Abstract
Background
The frequency of over-expression of human epidermal growth factor receptor-2 (HER-2) in bladder cancer is one of the highest among all human malignancies. This over-expression is thought to play a role in aberrant proliferation of cancer cells. Studies on HER-2 expression in bladder carcinoma have shown heterogeneous results.
Purpose
The aim of the study was to evaluate the status of HER-2 protein expression in patients with invasive carcinomas of the urinary bladder as related to tumor grade and stage.
Materials and methods
Archival samples from 39 patients (6 women, 33 males) with urinary bladder cancer were analyzed for HER-2 over-expression, using immunohistochemistry with the HercepTest.
Results
HER-2 over-expression was observed in 23/39 tumors (59%) and was more frequent in high-grade than in low-grade carcinomas, but the difference was not statistically significant. A significant correlation was established between HER-2 over-expression and tumor stage (p=0.011). HER-2 expression was more frequent in transitional cell carcinomas (TCC) and adenocarcinomas (AC) as compared with squamous cell carcinoma (SCC). Patients’ age and sex were not related to HER-2 over-expression.
Conclusion
Over-expression of HER-2 was frequent in carcinomas of the urinary bladder. Knowing the HER-2 status would be helpful in formulating a rational treatment strategy for patients with urinary bladder cancer.
Keywords: bladder cancer, human epidermal growth factor receptor-2, immunohistochemistry, over-expression
Bladder cancer is a common malignancy in the genitourinary tract, and transitional cell cancer (TCC) accounts for >90% of all bladder cancers. It is a prevalent disease and ranks ninth in the global cancer incidence, with 356,000 annual new cases and 145,000 annual deaths (1, 2). Bladder cancers are classified as superficial (80%) and invasive disease (20%) on the basis of their histological appearance. Transurethral resection (TUR) with or without intra-vesical treatment is the therapy-of-choice in superficial bladder cancers, while radical cystectomy is needed in invasive disease (3, 4). However, 5-year survival rates following radical treatment for tumors of all stages remain low, around 40–50%, due to a high risk of recurrence or occult metastasis (5, 6).
Despite the fact that the most common prognostic markers are conventional clinico-pathologic parameters, such as tumor stage and grade, which are subject to considerable intra- and inter-observer variation, it is difficult to predict accurate prognosis with any single factor. However, accurate estimation of the biological behavior of these tumors is important to select the appropriate treatment. Therefore, more reliable prognostic factors are urgently needed, the prime interest being currently focused on protein and genetic markers (7–9).
Human epidermal growth factor receptor-2 (HER-2) is a trans-membrane tyrosine kinase receptor of the epidermal growth factor (EGF) receptor family. HER-2 plays a fundamental role in cell growth, survival, and migration, and abnormal activation of HER-2 has been proposed to lead to oncogenic transformation. The role of HER-2 has been most thoroughly studied in breast cancer, where constitutively active HER-2 is over-expressed in 18–22% of cases and shown to correlate with a poor prognosis (10, 11).
It has been shown that this protein is involved in pathogenesis of urinary bladder carcinomas as well, to an extent almost comparable to breast cancer (12, 13). However, the prognostic value of this protein in bladder cancer has not been well established, and also the true prevalence of HER-2 expression in this disease remains uncertain. A possible involvement of HER-2 receptor in the proliferation of invasive urothelial carcinomas has prompted trials with HER-2 targeted therapies in locally advanced or metastatic disease (14).
In this retrospective study, we used immunohistochemistry (IHC) to evaluate the HER-2 protein expression in bladder carcinomas in relation to tumor histology, grade and stage.
Material and methods
Clinical samples
Archival samples of 39 invasive urinary bladder carcinomas was examined in the present study: All the tumor samples were collected from the Pathology Department, Faculty of Medicine, Garyounis University, Benghazi, Libya, derived from years 2006 to 2010. Only invasive bladder tumors were included based on availability of representative paraffin blocks. Of these 39 cases, 33 patients were male and 6 were women, with the mean age of 64.5 years (range: 47–80 years). Histological diagnosis and tumor grading were performed by an experienced pathologist, following the World Health Organization (WHO) classification. Clinical staging was made according to the American Joint Committee on Cancer system in a blinded fashion. Key features of the patients and their tumors are shown in Table 1.
