SUMMARY
Chronically increased sympathetic nerve activity is present during chronic kidney disease (CKD); however, its role in contributing to hypertension or the progression of CKD remains poorly understood. The aim of the present study was to determine if neonatal sympathectomy attenuates hypertension in 5/6 nephrectomized rats and affects renal structure and function in a blood pressure independent manner.
We performed 5/6 nephrectomy (referred to as CKD) in both sympathetically-intact and sympathectomized (injected neonatally with guanethidine, referred to as CKD+Sympath) male Sprague Dawley rats. Sham operated sympathetically-intact and sympathectomized rats (referred to as Sham and Sham+Sympath, respectively) were used as the control groups. Radiotelemetry was used to monitor blood pressure throughout the six week duration of the study, after which renal function and histology were assessed.
The overall average systolic arterial pressure and final urinary protein excretion was significantly lower in the CKD+Sympath rats (168±7 mmHg, 33±5 mg/24 hr) compared to the CKD rats (184±6 mmHg, 66±7 mg/24 hr), respectively. However, the level of proteinuria in the CKD+Sympath group was reduced to a greater extent than what would be expected solely on the basis of lower blood pressure. All other indices of renal function and histology were comparable between both CKD groups. All measurements were comparable between Sham and Sham+Sympath groups.
We conclude the following: (1) sympathectomy attenuated hypertension by approximately one-third in 5/6 nephrectomized rats, and (2) sympathetic nerves to the kidney during 5/6 nephrectomy may contribute to proteinuria in a blood pressure independent manner.
Keywords: sympathetic nervous system, pressure, glomerulosclerosis, renal failure
INTRODUCTION
Chronic kidney disease (CKD) is frequently accompanied by increased sympathetic nerve activity, which may contribute to the pathogenesis and progression of renal parenchymal hypertension via neurogenically-mediated vasoconstriction, and directly to renal damage, via renal ischaemia and trophic effects of noradrenaline (1). The premise that the sympathetic nervous system should be an important drug target in patients with CKD has stimulated interest in the greater use of centrally-acting sympatholytic agents and third generation α- and/or β-blockers (1–3). However, the body of scientific evidence to support this enthusiasm is limited. The evidence that sympatholytic agents can slow the progression of CKD is largely based on relatively small, uncontrolled clinical studies in patients with essential hypertension (4–6), not CKD, and there are no large clinical trials in CKD patients that address this issue. Although plasma catecholamine levels predict mortality in chronic hemodialysis patients (7), such studies are observational and cannot prove causality. Finally, direct evidence of increased sympathetic nerve activity during CKD in humans comes from measurements of muscle sympathetic nerve activity (8–10). Such measurements may not contribute to hypertension or reflect efferent sympathetic nerve activity to the kidney, the organ of interest.
When considering limitations that exist with rat studies in the 5/6 nephrectomy model of renal parenchymal hypertension, it is important to distinguish a key methodological detail. That is, 5/6 nephrectomy can be performed either using the renal excision method (uninephrectomy + removal of upper and lower pole of the contralateral kidney) or the renal infarction method (uninephrectomy + ligation of multiple branches of the renal artery of the contralateral kidney). In the renal excision model, resection of renal afferent neural pathways (via dorsal rhizotomy) ameliorated the hypertension and prevented the decline in renal function (11, 12). This suggests that renal afferent activation is a critical mechanism that leads to increases in efferent sympathetic nerve activity that are thought to occur in this model (13). However, the tail-cuff measure of blood pressure that was used is indirect and sensitive to the arousal state of the animal, making it difficult to quantify a sympathetic contribution to the hypertension. Furthermore, renal sympathetic nerve activity has been shown to contribute to the progression of CKD in a blood pressure independent manner, because non-hypotensive doses of sympatholytic agents reduce renal structural/functional damage in renal excision 5/6 nephrectomized rats (14, 15). Importantly, the renal excision model does not exhibit hypertension when blood pressure is measured with radiotelemetry (16). This is a key difference between the renal excision model and clinical CKD, wherein hypertension and pressure-induced renal damage are typical. Thus, the magnitude of the efferent sympathetic neural contribution to renal parenchymal hypertension is unknown, as is whether renal sympathetic nerves independently affect renal structure and function in the setting of profound hypertension.
Accordingly, we used neonatal sympathectomy, which renders efferent sympathetic nerves nonfunctional, to, 1) determine its effect on hypertension in the renal infarction 5/6 nephrectomy model while using radiotelemetry to measure blood pressure, and 2) to determine if sympathetic nerves directly contribute to decline in renal structure/function independent of their effect on blood pressure. We studied adult rats that were either intact or had been neonatally-sympathectomized, while monitoring blood pressure for six weeks following 5/6 nephrectomy. We show that sympathectomy attenuates hypertension during 5/6 nephrectomy by approximately one-third, and provide evidence that renal sympathetic nerves may effect proteinuria in a blood pressure independent manner.
