Abstract
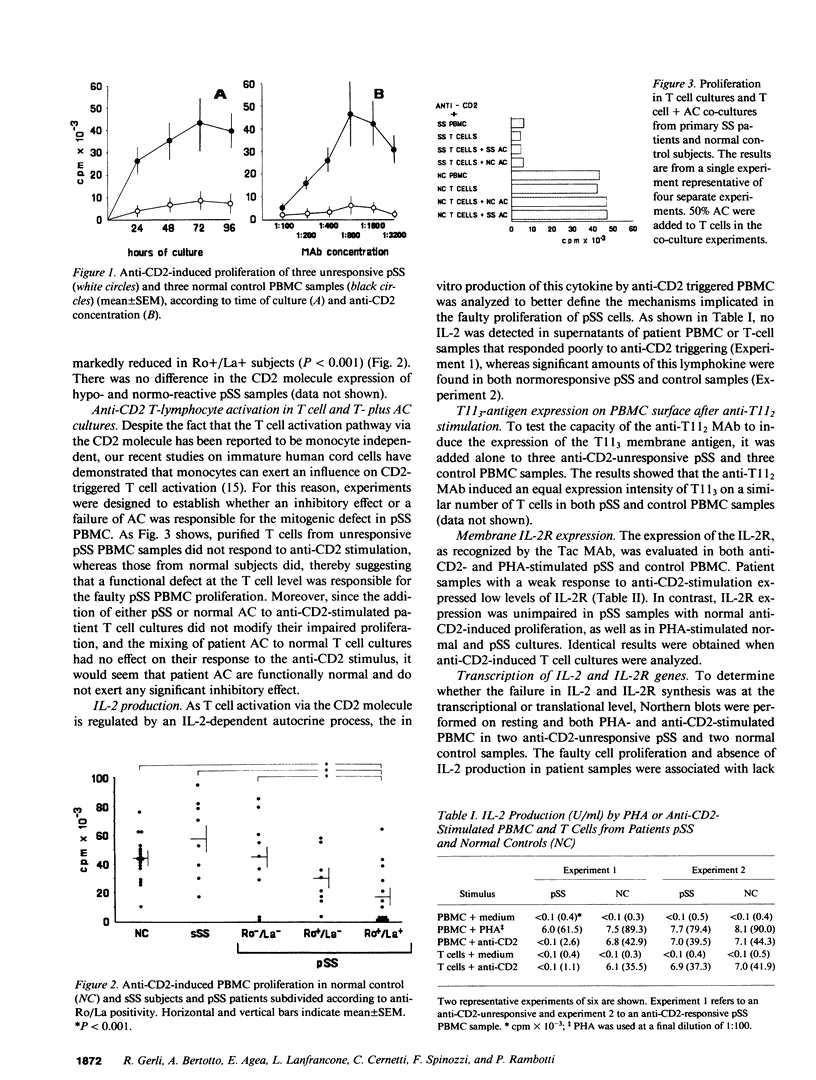
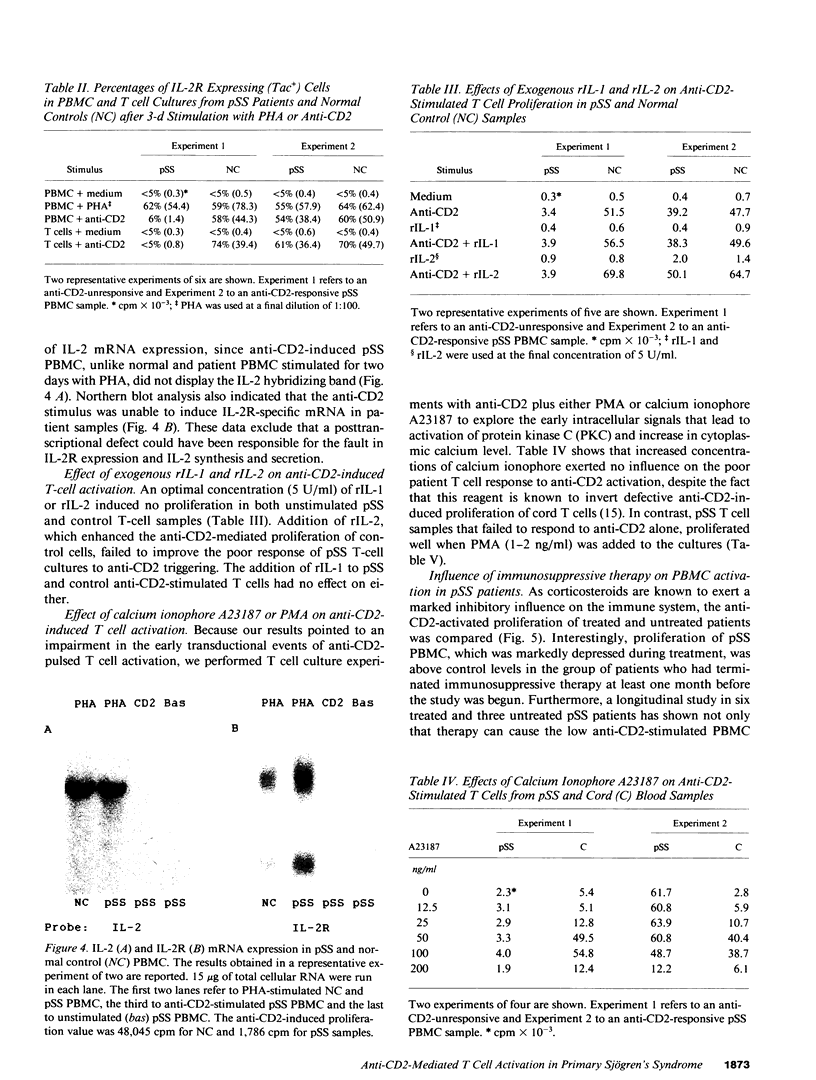
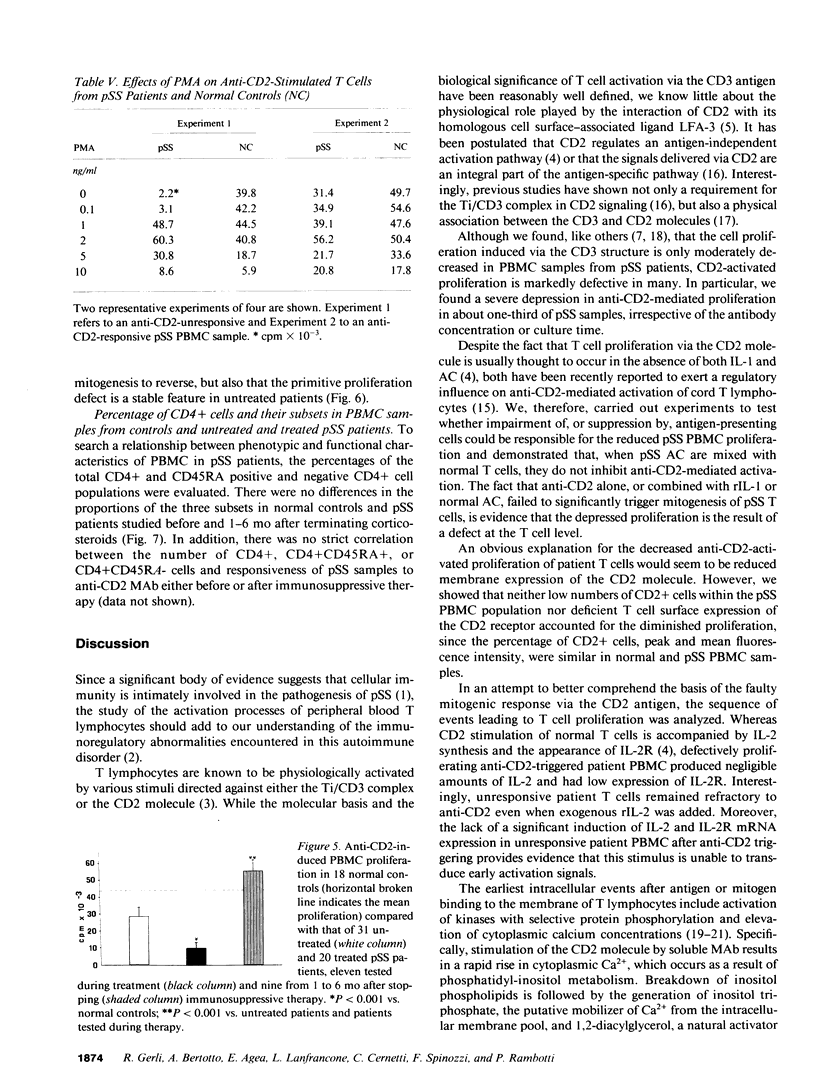
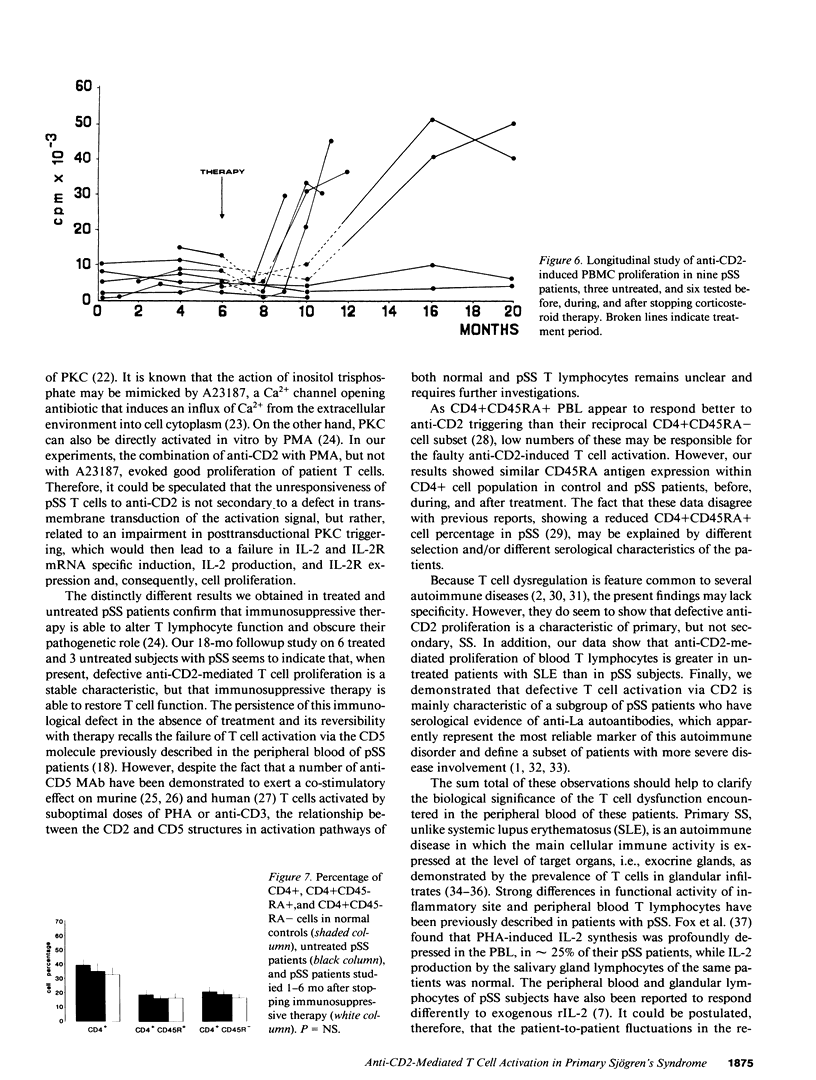
Anti-CD2-induced T cell proliferation was analyzed in the peripheral blood samples of 31 primary and 8 secondary untreated Sjögren's syndrome patients. Anti-CD2-stimulated PBMC proliferation was very low in about one-third of primary Sjögren's syndrome samples, despite the number of CD2+ cells being similar in primary and secondary Sjögren's syndrome and normal PBMC samples. The depressed response to anti-CD2 was mainly found in anti-Ro+/La+ patients. Experiments on purified T cells demonstrated that a defect at the T cell level was responsible for the anti-CD2 unresponsiveness. Cell proliferation failure was associated with poor IL-2 and IL-2 receptor mRNA expression and, consequently, IL-2 and IL-2 receptor synthesis. Since defective anti-CD2-induced mitogenesis could be reversed by phorbol myristate acetate, but not calcium ionophore A23187, it is probably correlated with impaired protein kinase C activation. Comparison of anti-CD2-triggered PBMC proliferation in treated and untreated patients and a long-term study of nine patients showed that the defect is a stable characteristic in primary Sjögren's syndrome