Table 1.
Key features of the patients and their tumors
| Variable | Number | Percentage% |
|---|---|---|
| Patients | ||
| Male | 33 | 84.6 |
| Female | 6 | 15.4 |
| Age (years) | ||
| Range | 47–80 | – |
| Mean | 64.51 | – |
| Median | 67.00 | – |
| Histological type | ||
| TCC | 36 | 92.3 |
| AC | 1 | 2.6 |
| SCC | 2 | 5.1 |
| Grade | ||
| Low grade | 27 | 69.2 |
| High grade | 12 | 30.8 |
| Stage | ||
| Stage 1 | 29 | 74.4 |
| Stage 2 | 9 | 23.1 |
| Stage 3 | 1 | 2.5 |
TCC, transitional cell carcinoma; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Immunohistochemical (IHC) method for HER-2
Paraffin-embedded tumor specimens were cut at 5 µm and subjected to IHC staining with the Food and Drug Administration (FDA)-approved HercepTest® (Ventana Medical System, Inc., Tucson, AZ, USA). The IHC analysis was done using an automated system (Bench-Mark XT, Ventana Medical System, Tucson, AZ, USA). In brief, tissue sections were incubated with the primary HER-2 antibody (Confirm™, Anti HER-2 primary antibody/4BS), followed by the secondary antibody, peroxidase-anti-peroxidase complex (PAP), and final detection of immunoreactivity by the diaminobenzidine (DAB) substrate. After staining, the sections were dehydrated in ethanol, cleared in xylene, and covered with Mountex and cover slips.
Scoring of HER-2 over-expression
Stained sections were reviewed independently by two pathologists, both blinded to the clinical outcome. Scoring was done using the following system: 1+, at most faint, equivocal, and incomplete membranous staining; 2+, unequivocal, complete membranous pattern, with moderate intensity; and 3+, complete and strong membranous pattern. Tumors with a score of 2 or 3 were considered as HER-2 positive (15). The staining index (I) was calculated using the following formula:
Where f0, f1 and f3 represent the fraction of the cells showing a defined level of staining intensity (from 0 to 3). Theoretically, the index ranges from 0 to 3 (16, 17).
Statistical analysis
SPSS for Windows SPSS 19.0.1 (IBM, NY, USA) was used for statistical analysis. Frequency tables were analyzed using the Chi-square test, with Fisher's exact test (where appropriate), or likelihood ratio (LR) statistics to assess the significance between categorical variables. Differences in the means of continuous variables were analyzed using ANOVA (analysis of variance) or non-parametric tests (Mann-Whitney, Kruskal-Wallis) tests. Reported p-values are from two-sided tests, and in all analysis p<0.05 was regarded as statistically significant.
Results
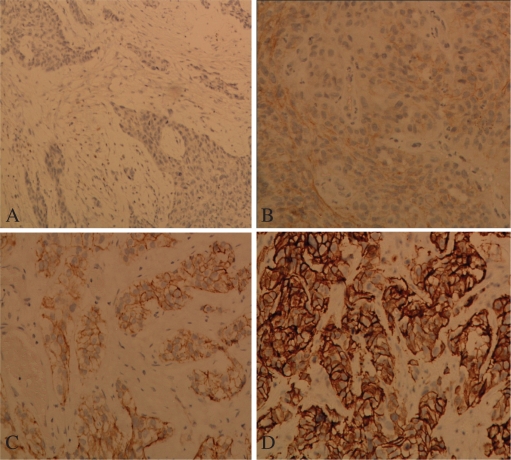
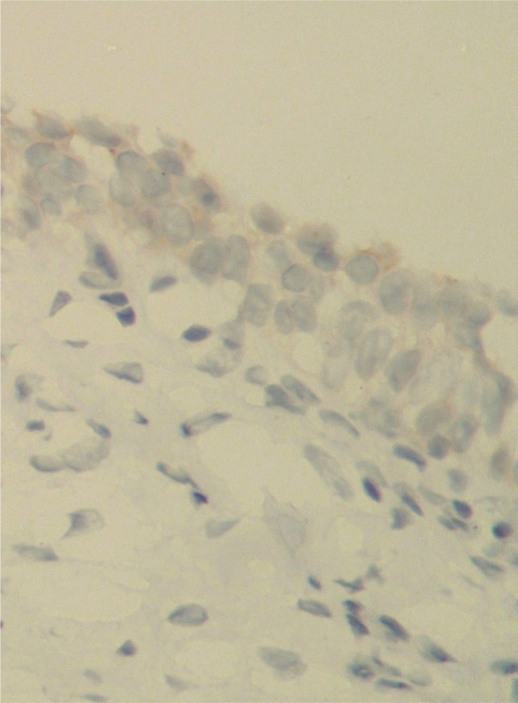
In interpreting HER-2 immunostaining, HER-2 positivity was indicated by membranous golden-brown staining. The cases with only cytoplasmic staining were considered negative, irrespective of the staining intensity. Of the 39 bladder carcinomas, 16 (41%) were considered negative (staining intensity 0 or 1+ in 4 and 12 patients, respectively) (Fig. 1 A,B), whereas 23 (59%) were considered positive (staining intensity 2+ or 3+ in 10 and 13 patients, respectively) (Fig. 1C,D). HER-2 expression was invariably negative in the normal urothelium adjacent to the tumor (Fig. 2).