METHODS
Methods used were in accordance with institutional guidelines, and all protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center. Pregnant Sprague Dawley rats were obtained from Charles River and rat pups were born at our facility. All experiments were performed on male Sprague-Dawley offspring (275–325 g). These rats received regular rodent chow and tap water ad libitum. All animals were housed in a temperature-controlled room on a 12:12 light-dark cycle.
Surgical and Experimental Protocols
Chronic Sympathectomy
Rats were pharmacologically sympathectomized by an established protocol in our laboratory (17–19) using repeated daily subcutaneous injections of guanethidine (50 mg/kg) to newborn Sprague Dawley pups from day 5 through day 27 after birth. Saline was injected into control (sympathetically-intact) rats. The permanent sympathectomy that results from this treatment protocol has been verified by numerous laboratories aside from our own (20–23).
Urine and Plasma Collection
At ~9 weeks of age and prior to surgery, male sympathectomized (n=28) and control (n=24) rats were placed in metabolic cages for three days, and during the last 24-hours, urine was collected to measure baseline urinary protein excretion. Blood samples were collected from the tail vein both before, and three days after 5/6 nephrectomy or sham surgery to assess serum creatinine levels as an index of renal mass reduction. After 6 weeks, another 24-hour urine collection was obtained to measure final urinary protein excretion.
Radiotelemetry
For telemetric monitoring of blood pressure, rats were anaesthetized with isoflurane gas, the abdomen was opened and the catheter of the radiotransmitter (Model TA11PA-C40, Data Sciences International, St. Paul, MN, USA) was inserted into the abdominal aorta, such that the tip was below the level of the renal arteries. The body of the radiotransmitter was placed intraperitoneally and sutured to the peritoneum. Rats were housed individually and the cages were placed on the telemetry receiver (Model RPC-1) which received the blood pressure signal from the radiotransmitter. The receiver was connected to a data exchange matrix which transmitted the information to the Data Quest Art data acquisition system (Gold v. 3.0). Blood pressure was sampled and averaged for 10 sec at 5 min intervals throughout the 6 week duration of the study.
5/6 Nephrectomy-Renal Infarction Model
During the same surgery for radiotransmitter implantation, the 5/6 nephrectomy or sham surgery was performed. For this procedure, the right kidney was removed, and 2–3 extrarenal branches of the left main renal artery were ligated such that approximately 2/3 of the left renal cortex was made ischaemic. For the sham operated rats, the left and right kidneys were isolated and the renal pedicles were manipulated prior to closing of the abdomen. Thus, there were four experimental groups: groups 1 and 2 were sympathetically-intact rats and will be referred to as Sham and CKD (5/6 nephrectomized) while groups 3 and 4 were sympathectomized rats and will be referred to as Sham+Sympath and CKD+Sympath.
Haemodynamic and Clearance Studies
At 16 weeks of age (during the 7th week after 5/6 nephrectomy), rats were anaesthetized with Inactin (100 mg/kg). Body temperature was maintained at 37°C using a heated surgical table. Polyethylene tubing (PE-240) was inserted into the trachea and rats were prepared for clearance studies. The left carotid artery was cannulated with PE-50 for continuous blood pressure measurement. The left femoral vein was cannulated with two PE-50 catheters. One venous catheter was used to infuse 0.9% NaCl at 0.042 ml/min to account for ongoing fluid loss. The other venous catheter was used to infuse [3H]methoxyinulin (priming dose of 0.2 ml followed by 0.009 ml/min, generously provided by R. Quigley). The left ureter was cannulated with PE-10 to collect urine samples. To measure whole kidney renal blood flow, a 1.0 mm R-series transit time ultrasound flow probe (Transonic Systems, Ithaca, NY) was placed around the left renal artery to measure renal blood flow (RBF). After a 45 minute equilibration period, two 20 minute urine collections were performed. At the midpoint of each collection, 200 µl of arterial blood was collected, centrifuged and used for liquid scintillation counting. When the studies were completed, rats were sacrificed with an overdose of pentobarbitone and perfused transcardially with 0.9% NaCl. The kidneys were harvested and fixed in formalin for histological analysis.
Histology
For histological procedures and analysis, we followed the methods of Griffin, Picken and Bidani (16). Briefly, transverse sections of kidney cut through the papilla were embedded in paraffin. Four µm sections were cut and stained with hematoxylin and eosin and periodic acid-Schiff stains. The stained slides were systematically analyzed for sclerotic glomeruli by an investigator blinded to the protocol (M.M.P.). At least 100 glomeruli per rat were analyzed and glomerular injury was assessed as a percentage of glomeruli exhibiting lesions.
Analytical Measurements and Calculations
Urinary protein was assayed quantitatively using the sulfosalicylic acid (SSA) method, which spectrophotometrically quantifies turbidity of the SSA-urine mixture. Human serum albumin was used as the standard. Serum creatinine was measured using a creatinine analyzer (Beckman Instruments, Inc., Fullerton, CA).