patients, but that it can be reversed by pharmacological immunosuppression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- Anderson P., Morimoto C., Breitmeyer J. B., Schlossman S. F. Regulatory interactions between members of the immunoglobulin superfamily. Immunol Today. 1988 Jul-Aug;9(7-8):199–203. doi: 10.1016/0167-5699(88)91213-3. [DOI] [PubMed] [Google Scholar]
- Breitmeyer J. B., Daley J. F., Levine H. B., Schlossman S. F. The T11 (CD2) molecule is functionally linked to the T3/Ti T cell receptor in the majority of T cells. J Immunol. 1987 Nov 1;139(9):2899–2905. [PubMed] [Google Scholar]
- Brown M. H., Cantrell D. A., Brattsand G., Crumpton M. J., Gullberg M. The CD2 antigen associates with the T-cell antigen receptor CD3 antigen complex on the surface of human T lymphocytes. Nature. 1989 Jun 15;339(6225):551–553. doi: 10.1038/339551a0. [DOI] [PubMed] [Google Scholar]
- Bunn C. C., Gharavi A. E., Hughes G. R. Antibodies to extractable nuclear antigens in 173 patients with DNA-binding positive SLE: an association between antibodies to ribonucleoprotein and Sm antigens observed by counterimmunoelectrophoresis. J Clin Lab Immunol. 1982 May;8(1):13–17. [PubMed] [Google Scholar]
