7-HSL, C8-HSL, C10-HSL, C12-HSL, C14-HSL 3-oxo-C8-HSL, 3-oxo-C6-HSL, 3-oxo-C10-HSL, 3-oxo-C12-HSL, 3-oxo-C14-HSL, 3-hydroxy-C8-HSL, and 3-hydroxy-C14-HSL. Due to the insensitivity of biosensor bacteria, activities toward several AHLs could not be quantified.
Table 2.
Hydrolytic activities of AiiAAI96 against various AHL substratesa
| Substrate | Biosensor bacterium | Enzyme activity (U/ml) |
|---|---|---|
| C4-HSL | KYC55/CV026 | D/44.97 |
| C6-HSL | KYC55/CV026 | D/2.28 |
| C7-HSL | KYC55/CV026 | 1.90/0.39 |
| C8-HSL | KYC55/CV026 | 1.29/0.33 |
| C10-HSL | KYC55 | 11.19 |
| C12-HSL | KYC55 | L |
| C14-HSL | KYC55 | D |
| 3-Oxo-C6-HSL | KYC55/CV026 | L/0.36 |
| 3-Oxo-C8-HSL | KYC55/CV026 | 9.13/8.54 |
| 3-Oxo-C10-HSL | KYC55 | 9.70 |
| 3-Oxo-C12-HSL | KYC55 | 3.08 |
| 3-Oxo-C14-HSL | KYC55 | 312.40 |
| 3-Hydroxy-C8-HSL | KYC55 | 5.22 |
| 3-Hydroxy-C14-HSL | KYC55 | L |
KYC55, A. tumefaciens KYC55(pJZ372)(pJZ384)(pJZ410); CV026, C. violaceum CV026; D, degraded but could not be quantified; L, little activity detected.
Assessment of AiiAAI96 as a feed additive for protection against A. hydrophila infection.
In protection ability detection experiments, no fish died after being fed the CK diet (the regular diet) (group A) or the experimental diet (groups C and D) for 25 days in the absence of environmental A. hydrophila NJ-1 (Fig. 5), suggesting that the CK diet, AiiAAI96, and AiiAB546 are not toxic. AiiAAI96 significantly attenuated A. hydrophila NJ-1 infection. Compared with group B, the percent survival of group F was significantly higher from day 4, increased about 20% at day 11, and maintained levels 20 to 38% higher until day 25. During the same period, significantly higher mortality rates were detected in group E than in group F, except for rates at days 10 to 14, suggesting the inability of AiiAB546 to protect zebrafish by oral administration. AiiAAI96 was stable in the experimental diet at 25°C. After a 2-month storage period at 25°C, AiiAAI96 retained ∼100% of its activity. In the bacteriological contamination test, A. hydrophila NJ-1 was detected only in the dead fish of groups B, E, and F and in A. hydrophila NJ-1-containing water.
Fig 5.
Protection of zebrafish infected with A. hydrophila NJ-1 by AiiAAI96. Groups were as follows: group A was fed the CK diet and immersed in clean water, group B was fed the CK diet and immersed in A. hydrophila NJ-1-containing water, group C was fed the CK diet supplemented with 3.74 U AiiAB546 per gram of feed and immersed in clean water, group D was fed the CK diet supplemented with 3.74 U AiiAAI96 per gram of feed and immersed in clean water, group E was fed the CK diet supplemented with 3.74 U AiiAB546 per gram of feed and immersed in A. hydrophila NJ-1-containing water, and group F was fed the CK diet supplemented with 3.74 U AiiAAI96 per gram of feed and immersed in A. hydrophila NJ-1-containing water. Each value is the mean ± SD (n = 4). Data marked with * (0.01 < P < 0.05) and ** (P < 0.01) are significantly different between groups B and F; data marked with # (0.01 < P < 0.05) and ## (P < 0.01) are significantly different between groups E and F.