Data and Statistical Analysis
Glomerular filtration rate (GFR) was calculated using standard formulae. Statistical analyses were performed by analysis of variance (ANOVA). When ANOVA indicated significance, a Bonferroni post-hoc test was utilized. The structural relationship between glomerulosclerosis or proteinuria and systolic arterial pressure was analyzed using ordinary least products (OLP) regression [Ludbrook 1997]. Estimates of slopes and standard errors were calculated as in [Kermack 1950], where the method is referred to as reduced major axis regression. Analysis of covariance was used to test whether the conditional distribution of proteinuria measurements, given the corresponding systolic arterial blood pressure measurements, differed between sympathetically intact and sympathectomized rats. Results are expressed as the mean ± SE. A value of P<0.05 was considered statistically significant.
RESULTS
Table 1 shows that baseline body weight, serum creatinine and 24-hour urinary protein excretion for the four experimental groups were not significantly different from each other. The serum creatinine levels three days post-nephrectomy in both sham operated groups were not different from baseline values. However, the serum creatinine levels during the same time point in both CKD and CKD+Sympath groups had significantly increased to similar values, indicating the magnitude of the reduction in renal mass was comparable in both groups. The final body weight in the sham group was significantly greater than all other groups.
Table 1.
Initial and final renal functional and structural data.
| Body Wt. g |
Serum Creatinine mg/dl |
Urine Protein mg/24 hr |
Renal Function ml/min/g Kid.Wt. |
Glomerulosclerosis % |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Initial | Final | Initial | Day 3 | Final | Initial | Final | Renal Blood Flow |
Glom. Filt. Rate |
Final | |
| Sham | 5–8 | 313±6 | 495±20 | 0.40±0.00 | 0.35±0.02 | 0.43±0.04 | 5.3±1.7 | 5.2±2.3 | 9.4±1.0 | 0.74±0.3 | 3.2±1.1 |
| Sham+Sympath | 5–14 | 289±7 | 388±6* | 0.43±0.01 | 0.39±0.02 | 0.47±0.02 | 3.1±0.5 | 4.3±0.9 | 12.5±1.1* | 0.76±0.5 | 1.0±0.3 |
| CKD | 9–16 | 291±8 | 395±12* | 0.41±0.01 | 0.86±0.02* | 1.09±0.08* | 7.7±1.0 | 65.7±6.8* | 4.1±1.2* | 0.35±0.6* | 38.2±4.8 |
| CKD+Sympath | 11–14 | 290±6 | 376±8* | 0.46±0.02 | 0.88±0.03* | 1.06±0.13* | 5.1±0.7 | 32.9±5.3*† | 4.6±0.6* | 0.36±0.6* | 30.2±6.5 |
The number of subjects for Renal Function data are: Sham, 5; Sham+Sympath, 5; CKD, 9; CKD+Sympath, 11. For all other parameters, the number of subjects/group is the maximal indicated for each individual group.
P <0.05 vs. Sham, 6 comparisons,
P<0.05 vs. CKD Final, 3 comparisons; Sympath = neonatal sympathectomy.
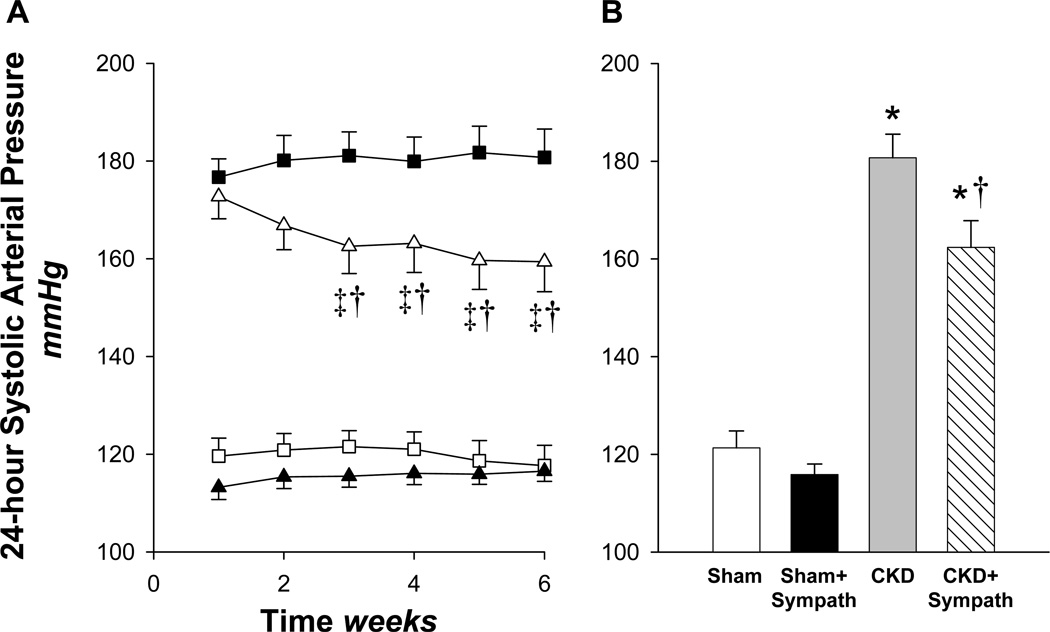
Figure 1A represents weekly averages of the systolic arterial pressure (SAP) following either sham surgery or 5/6 nephrectomy for the 6 week duration of the study. The average SAP, in both 5/6 nephrectomy groups was similar at the end of the first week, and exhibited marked hypertension compared with both sham groups. However, from that point forward, the two lines diverge, with SAP in the CKD group tending to increase slightly throughout the remainder of the study. In stark contrast, SAP in the CKD+Sympath declined progressively after the first week, such that at the end of the last week of the study, SAP was ~19 mmHg less compared with the CKD group. Note in Figure 1B that the overall pressure load in the CKD+Sympath group during the last 5 weeks of the study is significantly less than that in the sympathetically-intact CKD group. The systolic and diastolic pressure response patterns were similar to the mean arterial pressure response pattern for each CKD group (Table 2). In the sham groups, SAP (as well as mean and diastolic pressures, Table 2) was comparable throughout the study, indicating that neonatal sympathectomy did not affect baseline blood pressure.
