Abstract
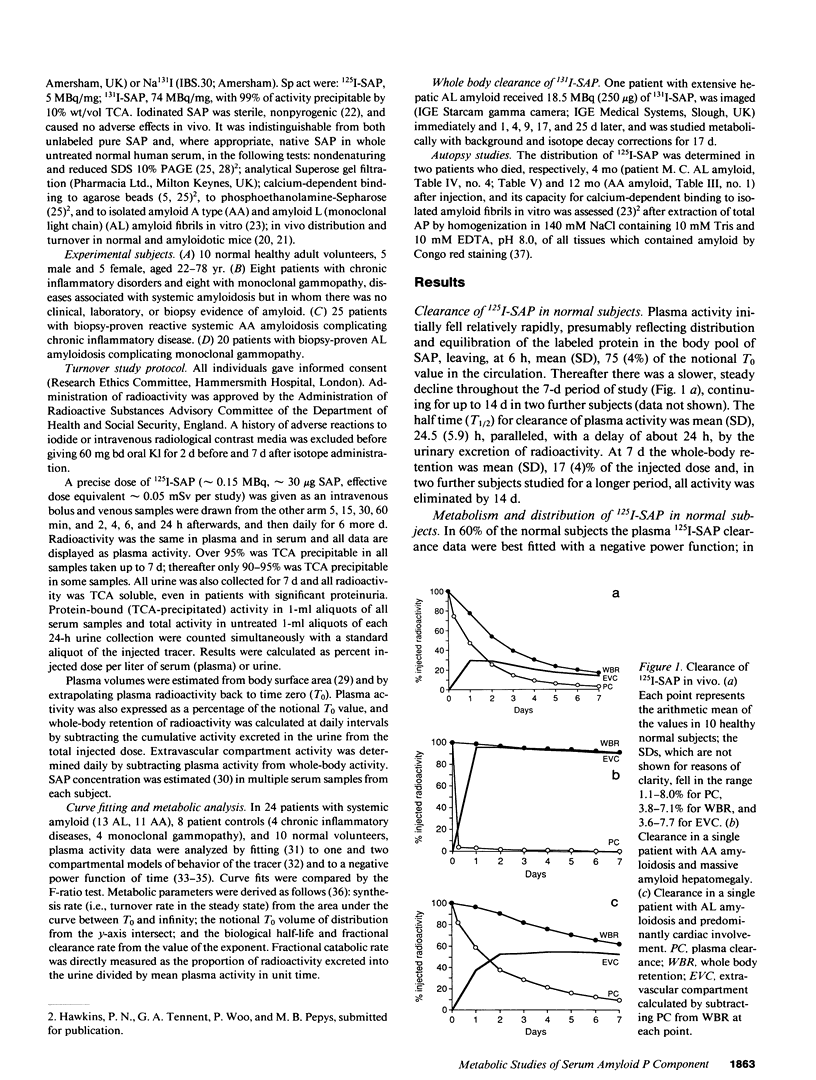
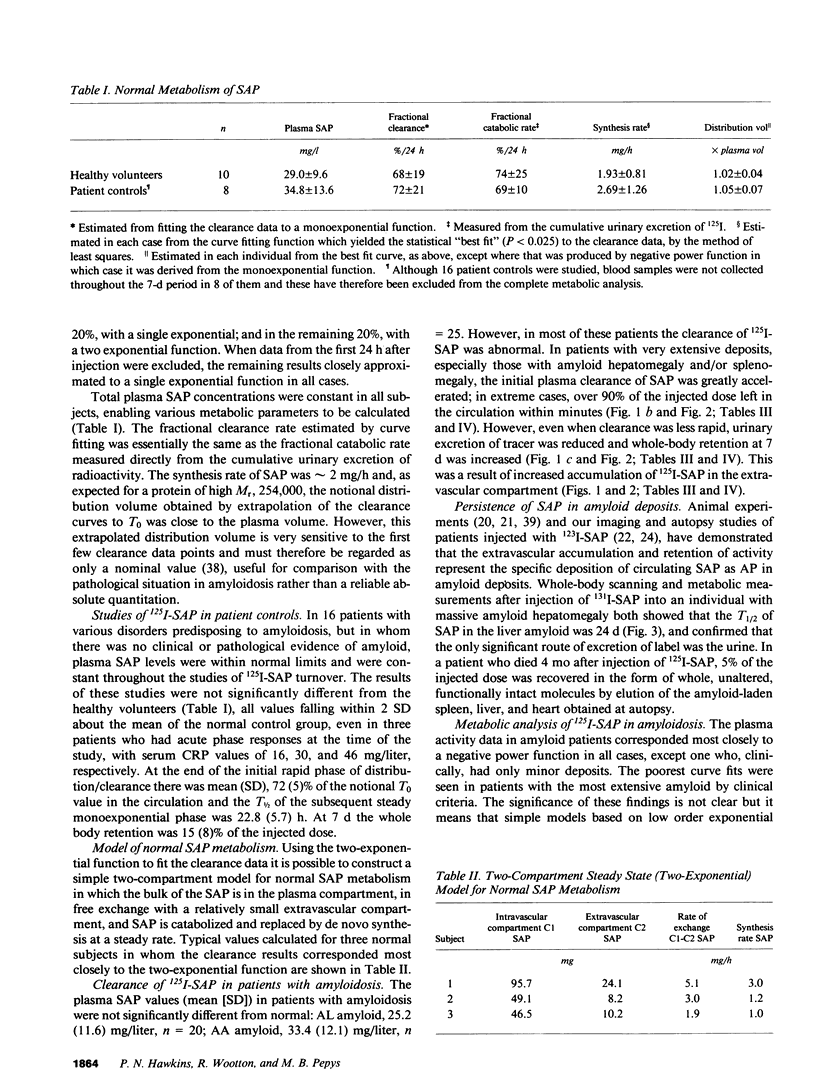
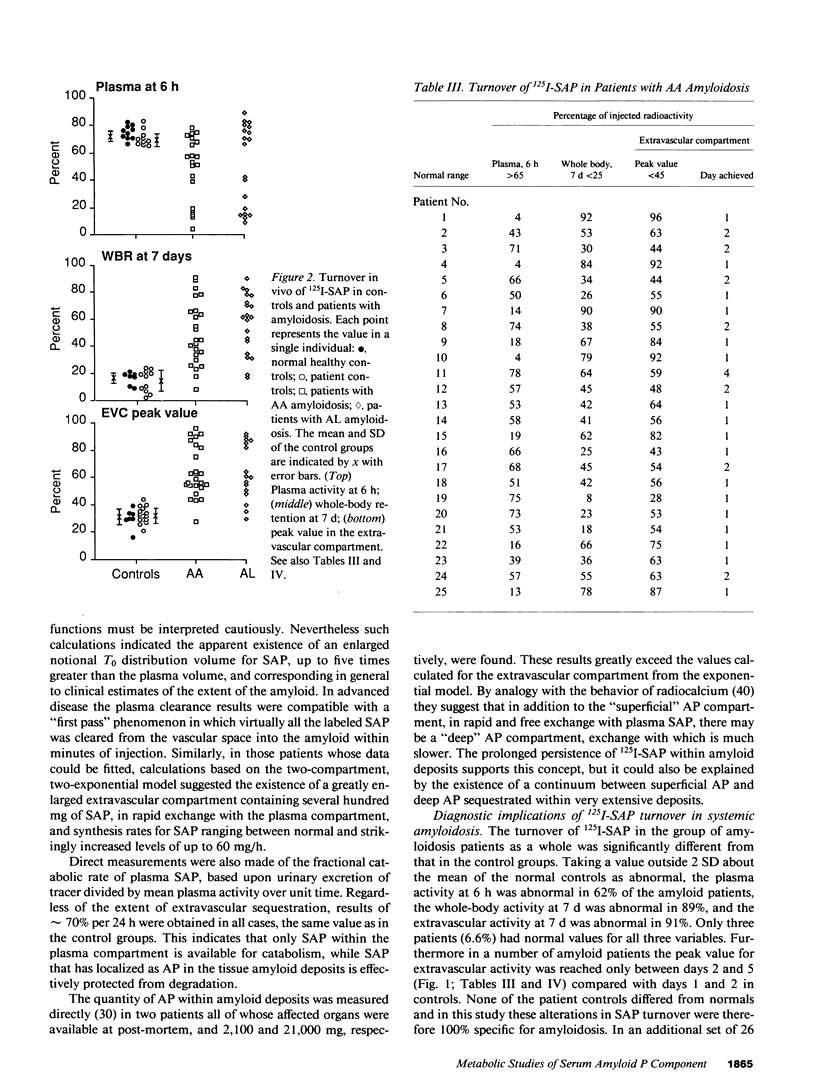
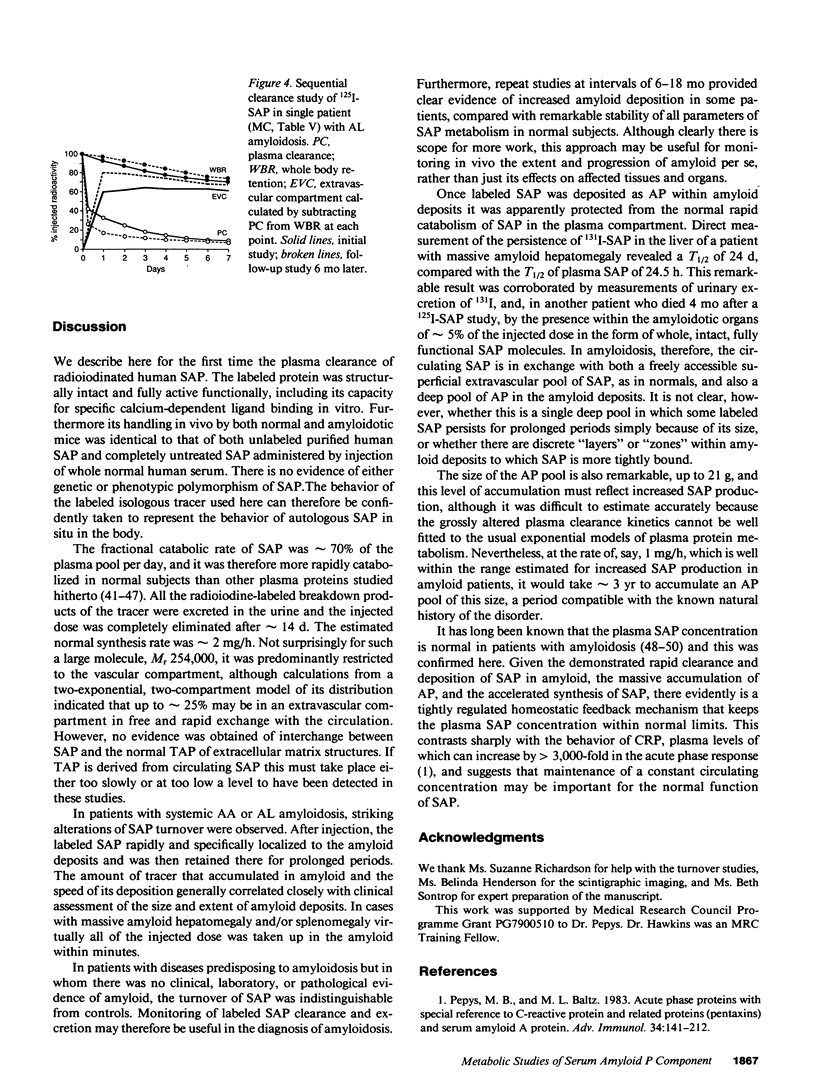
125I-Serum amyloid P component (SAP), injected intravenously into 10 normal subjects, remained predominantly intravascular with mean (SD) T1/2 (half time) in plasma of 24.5 (5.9) h. The fractional catabolic rate of 68 (19)% of the plasma pool per day was more rapid than other reported human plasma proteins. All radioactivity was excreted in the urine by 14 d. In 16 patients with monoclonal gammopathy or chronic inflammatory diseases, but without amyloidosis, 125I-SAP metabolism was normal. However, among 45 patients with biopsy-proven systemic amyloidosis (25, amyloid A type; 20, amyloid L type), 125I-SAP was cleared from the plasma more rapidly, accumulated in the amyloid deposits, and persisted there. The T1/2 in amyloid, measured directly with 131I-SAP, was 24 d. Repeat studies after 6-18 mo were notably consistent in normals but changed significantly in amyloid patients, generally correlating with clinical signs of disease progression. Measurements of 125I-SAP turnover may thus be of value for diagnosis and monitoring of amyloidosis. Analysis of SAP metabolism in amyloidosis suggests that plasma SAP is in dynamic equilibrium with a very large amyloid pool, and in two autopsies the total mass of SAP in the amyloid deposits was 2,100 and 21,000 mg, respectively.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz M. L., Caspi D., Evans D. J., Rowe I. F., Hind C. R., Pepys M. B. Circulating serum amyloid P component is the precursor of amyloid P component in tissue amyloid deposits. Clin Exp Immunol. 1986 Dec;66(3):691–700. [PMC free article] [PubMed] [Google Scholar]