Various amounts (on a feed-weight basis) of AiiAAI96 were added to the CK diet to assess the minimum amount of AiiAAI96 needed for protection. Compared with the percent survival of fish in group B (Fig. 6), groups C, D, E, and F all showed protection against A. hydrophila NJ-1 infection, with increased survival rates of 20, 10, 10, and 9%, respectively, at 35 days. Because the rates of survival of fish in groups D, E, and F did not show significant differences compared to those of group B, the smallest amount of AiiAAI96 needed for protection was defined as 3.74 U/gram of feed.
Fig 6.
Determination of the smallest amount of AiiAAI96 needed to protect zebrafish against A. hydrophila NJ-1 infection. Groups were as follows: group A was fed the CK diet and immersed in clean water; group B was fed the CK diet and immersed in A. hydrophila NJ-1-containing water; and groups C, D, E, and F were fed the CK diet supplemented with 3.74, 3.74 × 10−1, 3.74 × 10−2, and 3.74 × 10−3 U AiiAAI96 per gram of feed, respectively, and immersed in A. hydrophila NJ-1-containing water. Each value is the mean ± SD (n = 4). Data marked with * (0.01 < P < 0.05) and ** (P < 0.01) are significantly different between groups B and F, and data marked with # (0.01 < P < 0.05) and & (0.01 < P < 0.05) are significantly different between groups B and C and between groups B and D, respectively.
DISCUSSION
Here we isolated AHL-degrading Bacillus sp. AI96, which is closely related to B. pseudomycoides and B. mycoides. Broad-spectrum AHL-degrading AiiA-type enzymes were found to be widespread in B. thuringiensis and Bacillus cereus strains (14, 28). Recently, Bacillus amyloliquefaciens, B. subtilis, and B. marcorestinctum were shown to have AHL-degrading activities, whereas AHL-degrading activity has not yet been reported for B. pseudomycoides (23, 41, 56). Although B. mycoides was reported previously to produce AHL-inactivating proteins, their inactivating mechanism is still unknown (14).
The deduced amino acid sequence of AiiAAI96 is very similar to those of AiiA-like proteins from other Bacillus spp. (∼80% sequence identity) (Fig. 1). Given its sequence, AiiAAI96 belongs to the MBL superfamily, which currently contains 20 families, including the glyoxalase II, cyclase, arylsulfatase, alkylsulfatase, flavoprotein, and RNase families (3). MBL superfamily members have structural features in common, including a tertiary structure, two active-site zinc ions, and the conserved HXHXDH motif (37). Zinc atoms Zn1 and Zn2 in the active sites of AiiA-like proteins are coordinated by His-104, His-106, and His-169 as well as Asp-108, His-109, and His-235, respectively. Asp-191 and an H2O molecule or a hydroxide ion form two bridges between the two zinc ions (29). Moreover, Tyr-194 was also conserved, which plays a key role in hydrogen bonding with the substrate (AHLs) ester oxygen to form a complex (29). All these conserved amino acids can be identified in the corresponding sites of AiiAAI96. Moreover, certain residues, located near or far from the active site, e.g., Gly-12, Pro-34, Leu-39, Asp-50, Gly-52, Gly-91, Pro-94, and Asp-96, are also conserved (Fig. 1). Their functions remain to be determined. As shown in Fig. 1, the amino acids of the modified helix are conserved in AiiA-like proteins. However, the residues up- and downstream of this conserved motif (including the active-site His-104, His-106, Asp-108, and His-109) in AiiAAI96 are different from those of other AiiA-like proteins. This difference might lead to a subtle change from a helix to a random coil (Fig. 1, arrows) and consequently may affect the structure and function of AiiAAI96.
To date, only the Bacillus AHL lactonases AiiASS10 (AiiA of Bacillus thuringiensis serovar shandongiensis), AiiAB546, and AiiA240B1 have been characterized (7, 45, 52). These AHL lactonases are most active at 20°C to 30°C (at pH 8.0), a temperature range that is lower than that for AiiAAI96 (40°C). Moreover, AiiAAI96 retained 20% of its activity at 60°C, a temperature at which AiiAB564 and AiiASS10 are completely inactive (45, 52). The thermostability of AiiAAI96 is exceptional for an AHL lactonase. All other tested AHL lactonases, which had been expressed in E. coli, are not thermostable. For example, AiiA240B1 activity decreased sharply after incubation at 45°C for 2 h (16); AiiASS10 was inactive after incubation for 2 h at 40°C (45), and AiiAB564 retained 21.3% of its initial activity after incubation at 37°C for 30 min (7). Conversely, AiiAAI96 was stable at 70°C for 2 h and retained 80% of its activity at 80°C for 10 min and ∼67% of its activity at 90°C for 3 min.