Figure 1.
(A) Weekly averages of radiotelemetrically measured systolic arterial pressure over the six week duration of the study in the four groups. At time 0, all rats underwent either sham or 5/6 nephrectomy surgery and radiotransmitter implantation. CKD (■, 5/6 nephrectomy, n=22); CKD+Sympath (△, 5/6 nephrectomy+neonatal sympathectomy, n=19); Sham (▲, n=8); Sham+Sympath (□, sham+neonatal sympathectomy, n=14). ‡P < 0.05 vs. week 1, 11 comparisons; †P < 0.05 vs. CKD, 3 comparisons; (B) Average systolic arterial pressure during the final 5 weeks following 5/6 nephrectomy or sham. *P < 0.05 vs. Sham or Sham+Sympath, †P < 0.05 vs. CKD, 6 comparisons.
Table 2.
Arterial blood pressures measured by radiotelemetry during the first and last weeks of the study.
|
Arterial Blood Pressure mmHg | |||||||
|---|---|---|---|---|---|---|---|
| Systolic |
Mean |
Diastolic |
|||||
| n | Week 1 | Week 6 | Week 1 | Week 6 | Week 1 | Week 6 | |
| Sham | 8 | 121±4 | 118±4 | 99±4 | 96±4 | 81±4 | 81±3 |
| Sham+Sympath | 14 | 113±3 | 117±2 | 94±2 | 97±2 | 79±2 | 81±2 |
| CKD | 16 | 181±4 | 183±8 | 156±6 | 157±7 | 132±4 | 130±5 |
| CKD+Sympath | 14 | 177±5 | 164±7*† | 153±5 | 142±7*† | 131±5 | 121±7 |
P <0.05 vs. Week 1, 12 comparisons;
P<0.05 vs. CKD Week 6, 3 comparisons; Sympath = neonatal sympathectomy
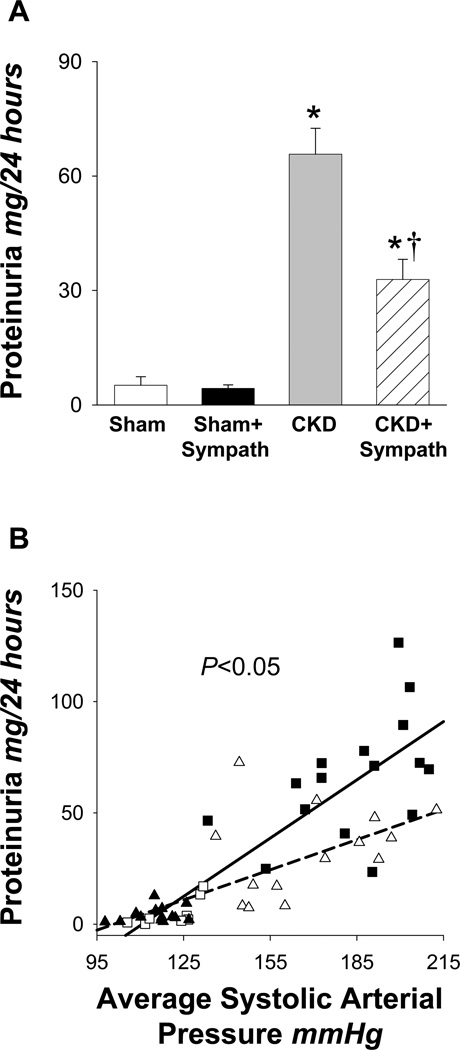
The final urinary protein levels for each group are presented in Figure 2A. The urinary protein levels in both CKD groups were markedly elevated compared to sham groups, although, these values were significantly less in the CKD+Sympath group compared with the CKD group (P<0.001). Figure 2B shows the correlation between proteinuria and the average systolic blood pressure during the last five weeks, respectively. An excellent direct correlation was observed between the combined Sham and CKD data (i.e., all data from sympathetically-intact rats, r=0.83, P<0.001), as well as the combined Sham+Sympath and CKD+Sympath data (i.e., all data from sympathectomized rats, r=0.73, P<0.001). However, the conditional distribution of proteinuria measurements given SAP pressure measurements differs between the sympathetically intact and sympathectomized rats (P=0.012). The structural relationship between proteinuria and SAP is characterized by a slope of 0.63 +/− 0.08 mg/24hr/mmHg in sympathectomized rats but by a slope of 1.02 +/− 0.12 mg/24hr/mmHg in sympathetically intact rats. That is, there was less proteinuria for any given blood pressure in the sympathectomized rats (dashed line) compared to sympathetically-intact rats (solid line). Despite the observed differences in proteinuria, the final serum creatinine, RBF, GFR and % glomeruli exhibiting sclerosis (Table 1) in both CKD groups were not significantly different from each other. Final serum creatinine, GFR, % glomeruli exhibiting sclerosis and urinary protein values (Table 1) in the Sham and Sham+Sympathectomy groups were similar; however, the RBF in the Sham+Sympath group was significantly greater compared to the Sham group.