- Breathnach S. M., Kofler H., Sepp N., Ashworth J., Woodrow D., Pepys M. B., Hintner H. Serum amyloid P component binds to cell nuclei in vitro and to in vivo deposits of extracellular chromatin in systemic lupus erythematosus. J Exp Med. 1989 Oct 1;170(4):1433–1438. doi: 10.1084/jem.170.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach S. M., Melrose S. M., Bhogal B., de Beer F. C., Dyck R. F., Tennent G., Black M. M., Pepys M. B. Amyloid P component is located on elastic fibre microfibrils in normal human tissue. Nature. 1981 Oct 22;293(5834):652–654. doi: 10.1038/293652a0. [DOI] [PubMed] [Google Scholar]
- Breathnach S. M., Pepys M. B., Hintner H. Tissue amyloid P component in normal human dermis is non-covalently associated with elastic fiber microfibrils. J Invest Dermatol. 1989 Jan;92(1):53–58. doi: 10.1111/1523-1747.ep13071087. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Tennent G. A., Pepys M. B. Pentraxin-chromatin interactions: serum amyloid P component specifically displaces H1-type histones and solubilizes native long chromatin. J Exp Med. 1990 Jul 1;172(1):13–18. doi: 10.1084/jem.172.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T. H., Simon T. L., Atencio A. C. In vivo behavior of human radioiodinated antithrombin III: distribution among three physiologic pools. Blood. 1985 Jul;66(1):13–19. [PubMed] [Google Scholar]
- Caspi D., Zalzman S., Baratz M., Teitelbaum Z., Yaron M., Pras M., Baltz M. L., Pepys M. B. Imaging of experimental amyloidosis with 131I-labeled serum amyloid P component. Arthritis Rheum. 1987 Nov;30(11):1303–1306. doi: 10.1002/art.1780301115. [DOI] [PubMed] [Google Scholar]
- Cathcart E. S., Wollheim F. A., Cohen A. S. Immunoassay of P-component in amyloidotic sera. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1123–1125. doi: 10.3181/00379727-125-32292. [DOI] [PubMed] [Google Scholar]