Distinct from other MBL superfamily members, AHL lactonases do not require Zn2+ for activity (7, 45, 52). In addition, inhibitors of MBL activity, e.g., EDTA and β-mercaptoethanol, have no effect on AHL lactonase activity (7, 45, 52). Although AHL lactonases have the typical MBL superfamily structure and contain the same conserved residues and Zn ions, the effects caused by metal ions that promote activity and inhibitors differ between the AHL lactonases and other MBLs. Therefore, whether the Zn ions function in AHL lactonase catalysis and whether AHL lactonases are truly metalloenzymes remain to be determined. AiiAAI96 is more resistant to the effects of heavy metal ions (Pb2+, Mn2+, Ni2+, Fe2+, Zn2+, and Cr3+) than are other AHL lactonases (7, 45, 52). Notably, Cu2+, which is an AHL lactonase inhibitor, had no effect on AiiAAI96 activity. To stimulate fish growth, metal ions, e.g., the macrominerals Ca2+, Mg2+, K+, and Na+ and the microminerals Cu2+, Fe2+, Zn2+, and Mn2+, are routinely added into fish feed (38). To be a useful fish feed additive, an additive enzyme must not be affected by these metal ions or by proteases. AiiAAI96 was strongly resistant to all the tested proteases and to carp intestinal juice.
Currently, only two groups of enzymes that are specific for AHL degradation are known, namely, AHL lactonases and AHL acylases (10, 14, 15). Only AHL lactonases hydrolyze many different AHL-signaling molecules. We found that AiiAAI96 degrades C4-HSL, C6-HSL, C7-HSL, C8-HSL, C10-HSL, C14-HSL, 3-oxo-C6-HSL, 3-oxo-C8-HSL, 3-oxo-C10-HSL, 3-oxo-C12-HSL, 3-oxo-C14-HSL, and 3-hydroxy-C8-HSL. Conversely, most AHL acylases hydrolyze only long-chain AHLs and penicillin G (42). PONs that have AHL lactonase activity hydrolyze many different lactones (19). Given the different substrate specificities of the above-mentioned AHL-degrading enzymes, AHL lactonases are more suitable for the control of AHL-producing bacteria.
Aquatic pathogenic and opportunistic bacteria can completely kill mollusk, fish, and shrimp populations that are reared in aquaculture facilities (2, 9, 12). The addition of antibiotics and disinfectants has led to bacterial resistance and has also caused aquaculture environmental pollution and food safety issues (12). Various strategies have been used, e.g., microbial-matured water, green-water systems, bacteriophage therapy, immunostimulants, and vaccines, to combat such problems. However, bacterial quorum quenching has not been attempted (12). The important Gram-negative fish pathogens Vibrio spp., Aeromonas spp., and Yersinia ruckeri use AHLs as signaling molecules (6, 12), and a quorum-quenching strategy might efficiently control these pathogens (10–12, 22, 40). A. hydrophila is an opportunistic, important zoonotic fish pathogen associated with hemorrhagic septicemia as well as fin and tail rot (2, 9). Previous studies of the AhyR/AhyI system, which is analogous to the LuxR/LuxI system, in A. hydrophila indicated that C4-HSL is the major signaling molecule and regulates the expression of virulence factors, biofilm maturation, extracellular protease expression, and so on (4, 25, 26, 48, 49, 51). The mutation of the A. hydrophila quorum-sensing system increased the rates of survival of infected Artemia shrimp (12). We have shown that the coinjection of recombinant AiiAB546 and A. hydrophila into common carp decreased carp mortality rates and delayed the time to death in comparison with the results for carp infected only with A. hydrophila (7). However, the oral administration of AHL lactonases is more viable for application in aquaculture. In a previous study, the addition of N-acyl homoserine lactone-degrading bacterial enrichment cultures (ECs) into water or feed increased the survival rate of Macrobrachium rosenbergii larvae with Vibrio harveyi infection (40). In our protection ability experiments (Fig. 5), two AHL lactonases were supplemented into experimental diets to control A. hydrophila infection. The results showed that the oral administration of AiiAAI96 significantly decreased the mortality of infected zebrafish, but AiiAB546 lost its protection ability by direct feeding. Therefore, AiiAAI96 might be used directly for the aquatic control of microbial pathogens. Compared with other AHL lactonases, AiiAAI96 has superior properties as a feed supplement; i.e., it is optimal over wide ranges of pHs and temperatures (pH 6.0 to 8.5 and 10°C to 40°C), is thermostable, is resistant to proteases, is C4-HSL hydrolyzed, and attenuates infection. These properties make AiiAAI96 an outstanding quorum-quenching tool for aquaculture. Supplementation with AiiAAI96 appears to be a feasible and economical way to decrease A. hydrophila infection.