Figure 2.
Final proteinuria and correlation between urinary protein and systolic arterial pressure. (A) Final proteinuria; Sham (□; n=8), Sham+Sympath (■; n=14), CKD ( ; n=16, CKD+Sympath (
; n=16, CKD+Sympath ( ; n=14). *P < 0.05 vs. Sham or Sham+Sympath; †P < 0.05 vs. CKD, 6 comparisons. Note that proteinuria levels in the CKD+Sympath group are reduced compare to the CKD group. (B) correlation of final proteinuria with the average systolic arterial pressure for each rat during the last five weeks of the study. Sham (□, n=8), Sham+Sympath (▲, n=14), CKD (■, n=16), CKD+Sympath (△, n=14). The lines show the least-squares mean of the conditional distribution of proteinuria measurements given systolic arterial pressure measurements for the Sham and CKD data combined (n=24, r=0.83, slope 1.02±0.12) and the Sham+Sympath and CKD+Sympath data combined (n=28, r=0.73, slope 0.63±0.08). The conditional distributions are significantly different from each other, *P=0.012.
; n=14). *P < 0.05 vs. Sham or Sham+Sympath; †P < 0.05 vs. CKD, 6 comparisons. Note that proteinuria levels in the CKD+Sympath group are reduced compare to the CKD group. (B) correlation of final proteinuria with the average systolic arterial pressure for each rat during the last five weeks of the study. Sham (□, n=8), Sham+Sympath (▲, n=14), CKD (■, n=16), CKD+Sympath (△, n=14). The lines show the least-squares mean of the conditional distribution of proteinuria measurements given systolic arterial pressure measurements for the Sham and CKD data combined (n=24, r=0.83, slope 1.02±0.12) and the Sham+Sympath and CKD+Sympath data combined (n=28, r=0.73, slope 0.63±0.08). The conditional distributions are significantly different from each other, *P=0.012.
DISCUSSION
Our data in which blood pressure was accurately measured with radiotelemetry indicate that neonatal sympathectomy attenuated hypertension in the 5/6 nephrectomy infarction model of CKD by approximately one-third. The levels of glomerular damage and most indices of renal function were indistinguishable between the sympathectomized and sympathetically-intact 5/6 nephrectomized rats. Despite this, the level of proteinuria in the CKD+Sympath group was attenuated by 50% compared to the CKD group, which is larger than what could be explained solely on the basis of a reduced blood pressure. We conclude that neonatal sympathectomy attenuates hypertension in the 5/6 nephrectomy model of CKD, and by inference, that increased sympathetic nerve activity likely accounts for approximately one-third of the hypertension. In addition, renal sympathetic nerves may exert blood pressure independent effects to increase proteinuria during 5/6 nephrectomy.
Although it has been hypothesized for several decades that increased sympathetic nerve activity contributes to hypertension during renal disease (11, 24, 25), this is the first study to use both radiotelemetry and the neonatal sympathectomy model to quantify the relative roles of sympathetically-mediated versus non-sympathetically-mediated elevations in blood pressure. Initially, one week after 5/6 nephrectomy, the systolic arterial pressure in the CKD and CKD+Sympath groups was nearly identical, but stabilized thereafter in the CKD group, while in the CKD+Sympath group, it decreased progressively throughout the remainder of the study. We interpret this to mean that, for the duration of our study, the maintenance of hypertension in the CKD group became progressively more dependent upon increased efferent sympathetic nerve activity. Our results are generally in line with those of Campese (11, 12) who demonstrated that resection of the renal afferent nerves (lesioning of dorsal roots from T10-L2 where renal afferent nerves travel that reflexively increase efferent sympathetic nerve activity) of 5/6 nephrectomized rats led to lower indices of sympathetic outflow and attenuation, but not normalization of blood pressure. Thus, although the methods of interrupting efferent sympathetic outflow were different, their effect to attenuate the hypertension were comparable. Thus, our study confirms and extends those by Campese (11, 12), and quantifies the magnitude of the sympathetic component to hypertension in 5/6 nephrectomized rats.