- Cavalieri R. R., McMahon F. A., Castle J. N. Preparation of 125-I-labeled human thyroxine-binding alpha globulin and its turnover in normal and hypothyroid subjects. J Clin Invest. 1975 Jul;56(1):79–87. doi: 10.1172/JCI108082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth J. A., Williams D. G., Sherington E., Lachmann P. J., Peters D. K. Metabolic studies of the third component of complement and the glycine-rich beta glycoprotein in patients with hypocomplementemia. J Clin Invest. 1974 Jun;53(6):1578–1587. doi: 10.1172/JCI107708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coria F., Castaño E., Prelli F., Larrondo-Lillo M., van Duinen S., Shelanski M. L., Frangione B. Isolation and characterization of amyloid P component from Alzheimer's disease and other types of cerebral amyloidosis. Lab Invest. 1988 Apr;58(4):454–458. [PubMed] [Google Scholar]
- De Beer F. C., Pepys M. B. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Methods. 1982;50(1):17–31. doi: 10.1016/0022-1759(82)90300-3. [DOI] [PubMed] [Google Scholar]
- Dische F. E., Wernstedt C., Westermark G. T., Westermark P., Pepys M. B., Rennie J. A., Gilbey S. G., Watkins P. J. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988 Mar;31(3):158–161. doi: 10.1007/BF00276849. [DOI] [PubMed] [Google Scholar]
- Dyck R. F., Lockwood C. M., Kershaw M., McHugh N., Duance V. C., Baltz M. L., Pepys M. B. Amyloid P-component is a constituent of normal human glomerular basement membrane. J Exp Med. 1980 Nov 1;152(5):1162–1174. doi: 10.1084/jem.152.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz M. A., Skinner M., Cohen A. S., Kyle R. A. Nephelometric measurement of human serum amyloid P component (SAP). J Lab Clin Med. 1983 Nov;102(5):773–778. [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Hamazaki H. Ca2+-mediated association of human serum amyloid P component with heparan sulfate and dermatan sulfate. J Biol Chem. 1987 Feb 5;262(4):1456–1460. [PubMed] [Google Scholar]
- Hawkins P. N., Lavender J. P., Pepys M. B. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990 Aug 23;323(8):508–513. doi: 10.1056/NEJM199008233230803. [DOI] [PubMed] [Google Scholar]