ACKNOWLEDGMENTS
The Conversion of Agricultural Science and Technology Achievement (Ministry of Science and Technology of China) (grant number 2010GB23260591), the Agricultural Science and Technology Achievement Transformation and Promotion in Tianjin (grant number 201004040), and the China Postdoctoral Science Foundation (grant number 20090450472) supported this work.
Footnotes
Published ahead of print 13 January 2012
REFERENCES
- 1. Asad S, Opal SM. 2008. Bench-to-bedside review: quorum sensing and the role of cell-to-cell communication during invasive bacterial infection. Crit. Care 12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Austin B, Austin DA. 1999. Bacterial fish pathogens: diseases in farmed and wild fish. Praxis Publishing, Chichester, United Kingdom [Google Scholar]
- 3. Bebrone C. 2007. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem. Pharmacol. 74:1686–1701 [DOI] [PubMed] [Google Scholar]
- 4. Bi ZX, Liu YJ, Lu CP. 2007. Contribution of AhyR to virulence of Aeromonas hydrophila J-1. Res. Vet. Sci. 83:150–156 [DOI] [PubMed] [Google Scholar]
- 5. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 6. Bruhn JB, et al. 2005. Quorum sensing signal molecules (acylated homoserine lactones) in gram-negative fish pathogenic bacteria. Dis. Aquat. Organ. 65:43–52 [DOI] [PubMed] [Google Scholar]
- 7. Chen R, Zhou Z, Cao Y, Bai Y, Yao B. 2010. High yield expression of an AHL lactonase from Bacillus sp. B546 in Pichia pastoris and its application to reduce Aeromonas hydrophila mortality in aquaculture. Microb. Cell Fact. 9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choudhary S, Schmidt-Dannert C. 2010. Applications of quorum sensing in biotechnology. Appl. Microbiol. Biotechnol. 86:1267–1279 [DOI] [PubMed] [Google Scholar]
- 9. Colwell RR, MacDonell MT, Deley J. 1986. Proposal to recognize the family Aeromonadaceae fam. nov. Int. J. Syst. Bacteriol. 36:473–477 [Google Scholar]
- 10. Czajkowski R, Jafra S. 2009. Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim. Pol. 56:1–16 [PubMed] [Google Scholar]
- 11. Defoirdt T, Boon N, Bossier P, Verstraete W. 2004. Disruption of bacterial quorum sensing: an unexplored strategy to fight infections in aquaculture. Aquaculture 240:69–88 [Google Scholar]
- 12. Defoirdt T, Bossier P, Sorgeloos P, Verstraete W. 2005. The impact of mutations in the quorum sensing systems of Aeromonas hydrophila, Vibrio anguillarum and Vibrio harveyi on their virulence towards gnotobiotically cultured Artemia franciscana. Environ. Microbiol. 7:1239–1247 [DOI] [PubMed] [Google Scholar]
- 13. de Kievit TR, Iglewski BH. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong YH, Gusti AR, Zhang Q, Xu JL, Zhang LH. 2002. Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl. Environ. Microbiol. 68:1754–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong YH, Zhang LH. 2005. Quorum sensing and quorum quenching enzymes. J. Microbiol. 43:101–109 [PubMed] [Google Scholar]
- 16. Dong YH, Xu JL, Li XZ, Zhang LH. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorumsensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. U. S. A. 97:3526–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong YH, et al. 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813–817 [DOI] [PubMed] [Google Scholar]
- 18. Dong YH, Wang LY, Zhang LH. 2007. Quorum-quenching microbial infections: mechanisms and implications. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362:1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Draganov DI, et al. 2005. Human paraoxonases (PON1, PON2 and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 46:1239–1247 [DOI] [PubMed] [Google Scholar]
- 20. Eneyskaya EV, et al. 1999. Acid protease from Trichoderma reesei: limited proteolysis of fungal carbohydrases. Appl. Microbiol. Biotechnol. 52:226–231 [Google Scholar]