Previous studies using the 5/6 nephrectomy model have demonstrated that pharmacological interruption of the renin-angiotensin-aldosterone system generally leads to reductions in blood pressure and parallel reductions in urinary protein and glomerulosclerosis (26–28). However, in our study, despite the lower blood pressure and marked attenuation of proteinuria in the CKD+Sympath group, there was no change in the level of glomerulosclerosis as compared with the CKD group. We believe one reason for this difference is that a portion of the reduction in proteinuria in the CKD+Sympath group resulted directly from the lowering of blood pressure. That is, if two experimental groups (CKD and CKD+Sympath) have similar levels of renal damage but systemic blood pressure is lower in one, then this should translate to lower intraglomerular pressures and less protein crossing the glomerular capillary membrane. However, the flattening of the regression line between proteinuria and SAP in the CKD+Sympath group means that for any given blood pressure there is less protein in the urine. Thus, the magnitude of the attenuation in urinary protein in the CKD+Sympath group is greater than what could be explained solely on the basis of a reduction in blood pressure. This indicates that there were likely either changes in intraglomerular haemodynamics or in glomerular capillary permeability to protein. The fact that final RBF and GFR values in both remnant kidney groups were similar suggests that there were not gross differences in intraglomerular haemodynamics between these two groups, although micropuncture studies would be needed to definitively prove this. A recent study in healthy subjects found an association between elevated adrenergic activity and urinary albumin excretion (29), and it has recently been hypothesized that noradrenaline may act directly upon the glomerular capillary membrane to increase permeability to albumin (30). While this has yet to be shown in CKD patients, it is interesting to speculate that the lack of noradrenaline release from renal sympathetic nerve endings in our CKD+Sympath rats resulted in reduced glomerular capillary permeability to protein. This would suggest an important blood pressure independent effect of excess sympathetic activation on proteinuria in the 5/6 nephrectomy model of CKD. Along these lines, it was recently shown in a rat model of glomerulonephritis that renal denervation significantly reduced albuminuria while having only minimal effects upon mean arterial pressure (~10 mmHg) (31).
Why did the lower blood pressure in the CKD+Sympath rats not lead to a significant reduction in glomerulosclerosis (see Table 1)? Bidani and Griffin have established that there is a linear relationship between systolic arterial pressure and glomerular damage in the 5/6 nephrectomy model (26–28, 32). Yet, in our current study, renal histology, GFR, RBF and final serum creatinine levels in the CKD+Sympath rats were similar in comparison to the CKD group, despite the fact that the overall systolic arterial pressure was about 19 mmHg less in the CKD+Sympath rats. This indicates that presence or absence of sympathetic innervation of the kidney had no marked effect on glomerulosclerosis. The most likely explanation is that the magnitude of the drop in blood pressure was not great enough to produce such an effect. Indeed, previous studies in 5/6 nephrectomized rats in which blood pressure was measured with radiotelemetry have found that although even larger pharmacologically-mediated reductions in blood pressure (40–50 mmHg) have tended to improve renal structural and functional indices, but this often did not reach statistical significance (26, 27).
However, it is important to discuss work by Amann, Ritz and colleagues (14, 15, 33) which provide supporting evidence that sympathetic overactivity does indeed contribute to the progression of renal structural damage in a blood pressure independent manner. Specifically, subantihypertensive doses of either moxonodine (14), a central sympatholytic agent, or combined α– and β-adrenergic receptor blockade (15) exert significant improvements in renal morphology and reductions in albuminuria in renal excision 5/6 nephrectomized rats. Furthermore, in the same model, they have recently shown that angiotensin converting enzyme inhibition (ACEI) plus sympathetic denervation provides superior renoprotection to ACEI monotherapy (33). While our urinary protein data confirm these findings, the similarity in glomerulosclerosis levels between both CKD groups in our study appears to be at direct odds with theirs. We believe that this discrepancy is likely explained by differences in the 5/6 nephrectomy model employed, i.e. renal excision vs. renal infarction. Renal excision rats are normotensive when arterial pressure is measured by radiotelemetry and have much less renal damage (16) in comparison to renal infarction rats which have profound hypertension and renal injury (16, 26–28, 32). It is possible in our study that blood pressure independent effects of increased sympathetic nerve activity on renal structure did indeed occur but were overshadowed by hypertension-induced renal damage. If this is the case, it points to hypertension as a primary culprit in renal structural damage that supersedes other potential blood pressure independent mechanisms.
In summary, our data demonstrate that neonatal sympathectomy attenuates hypertension in the 5/6 nephrectomy model of CKD by approximately one-third, thereby suggesting, that increased sympathetic nerve activity accounts for about one-third of the hypertension. In addition, the attenuation of the increase in urinary protein in the CKD+Sympath group compared to the CKD group was greater than can be expected exclusively on the basis of the lower blood pressure. These results suggest that increased sympathetic nerve activity plays an important role in the development of hypertension during CKD and that activation of renal sympathetic nerves may exert blood pressure independent effects on proteinuria.