- Ceuppens J. L., Baroja M. L. Monoclonal antibodies to the CD5 antigen can provide the necessary second signal for activation of isolated resting T cells by solid-phase-bound OKT3. J Immunol. 1986 Sep 15;137(6):1816–1821. [PubMed] [Google Scholar]
- Chisholm D. M., Mason D. K. Labial salivary gland biopsy in Sjögren's disease. J Clin Pathol. 1968 Sep;21(5):656–660. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G., Reichlin M., Tomasi T. B., Jr Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythmatosus. J Immunol. 1969 Jan;102(1):117–122. [PubMed] [Google Scholar]
- Dauphinée M. J., Tovar Z., Ballester A., Talal N. The expression and function of CD3 and CD5 in patients with primary Sjögren's syndrome. Arthritis Rheum. 1989 Apr;32(4):420–429. doi: 10.1002/anr.1780320411. [DOI] [PubMed] [Google Scholar]
- Flescher E., Bowlin T. L., Ballester A., Houk R., Talal N. Increased polyamines may downregulate interleukin 2 production in rheumatoid arthritis. J Clin Invest. 1989 Apr;83(4):1356–1362. doi: 10.1172/JCI114023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D. A., Schlossman S. F., Reinherz E. L. Regulation of the alternative pathway of T cell activation by anti-T3 monoclonal antibody. J Immunol. 1986 Mar 15;136(6):1945–1950. [PubMed] [Google Scholar]