Fig. 1.
HER-2 immunostaining of invasive urothelial carcinoma cells showing: (A) IHC 0 score (×100), (B) IHC +1 score (×200), (C) IHC +2 score (×200) and (D) IHC +3 score (×200).
Fig. 2.
Normal urothelium showing negative HER-2 immunostaining (×400)
The present study revealed a significant correlation between HER-2 expression and the tumor stage (p=0.011). Although there was no statistically significant association (p=0.21) between HER-2 and tumor grade, HER-2 showed over-expression more often in high-grade (8/12, 66.6%) than in low-grade tumors (12/27 44.4%). HER-2 did not show any correlation with age or sex. However, HER-2 expression was higher in TCC and adenocarcinomas (AC) as compared to squamous cell carcinoma (SCC) (p=0.029) despite the small number of AC and SCC samples used. The associations of HER-2 expression with clinical and pathological variables are shown in Table 2.
Table 2.
The associations of HER-2 expression with clinical and pathological variables
| HER-2 expression status N (%) | ||||||
|---|---|---|---|---|---|---|
| Variable | N (%) | 0 | +1 | +2 | +3 | P |
| Total | 39 (100) | 4 (10.2) | 12 (30.8) | 10 (25.7) | 13 (33.3) | _ |
| Gender | ||||||
| Female | 6 (15.4) | 1 (16.7) | 0 (0) | 2 (33.3) | 3 (50) | 0.17 |
| Male | 33 (84.6) | 3 (9.1) | 12 (36.3) | 8 (24.2) | 10 (30.4) | |
| Stage | ||||||
| I | 29 (74.4) | 3 (10.3) | 11 (38) | 9 (31) | 6 (20.7) | 0.011 |
| II | 9 (23.1) | 1 (11.1) | 1 (11.1) | 1 (11.1) | 6 (66.7) | |
| III | 1 (2.5) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |
| Grade | ||||||
| Low grade | 27 (69.2) | 4 (14.8) | 11 (40.7) | 7 (26) | 5 (18.5) | 0.21 |
| High grade | 12 (30.8) | 2 (16.6) | 2 (16.6) | 2 (16.6) | 6 (50) | |
| Histological type | ||||||
| TCC | 36 (92.3) | 4 (11.1) | 13 (36.1) | 9 (25) | 10 (27.8) | 0.029 |
| AC | 1 (2.6) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |
| SCC | 2 (5.1) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | |
TCC, transitional cell carcinoma; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Discussion
The first report of increased amplification and expression of the HER-2 in bladder carcinoma (18) enthused interest in the role of this oncogene in tumor progression. Prevalence of HER-2 expression in urothelial carcinomas has ranged from 2% to 81% (19–34). Moreover, its prognostic significance is highly controversial, with several conflicting results in the literature. Thus, although some studies (24, 26) have found correlation between HER-2 amplification/over-expression and more aggressive clinical behavior, others (19, 21, 25) have found no such prognostic significance, and still some others (20, 22, 23, 27) have linked HER-2 with a more favorable clinical outcome. Differences in the frequency of HER-2 expression and its prognostic significance in urothelial cancer are most likely explained by the use of different techniques, including assessment of the HER-2 status (i.e. gene amplification or protein over-expression), detection methods (i.e. PCR, fluorescence in situ hybridization, and IHC), as well as definition of HER-2 positivity. Thus, the available literature in this field is extremely difficult to compare, and definite conclusions are hard to draw from the accumulated data.