- 21. Fuqua C, Parsek MR, Greenberg EP. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439–468 [DOI] [PubMed] [Google Scholar]
- 22. Geske GD, O'Neill JC, Blackwell HE. 2008. Expanding dialogues: from natural autoinducers to non-natural analogues that modulate quorum sensing in Gram-negative bacteria. Chem. Soc. Rev. 37:1432–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han Y, Chen F, Li N, Zhu B, Li X. 2010. Bacillus marcorestinctum sp. nov., a novel soil acylhomoserine lactone quorum-sensing signal quenching bacterium. Int. J. Mol. Sci. 11:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelly MT, Stroh EM, Jessop J. 1988. Comparison of blood agar, ampicillin blood agar, MacConkey-ampicillin-Tween agar, and modified cefsulodin-Irgasan-novobiocin agar for isolation of Aeromonas spp. from stool specimens. J. Clin. Microbiol. 26:1738–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khajanchi BK, et al. 2009. N-Acylhomoserine lactones involved in quorum sensing control the type VI secretion system, biofilm formation, protease production, and in vivo virulence in a clinical isolate of Aeromonas hydrophila. Microbiology 155:3518–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirke DF, Swift S, Lynch MJ, Williams P. 2004. The Aeromonas hydrophila LuxR homologue AhyR regulates the N-acyl homoserine lactone synthase, AhyI positively and negatively in a growth phase-dependent manner. FEMS Microbiol. Lett. 241:109–117 [DOI] [PubMed] [Google Scholar]
- 27. Ledoux M, Lamy F. 1986. Determination of proteins and sulfobetaine with the Folin-phenol reagent. Anal. Biochem. 157:28–31 [DOI] [PubMed] [Google Scholar]
- 28. Lee SJ, et al. 2002. Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Appl. Environ. Microbiol. 68:3919–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liao RZ, Yu JG, Himo F. 2009. Reaction mechanism of the dinuclear zinc enzyme N-acyl-l-homoserine lactone hydrolase: a quantum chemical study. Inorg. Chem. 48:1442–1448 [DOI] [PubMed] [Google Scholar]
- 30. Liu D, et al. 2008. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 1. Product-bound structures. Biochemistry 47:7706–7714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu D, et al. 2007. Structure and specificity of a quorum-quenching lactonase (AiiB) from Agrobacterium tumefaciens. Biochemistry 46:11789–11799 [DOI] [PubMed] [Google Scholar]
- 32. McClean KH, et al. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703–3711 [DOI] [PubMed] [Google Scholar]
- 33. Miller MB, Bassler BL. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165–199 [DOI] [PubMed] [Google Scholar]
- 34. Molina L, Rezzenico F, Defago G, Duffy B. 2005. Autoinduction in Erwinia amylovora: evidence of an acylhomoserine lactone signal in the fire blight pathogen. J. Bacteriol. 187:3206–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Momb J, et al. 2008. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 2. Substrate modeling and active site mutations. Biochemistry 47:7715–7725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moreno C, Romero J, Espejo RT. 2002. Polymorphism in repeated 16S rRNA genes is a common property of type strains and environmental isolates of the genus Vibrio. Microbiology 148:1233–1239 [DOI] [PubMed] [Google Scholar]
- 37. Murphy TA, Simm AM, Toleman MA, Jones RN, Walsh TR. 2003. Biochemical characterization of the acquired metallo-β-lactamase SPM-1 from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 47:582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. National Research Council 1993. Nutrient requirements of fish. National Academy Press, Washington, DC [Google Scholar]