- Hawkins P. N., Myers M. J., Epenetos A. A., Caspi D., Pepys M. B. Specific localization and imaging of amyloid deposits in vivo using 123I-labeled serum amyloid P component. J Exp Med. 1988 Mar 1;167(3):903–913. doi: 10.1084/jem.167.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. N., Myers M. J., Lavender J. P., Pepys M. B. Diagnostic radionuclide imaging of amyloid: biological targeting by circulating human serum amyloid P component. Lancet. 1988 Jun 25;1(8600):1413–1418. doi: 10.1016/s0140-6736(88)92235-0. [DOI] [PubMed] [Google Scholar]
- Hind C. R., Collins P. M., Renn D., Cook R. B., Caspi D., Baltz M. L., Pepys M. B. Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. J Exp Med. 1984 Apr 1;159(4):1058–1069. doi: 10.1084/jem.159.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnum S. Turnover of plasma proteins. J Clin Pathol Suppl (Assoc Clin Pathol) 1975;6:13–21. doi: 10.1136/jcp.s1-6.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Mantzouranis E. C., Dowton S. B., Whitehead A. S., Edge M. D., Bruns G. A., Colten H. R. Human serum amyloid P component. cDNA isolation, complete sequence of pre-serum amyloid P component, and localization of the gene to chromosome 1. J Biol Chem. 1985 Jun 25;260(12):7752–7756. [PubMed] [Google Scholar]
- Mather S. J., Ward B. G. High efficiency iodination of monoclonal antibodies for radiotherapy. J Nucl Med. 1987 Jun;28(6):1034–1036. [PubMed] [Google Scholar]
- McDougal J. S., Martin L. S., Cort S. P., Mozen M., Heldebrant C. M., Evatt B. L. Thermal inactivation of the acquired immunodeficiency syndrome virus, human T lymphotropic virus-III/lymphadenopathy-associated virus, with special reference to antihemophilic factor. J Clin Invest. 1985 Aug;76(2):875–877. doi: 10.1172/JCI112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najean Y., Cacchione R. Blood volume in health and disease. Clin Haematol. 1977 Oct;6(3):543–566. [PubMed] [Google Scholar]