In the present series, there was a positive HER-2 status in 23 (59%) cases of bladder cancer. This rate is within the range of previously published data. Previous studies on HER-2 expression in bladder carcinoma using Western blot analysis and IHC have found a correlation between increased HER-2 expression and both higher tumor stage and grade (35–39). Our study confirmed these findings. We assessed HER-2 expression by IHC, which is the method most commonly used also for HER-2 status determination in breast cancer, and we found a significant correlation between HER-2 over-expression and tumor stage (p=0.011), and a trend (albeit not significant) for higher HER-2 expression with high-grade tumors. Others (19, 27, 40) have reported a significant association between HER-2 over-expression and a higher tumor grade, but not with the tumor stage. Thus, Krüger et al. (41) studied HER-2 expression in patients undergoing radical cystectomy for muscle-invasive carcinoma using the HercepTest. HER-2 over-expression was associated with high-grade bladder cancers but showed no correlation with the tumor stage. Similarly, in a recent study (42) on 59 patients, HER-2 over-expression was significantly correlated with the differentiation grade but not with the tumor stage.
In two other studies (43, 44) there was no significant association between HER-2 and either pathological staging or tumor grading. Similarly, in a series of 53 bladder cancers, no significant correlation was found between HER-2 and stage or grade (45). Furthermore, in that study, the authors reported no significant difference between TCC and SCC groups, which is in contrast to our current study, where HER-2 expression was significantly more frequent in TCC and AC than in SCC (p=0.029), notwithstanding the small number of AC and SCC samples in the current study.
It seems likely that the reported variations in HER-2 expression in urothelial cancer may be the result not only of true biological variations but also of several confounding variables in these studies. These include the use of different antibodies for IHC, different criteria for IHC-positivity (i.e. cytoplasmic or membrane staining), and different scoring criteria.
Despite the inconclusive data on the prognostic value of HER-2 as an independent marker of tumor progression, there may be a therapeutic role for an anti HER-2 agent such as trastuzumab in cancer treatment. Available data clearly demonstrate that the development of new drugs will have little, if any, chance of success if it is not guided by in-depth knowledge of disease biology. However, using biologic agents to target key molecular pathways, such as those regulated by HER family members, may be effective. Indeed, the positive results achieved by trastuzumab in breast and gastric HER-2 positive tumors support this approach (46). Combining trastuzumab with chemotherapy in HER-2 positive advanced gastric cancer was significantly more effective than chemotherapy alone (47) and has a favorable toxicity profile (48). The efficacy of trastuzumab in breast and gastric cancer patients has led to investigation of its antitumor activity in patients with HER-2 positive cancers, including urothelial carcinomas. Therefore, all patients with advanced or metastatic bladder cancers should be tested for HER-2 status for selection of proper candidates who may benefit from adjuvant HER-2 targeted therapy.
Conclusion
The present study demonstrates a statistically significant (p=0.011) association between HER-2 over-expression and increased tumor stage, as well as a statistically insignificant (p=0.21) increase in protein expression in higher grade tumors. Assessment of HER-2 status can be helpful in identifying patients at high-risk of disease progression who may benefit from adjuvant HER-2-targeted therapy after radical cystectomy. Future prospective studies on HER-2 expression with chemosensitivity and efficacy of HER-2-targeted therapies in urothelial carcinomas are warranted.