Perspectives
Several lines of evidence support the existence of increased sympathetic nerve activity is present during CKD. Although sympathetic activation is thought to contribute to both hypertension and the progression of CKD, the clinical evidence to support this is largely obtained from subjects that have heart failure or essential hypertension, not overt renal disease. Our data and that of others (3, 14, 15) suggest that the sympathetic nervous system may be an important therapeutic target to reduce both blood pressure and proteinuria during CKD, each of which are risk factors for renal and cardiovascular disease (34–36). Indeed, Krum et al., recently found that catheter-based renal sympathetic denervation in patients with resistant hypertension caused substantial and sustained blood pressure reduction. There is a need to critically test the effectiveness of newer central sympatholytic agents, 3rd generation α/β-blockers or catheter-base renal sympathetic denervation in patients with diabetic or non-diabetic CKD.
ACKNOWLEDGEMENTS
The authors wish to thank R. Quigley and K.A. Griffin for methodological suggestions and N.F. Rossi and P.J. Mueller for critical review of the manuscript. The work in this paper was supported by the Donald W. Reynolds Foundation and National Heart, Lung and Blood Institute grants HL44010 (R.G.V.) and HL71462 (R.A.A.).
REFERENCES
- 1.Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. 2004;65:1568–1576. doi: 10.1111/j.1523-1755.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 2.Bakris GL, Hart P, Ritz E. Beta blockers in the management of chronic kidney disease. Kidney Int. 2006;70:1905–1913. doi: 10.1038/sj.ki.5001835. [DOI] [PubMed] [Google Scholar]
- 3.Campese VM, Mitra N, Sandee D. Hypertension in renal parenchymal disease: why is it so resistant to treatment? Kidney Int. 2006;69:967–973. doi: 10.1038/sj.ki.5000177. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal B, Wolf K, Berger A, Luft FC. Effect of antihypertensive treatment on qualitative estimates of microalbuminuria. J Hum Hypertens. 1996;10:551–555. [PubMed] [Google Scholar]
- 5.Fassbinder W, Quarder O, Waltz A. Treatment with carvedilol is associated with a significant reduction in microalbuminuria: a multicentre randomised study. Int J Clin Pract. 1999;53:519–522. [PubMed] [Google Scholar]
- 6.Marchi F, Ciriello G. Efficacy of carvedilol in mild to moderate essential hypertension and effects on microalbuminuria: a multicenter, randomized, open-label, controlled study versus atenolol. Adv Ther. 1995;12:212–221. [PubMed] [Google Scholar]
- 7.Zoccali C, Mallamaci F, Parlongo S, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- 8.Converse RL, Jr, Jacobsen TN, Toto RD, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 9.Neumann J, Ligtenberg G, Klein IH, et al. Sympathetic hyperactivity in hypertensive chronic kidney disease patients is reduced during standard treatment. Hypertension. 2007;49:506–510. doi: 10.1161/01.HYP.0000256530.39695.a3. [DOI] [PubMed] [Google Scholar]
- 10.Ligtenberg G, Blankestijn PJ, Oey PL, et al. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N Engl J Med. 1999;340:1321–1328. doi: 10.1056/NEJM199904293401704. [DOI] [PubMed] [Google Scholar]
- 11.Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995;25:878–882. doi: 10.1161/01.hyp.25.4.878. [DOI] [PubMed] [Google Scholar]
- 12.Campese VM, Kogosov E, Koss M. Renal afferent denervation prevents the progression of renal disease in the renal ablation model of chronic renal failure in the rat. Am J Kidney Dis. 1995;26:861–865. doi: 10.1016/0272-6386(95)90456-5. [DOI] [PubMed] [Google Scholar]
- 13.Yuhara M, Ikeda T, Toya Y, Sakurai J, Gomi T. Participation of the sympathetic nervous system in hypertension in rats with subtotal renal ablation. J Hypertens. 1989;7:443–446. doi: 10.1097/00004872-198906000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Amann K, Rump LC, Simonaviciene A, et al. Effects of low dose sympathetic inhibition on glomerulosclerosis and albuminuria in subtotally nephrectomized rats. J Am Soc Nephrol. 2000;11:1469–1478. doi: 10.1681/ASN.V1181469. [DOI] [PubMed] [Google Scholar]
- 15.Amann K, Koch A, Hofstetter J, et al. Glomerulosclerosis and progression: effect of subantihypertensive doses of alpha and beta blockers. Kidney Int. 2001;60:1309–1323. doi: 10.1046/j.1523-1755.2001.00936.x. [DOI] [PubMed] [Google Scholar]
- 16.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994;4:2023–2031. doi: 10.1681/ASN.V4122023. [DOI] [PubMed] [Google Scholar]