- Nexö E., Gimsing P. Turnover in humans of iodine- and cobalamin-labeled transcobalamin I and of iodine-labeled albumin. Scand J Clin Lab Invest. 1975 Sep;35(5):391–398. [PubMed] [Google Scholar]
- Norwich K. H., Siu S. Power functions in physiology and pharmacology. J Theor Biol. 1982 Mar 21;95(2):387–398. doi: 10.1016/0022-5193(82)90253-3. [DOI] [PubMed] [Google Scholar]
- Ohnishi S., Maeda S., Shimada K., Arao T. Isolation and characterization of the complete complementary and genomic DNA sequences of human serum amyloid P component. J Biochem. 1986 Oct;100(4):849–858. doi: 10.1093/oxfordjournals.jbchem.a121797. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L., de Beer F. C., Dyck R. F., Holford S., Breathnach S. M., Black M. M., Tribe C. R., Evans D. J., Feinstein A. Biology of serum amyloid P component. Ann N Y Acad Sci. 1982;389:286–298. doi: 10.1111/j.1749-6632.1982.tb22144.x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Butler P. J. Serum amyloid P component is the major calcium-dependent specific DNA binding protein of the serum. Biochem Biophys Res Commun. 1987 Oct 14;148(1):308–313. doi: 10.1016/0006-291x(87)91111-9. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C., Markham R. E., Thomas H. C., Williams B. D., Petrie A. Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin Exp Immunol. 1978 Apr;32(1):119–124. [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Dyck R. F., de Beer F. C., Skinner M., Cohen A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979 Nov;38(2):284–293. [PMC free article] [PubMed] [Google Scholar]
- Potter B. J., Elias E., Jones E. A. Hypercatabolism of the third component of complement in patients with primary biliary cirrhosis. J Lab Clin Med. 1976 Sep;88(3):427–439. [PubMed] [Google Scholar]