Acknowledgements
We thank Ms. Huda El-Labbar for expert technical assistance in conducting the immunohistochemical staining.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–93. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl. 2008;218:12–20. doi: 10.1080/03008880802285032. [DOI] [PubMed] [Google Scholar]
- 3.Canoglu A, Gogus C, Beduk Y, Orhan D, Tulunay O, Baltaci S. Microvessel density as a prognostic marker in bladder carcinoma: correlation with tumor grade, stage and prognosis. Int Urol Nephrol. 2004;36:401–5. doi: 10.1007/s11255-004-8869-9. [DOI] [PubMed] [Google Scholar]
- 4.Von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 5.Duncan W, Quilty PM. The results of a series of 963 patients with transitional cell carcinoma of the urinary bladder primarily treated by radical megavoltage X-ray therapy. Radiother Oncol. 1986;7:299–310. doi: 10.1016/s0167-8140(86)80059-7. [DOI] [PubMed] [Google Scholar]
- 6.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 7.Kassouf W, Black PC, Tuziak T, Bondaruk J, Lee S, Brown GA, et al. Distinctive expression pattern of Erb-b famil receptors signify an aggressive variant of bladder cancer. J Urol. 2008;179:353–8. doi: 10.1016/j.juro.2007.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latif Z, Watters AD, Dunn I, Grigor KM, Underwood MA, Bartlett JM. HER2/neu overexpression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br J Cancer. 2003;89:1305–9. doi: 10.1038/sj.bjc.6601245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reznikoff CA, Sarkar S, Knut PJ, Burger MS, Newton MA. Genetic alterations and biological pathways in Human bladder cancer pathogenesis. Urol Oncol. 2000;5:191–203. doi: 10.1016/s1078-1439(00)00079-x. [DOI] [PubMed] [Google Scholar]
- 10.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–67. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61(Suppl. 2):1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
- 12.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–9. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger MS, Greenfield C, Gullick WJ, Haley J, Downward J, Neal DE, et al. Evaluation of epidermal growth factor receptors in bladder tumors. Br J Cancer. 1987;56:533–7. doi: 10.1038/bjc.1987.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitra AP, Lin H, Datar RH, Cote RJ. Molecular biology of bladder cancer: prognostic and clinical implications. Clin Genitourin Cancer. 2006;5:67–77. doi: 10.3816/CGC.2006.n.020. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez RE, Hussain M, Bianco FJ, Jr, Vaishampayan U, Tabazcka P, Sakr WA, et al. Her2/neu overexpression in muscle-invasive urothelial carcinoma of the bladder prognostic significance and comparative analysis in primary and metastatic tumors. Clin Cancer Res. 2001;7:2440–7. [PubMed] [Google Scholar]
- 16.Elzagheid A, Kuopio T, Ilmen M, Collan Y. Prognostication of invasive ductal breast cancer by quantification of E-cadherin immunostaining: the methodology and clinical relevance. Histopathology. 2002;41:127–33. doi: 10.1046/j.1365-2559.2002.01448.x. [DOI] [PubMed] [Google Scholar]
- 17.Lipponen P, Collan Y. Simple quantitation of immunohistochemical staining positivity in microscopy. Acta Stereol. 1992;11:125–32. [Google Scholar]
- 18.Zhau HE, Zhang X, von Eschenbach AC, Scorsone K, Babaian RJ, Ro JY, et al. Amplification and expression of the c-erb B-2/neu proto-oncogene in human bladder cancer. Mol Carcinog. 1990;3:254–7. doi: 10.1002/mc.2940030503. [DOI] [PubMed] [Google Scholar]
- 19.Tetu B, Fradet Y, Allard P, Vellleux C, Roberge N, Bernard P. Prevalence and clinical significance of HER/2neu, p53 and Rb expression in primary superficial bladder cancer. J Urol. 1996;155:1784–8. [PubMed] [Google Scholar]