- 39. Nealson KH, Platt T, Hastings JW. 1970. Cellular control of the synthesis and activity of the bacterial luminescence system. J. Bacteriol. 104:313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nhan DT, et al. 2010. Quorum quenching bacteria protect Macrobrachium rosenbergii larvae from Vibrio harveyi infection. J. Appl. Microbiol. 109:1007–1016 [DOI] [PubMed] [Google Scholar]
- 41. Pan J, et al. 2008. Expression and characterization of aiiA gene from Bacillus subtilis BS-1. Microbiol. Res. 163:711–716 [DOI] [PubMed] [Google Scholar]
- 42. Park SY, et al. 2005. Identification of extracellular N-acylhomoserine lactone acylase from a Streptomyces sp. and its application to quorum quenching. Appl. Environ. Microbiol. 71:2632–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parker CT, Sperandio V. 2009. Cell-to-cell signaling during pathogenesis. Cell. Microbiol. 11:363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patton JS, Nevenzel JC, Benson AA. 1975. Specificity of digestive lipases in hydrolysis of wax esters and triglycerides studied in anchovy and other selected fish. Lipids 10:575–583 [DOI] [PubMed] [Google Scholar]
- 45. Qiu J, et al. 2007. Enzymatic characterization and function of N-acylhomoserine lactonase SS10. Acta Phytopathol. Sinica 37:629–636 [Google Scholar]
- 46. Ravn L, Christensen AB, Molin S, Givskov M, Gram L. 2001. Methods for detecting acylated homoserine lactones produced by Gram-negative bacteria and their application in studies of AHL-production kinetics. J. Microbiol. Methods 44:239–251 [DOI] [PubMed] [Google Scholar]
- 47. Reimmann C, et al. 2002. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 148:923–932 [DOI] [PubMed] [Google Scholar]
- 48. Swift S, et al. 1997. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J. Bacteriol. 179:5271–5281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Swift S, et al. 1999. Quorum sensing-dependent regulation and blockade of exoprotease production in Aeromonas hydrophila. Fect. Immun. 67:5192–5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tempé J, Petit A, Holsters M, Montagu M, Schell J. 1977. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: possible relation to transformation in crown gall. Proc. Natl. Acad. Sci. U. S. A. 74:2848–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vilches S, Jimenez N, Tomás JM, Merino S. 2009. Aeromonas hydrophila AH-3 type III secretion system expression and regulatory network. Appl. Environ. Microbiol. 75:6382–6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang LH, Weng LX, Dong YH, Zhang LH. 2004. Specificity and enzyme kinetics of the quorum-quenching N-acyl homoserine lactone lactonase (AHL lactonase). J. Biol. Chem. 279:13645–13651 [DOI] [PubMed] [Google Scholar]
- 53. Williams P, et al. 2000. Quorum sensing and the population-dependent control of virulence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:667–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wopperer J, et al. 2006. A quorum-quenching approach to investigate the conservation of quorum-sensing-regulated functions within the Burkholderia cepacia complex. Appl. Environ. Microbiol. 72:1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang F, et al. 2005. Quorum quenching activity is widely conserved in the sera of mammalian species. FEBS Lett. 579:3713–3717 [DOI] [PubMed] [Google Scholar]
- 56. Yin XT, et al. 2010. Isolation and characterization of an AHL lactonase gene from Bacillus amyloliquefaciens. World J. Microbiol. Biotechnol. 26:1361–1367 [Google Scholar]
- 57. Zhu J, Chai Y, Zhong Z, Li S, Winans SC. 2003. Agrobacterium bioassay strain for ultrasensitive detection of N-acylhomoserine lactone-type quorum-sensing molecules: detection of autoinducers in Mesorhizobium huakuii. Appl. Environ. Microbiol. 69:6949–6953 [DOI] [PMC free article] [PubMed] [Google Scholar]