- Prelli F., Pras M., Frangione B. The primary structure of human tissue amyloid P component from a patient with primary idiopathic amyloidosis. J Biol Chem. 1985 Oct 25;260(24):12895–12898. [PubMed] [Google Scholar]
- Skinner M., Vaitukaitis J. L., Cohen A. S., Benson M. D. Serum amyloid P-component levels in amyloidosis, connective tissue diseases, infection, and malignancy as compared to normal serum. J Lab Clin Med. 1979 Oct;94(4):633–638. [PubMed] [Google Scholar]
- Socolow E. L., Woeber K. A., Purdy R. H., Holloway M. T., Ingbar S. H. Preparation of I-131-labeled human serum prealbumin and its metabolism in normal and sick patients. J Clin Invest. 1965 Oct;44(10):1600–1609. doi: 10.1172/JCI105266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Shirahama T., Skinner M., Norén P., Cohen A. S. Amyloid P-component (protein AP) in localized amyloidosis as revealed by an immunocytochemical method. Histochemistry. 1981;71(2):171–175. doi: 10.1007/BF00507821. [DOI] [PubMed] [Google Scholar]
- Wise M. E. Negative power functions of time in pharmacokinetics and their implications. J Pharmacokinet Biopharm. 1985 Jun;13(3):309–346. doi: 10.1007/BF01065658. [DOI] [PubMed] [Google Scholar]
- Wise M. E. The form and interpretation of clearance curves for injected radioisotopes based on negative power laws, especially for 47Ca and estimating bone accretion rate. Curr Top Radiat Res Q. 1978 Jan;12(1-4):63–82. [PubMed] [Google Scholar]
- Wootton R., Flecknell P. A., Mehta A. A comparison in neonatal piglets of model-dependent and model-independent methods for measuring glucose turnover. Clin Phys Physiol Meas. 1987 Nov;8(4):355–365. doi: 10.1088/0143-0815/8/4/008. [DOI] [PubMed] [Google Scholar]
- de Beer F. C., Baltz M. L., Holford S., Feinstein A., Pepys M. B. Fibronectin and C4-binding protein are selectively bound by aggregated amyloid P component. J Exp Med. 1981 Oct 1;154(4):1134–1139. doi: 10.1084/jem.154.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]