- 20.Lee SE, Chow NH, Chi YC, Tzai TS, Yang WH, Lin SN. Expression of c-erbB-2 protein in normal and neoplastic urothelium: lack of adverse prognostic effect in human urinary bladder cancer. Anticancer Res. 1994;14:1317–24. [PubMed] [Google Scholar]
- 21.Mellon JK, Lunec J, Wright C, Home CH, Kelly P, Neal DE. C-erbB-2 in bladder cancer: molecular biology, correlation with epidermal growth factor receptors and prognostic value. J Urol. 1996;155:321–6. doi: 10.1016/s0022-5347(01)66653-9. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen PL, Swanson PE, Jaszcz W, Aeppli DM, Zhang G, Singleton TP, et al. Expression of epidermal growth factor receptor in invasive transitional cell carcinoma of the urinary bladder. A multivariate survival analysis. Am J Clin Pathol. 1994;101:166–76. doi: 10.1093/ajcp/101.2.166. [DOI] [PubMed] [Google Scholar]
- 23.Korkolopoulou P, Christodoulou P, Kapralos P, Exarchakos M, Bisbiroula A, Hadjiyannakis M, et al. The role of p53, MDM2 and c-erb B-2 oncoproteins, epidermal growth factor receptor and proliferation markers in the prognosis of urinary bladder cancer. Pathol Res Pract. 1997;193:767–75. doi: 10.1016/S0344-0338(97)80055-6. [DOI] [PubMed] [Google Scholar]
- 24.Lipponen P, Eskelinen M, Syrjanen S, Tervahauta A, Syrjanen K. Use of immunohistochemically demonstrated c-erb B-2 oncoprotein expression as a prognostic factor in transitional cell carcinoma of the urinary bladder. Eur Urol. 1991;20:238–42. doi: 10.1159/000471706. [DOI] [PubMed] [Google Scholar]
- 25.Underwood M, Bartlett J, Reeves J, Gardiner DS, Scott R, Cooke T. C-erbB-2 gene amplification: a molecular marker in recurrent bladder tumors? Cancer Res. 1995;55:2422–30. [PubMed] [Google Scholar]
- 26.Lonn U, Lonn S, Friberg S, Nilsson B, Silfersward C, Stenkvist B. Prognostic value of amplification of c-erb-B2 in bladder carcinoma. Clin Cancer Res. 1995;1:1189–94. [PubMed] [Google Scholar]
- 27.Vollmer RT, Humphrey PA, Swanson PE, Wick MR, Hudson ML. Invasion of the bladder by transitional cell carcinoma: its relation to histologic grade and expression of p53, MIB-1, c-erb B-2, epidermal growth factor receptor, and bcl-2. Cancer. 1998;82:715–23. doi: 10.1002/(sici)1097-0142(19980215)82:4<715::aid-cncr15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.McCann A, Dervan PA, Johnston PA, Gullick WJ, Carney DN. c- erbB-2 oncoprotein expression in primary human tumors. Cancer. 1990;65:88–92. doi: 10.1002/1097-0142(19900101)65:1<88::aid-cncr2820650119>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 29.Matsubara H, Yamada Y, Naruse K, Nakamura K, Aoki S, Taki T, et al. Potential for HER-2/neu molecular targeted therapy for invasive bladder carcinoma: Comparative study of immunohistochemistry and fluorescent in situ hybridization. Oncol Rep. 2008;19:57–63. [PubMed] [Google Scholar]
- 30.Wester K, Sjostrom A, de la Torre M, Carlsson J, Malmstrom PU. HER-2, a possible target for therapy of metastatic urinary bladder carcinoma. Acta Oncol. 2002;41:282–8. doi: 10.1080/02841860260088836. [DOI] [PubMed] [Google Scholar]
- 31.Gardmark T, Wester K, De la Torre M, Carlsson J, Malmström PU. Analysis of HER2 expression in primary urinary bladder carcinoma and corresponding metastases. BJU Int. 2005;95:982–6. doi: 10.1111/j.1464-410X.2005.05452.x. [DOI] [PubMed] [Google Scholar]
- 32.Bolenz C, Shariat SF, Karakiewicz PI, Ashfaq R, Ho R, Sagalowsky AI, et al. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int. 2010;106:1216–22. doi: 10.1111/j.1464-410X.2009.09190.x. [DOI] [PubMed] [Google Scholar]