- 17.Sander M, Hansen J, Victor RG. The sympathetic nervous system is involved in the maintenance but not initiation of the hypertension induced by N(omega)-nitro-L-arginine methyl ester. Hypertension. 1997;30:64–70. doi: 10.1161/01.hyp.30.1.64. [DOI] [PubMed] [Google Scholar]
- 18.Sander M, Hansen PG, Victor RG. Sympathetically mediated hypertension caused by chronic inhibition of nitric oxide. Hypertension. 1995;26:691–695. doi: 10.1161/01.hyp.26.4.691. [DOI] [PubMed] [Google Scholar]
- 19.Dobbins RL, Szczepaniak LS, Zhang W, McGarry JD. Chemical sympathectomy alters regulation of body weight during prolonged ICV leptin infusion. Am J Physiol Endocrinol Metab. 2003;284:E778–E787. doi: 10.1152/ajpendo.00128.2002. [DOI] [PubMed] [Google Scholar]
- 20.Johnson EM, Jr, Cantor E, Douglas JR., Jr Biochemical and functional evaluation of the sympathectomy produced by the administration of guanethidine to newborn rats. J Pharmacol Exp Ther. 1975;193:503–512. [PubMed] [Google Scholar]
- 21.Johnson EM, Jr, O'Brien F, Werbitt R. Modification and characterization of the permanent sympathectomy produced by the administration of guanethidine to newborn rats. Eur J Pharmacol. 1976;37:45–54. doi: 10.1016/0014-2999(76)90006-6. [DOI] [PubMed] [Google Scholar]
- 22.Levin BE, Sullivan AC. Dietary obesity and neonatal sympathectomy. II. Thermoregulation and brown adipose metabolism. Am J Physiol. 1984;247:R988–R994. doi: 10.1152/ajpregu.1984.247.6.R988. [DOI] [PubMed] [Google Scholar]
- 23.Powley TL, Walgren MC, Laughton WB. Effects of guanethidine sympathectomy on ventromedial hypothalamic obesity. Am J Physiol. 1983;245:R408–R420. doi: 10.1152/ajpregu.1983.245.3.R408. [DOI] [PubMed] [Google Scholar]
- 24.McGrath BP, Tiller DJ, Bune A, Chalmers JP, Korner PI, Uther JB. Autonomic blockade and the Valsalva maneuver in patients on maintenance hemodialysis: a hemodynamic study. Kidney Int. 1977;12:294–302. doi: 10.1038/ki.1977.114. [DOI] [PubMed] [Google Scholar]
- 25.Schohn D, Weidmann P, Jahn H, Beretta-Piccoli C. Norepinephrine-related mechanism in hypertension accompanying renal failure. Kidney Int. 1985;28:814–822. doi: 10.1038/ki.1985.203. [DOI] [PubMed] [Google Scholar]
- 26.Bidani AK, Griffin KA, Bakris G, Picken MM. Lack of evidence of blood pressureindependent protection by renin-angiotensin system blockade after renal ablation. Kidney Int. 2000;57:1651–1661. doi: 10.1046/j.1523-1755.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 27.Griffin KA, Picken M, Bidani AK. Radiotelemetric BP monitoring, antihypertensives and glomeruloprotection in remnant kidney model. Kidney Int. 1994;46:1010–1018. doi: 10.1038/ki.1994.361. [DOI] [PubMed] [Google Scholar]
- 28.Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793–800. doi: 10.1172/JCI118125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mena-Martin FJ, Martin-Escudero JC, Simal-Blanco F, Carretero-Ares JL, Arzua-Mouronte D, Castrodeza Sanz JJ. Influence of sympathetic activity on blood pressure and vascular damage evaluated by means of urinary albumin excretion. J Clin Hypertens (Greenwich) 2006;8:619–624. doi: 10.1111/j.1524-6175.2006.05569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao F, Wessel J, Wen G, et al. Renal albumin excretion: twin studies identify influences of heredity, environment, and adrenergic pathway polymorphism. Hypertension. 2007;49:1015–1013. doi: 10.1161/HYPERTENSIONAHA.106.081679. [DOI] [PubMed] [Google Scholar]
- 31.Veelken R, Vogel EM, Hilgers K, et al. Autonomic renal denervation ameliorates experimental glomerulonephritis. J Am Soc Nephrol. 2008;19:1371–1378. doi: 10.1681/ASN.2007050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol. 1993;265:F391–F398. doi: 10.1152/ajprenal.1993.265.3.F391. [DOI] [PubMed] [Google Scholar]
- 33.Hamar P, Kokeny G, Liptak P, et al. The combination of ACE inhibition plus sympathetic denervation is superior to ACE inhibitor monotherapy in the rat renal ablation model. Nephron Exp Nephrol. 2007;105:e124–e136. doi: 10.1159/000100494. [DOI] [PubMed] [Google Scholar]
- 34.de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17:2100–2105. doi: 10.1681/ASN.2006050517. [DOI] [PubMed] [Google Scholar]
- 35.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 36.Marin R, Rodriguez P, Tranche S, et al. Prevalence of abnormal urinary albumin excretion rate in hypertensive patients with impaired fasting glucose and its association with cardiovascular disease. J Am Soc Nephrol. 2006;17:S178–S188. doi: 10.1681/ASN.2006080912. [DOI] [PubMed] [Google Scholar]