- 33.Janane A, Hajji F, Ismail TO, Elondo JC, Ghadouane M, Ameur A, et al. Evaluation of HER2 protein overexpression in non-muscle invasive bladder cancer with emphasis on tumour grade and recurrence. Actas Urol Esp. 2011;35:189–94. doi: 10.1016/j.acuro.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Naik DS, Sharma S, Ray A, Hedau S. Epidermal growth factor receptor expression in urinary bladder cancer. Indian J Urol. 2011;27:208–14. doi: 10.4103/0970-1591.82839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skagias L, Politi E, Karameris A, Sambaziotis D, Archondakis AO, et al. Prognostic impact of HER2/neu protein in urothelial bladder cancer. Survival analysis of 80 cases and an overview of almost 20 years’ research. J BUON. 2009;14:457–62. [PubMed] [Google Scholar]
- 36.Kolla SB, Seth A, Singh MK, Gupta NP, Hemal AK, Dogra PN, et al. Prognostic significance of Her2/neu overexpression in patients with muscle invasive urinary bladder cancer treated with radical cystectomy. Int Urol Nephrol. 2008;40:321–7. doi: 10.1007/s11255-007-9283-x. [DOI] [PubMed] [Google Scholar]
- 37.Gorgoulis VG, Barbatis C, Poulias I, Karameris AM. Molecular and immunohistochemical evaluation of epidermal growth factor receptor and c-erb-B-2 gene product in transitional cell carcinomas of the urinary bladder: a study in Greek patients. Mod Pathol. 1995;8:758–64. [PubMed] [Google Scholar]
- 38.Moch H, Sauter G, Moore D, Mihatsch MJ, Gudat F, Waldman F. P53 and erbB-2 protein overexpression are associated with early invasion and metastasis in bladder cancer. Virchows Arch A Pathol Anat Histopathol. 1993;423:329–34. doi: 10.1007/BF01607144. [DOI] [PubMed] [Google Scholar]
- 39.Moriyama M, Akiyama T, Yamamoto T, et al. Expression of C-ERBB-2 gene product in urinary bladder cancer. J Urol. 1991;145:423–7. doi: 10.1016/s0022-5347(17)38356-8. [DOI] [PubMed] [Google Scholar]
- 40.Gandour-Edwards R, Lara PN, Jr, Folkins AK, LaSalle JM, Beckett L, Li Y, et al. Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer. 2002;95:1009–15. doi: 10.1002/cncr.10808. [DOI] [PubMed] [Google Scholar]
- 41.Kruger S, Weitsch G, Buttner H, Matthiensen A, Bohmer T, Marquardt T, et al. HER2 overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic implications. Int J Cancer. 2002;102:514–8. doi: 10.1002/ijc.10731. [DOI] [PubMed] [Google Scholar]
- 42.Alexa A, Baderca F, Zahoi DE, Lighezan R, Izvernariu D, Raica M. Clinical significance of Her2/neu overexpression in urothelial carcinomas. Rom J Morphol Embryol. 2010;51:277–82. [PubMed] [Google Scholar]
- 43.Tommasi S, Ditonno P, Sisto M, Paradiso A, Gentile A, Ricco R, et al. HER-2/neu in bladder carcinoma. Int J Oncol. 1996;8:957–61. doi: 10.3892/ijo.8.5.957. [DOI] [PubMed] [Google Scholar]
- 44.Badr KM, Nolen JD, Derose PB, Cohen C. Muscle invasive schistosomal squamous cell carcinoma of the urinary bladder: frequency and prognostic significance of p53, BCL-2, HER2/neu, and proliferation (MIB-1) Hum Pathol. 2004;35:184–9. doi: 10.1016/j.humpath.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Eissa S, Ali HS, Al Tonsi AH, Zaglol A, El Ahmady O. HER2/neu expression in bladder cancer: relationship to cell cycle kinetics. Clin Biochem. 2005;38:142–8. doi: 10.1016/j.clinbiochem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Fornaro L, Lucchesi M, Caparello C, Vasile E, Caponi S, Ginocchi L, et al. Anti-HER agents in gastric cancer: from bench to bedside. Nat Rev Gastroenterol Hepatol. 2011;8:369–83. doi: 10.1038/nrgastro.2011.81. [DOI] [PubMed] [Google Scholar]
- 47.De Vita F, Giuliani F, Silvestris N, Catalano G, Ciardiello F, Orditura M. Human epidermal growth factor receptor 2 (HER2) in gastric cancer: a new therapeutic target. Cancer Treat Rev. 2010;36(Suppl. 3):11–5. doi: 10.1016/S0305-7372(10)70014-1. [DOI] [PubMed] [Google Scholar]
- 48.Gravalos C, Gomez-Martin C, Rivera F, Ales I, Queralt B, Marquez A, et al. Phase II study of trastuzumab and cisplatin as first-line therapy in patients with HER2-positive advanced gastric or gastroesophageal junction cancer. Clin Transl Oncol. 2011;13:179–84. doi: 10.1007/s12094-011-0637-6. [DOI] [PubMed] [Google Scholar]