- Fox R. I., Theofilopoulos A. N., Altman A. Production of interleukin 2 (IL 2) by salivary gland lymphocytes in Sjögren's syndrome. Detection of reactive cells by using antibody directed to synthetic peptides of IL 2. J Immunol. 1985 Nov;135(5):3109–3115. [PubMed] [Google Scholar]
- Gerli R., Bertotto A., Crupi S., Arcangeli C., Marinelli I., Spinozzi F., Cernetti C., Angelella P., Rambotti P. Activation of cord T lymphocytes. I. Evidence for a defective T cell mitogenesis induced through the CD2 molecule. J Immunol. 1989 Apr 15;142(8):2583–2589. [PubMed] [Google Scholar]
- Gerli R., Bertotto A., Rambotti P., Barbieri P., Ciompi M. L., Bombardieri S. T cell immunoregulation in rheumatoid synovitis. Arthritis Rheum. 1988 Aug;31(8):1075–1076. doi: 10.1002/art.1780310823. [DOI] [PubMed] [Google Scholar]
- Gerli R., Bertotto A., Spinozzi F., Cernetti C., Grignani F., Rambotti P. Phenotypic dissection of cord blood immunoregulatory T-cell subsets by using a two-color immunofluorescence study. Clin Immunol Immunopathol. 1986 Sep;40(3):429–435. doi: 10.1016/0090-1229(86)90187-x. [DOI] [PubMed] [Google Scholar]
- Gerli R., Darwish S., Broccucci L., Minotti V., Spinozzi F., Cernetti C., Bertotto A., Rambotti P. Analysis of CD4-positive T cell subpopulation in sarcoidosis. Clin Exp Immunol. 1988 Aug;73(2):226–229. [PMC free article] [PubMed] [Google Scholar]
- Gerli R., Darwish S., Broccucci L., Spinozzi F., Rambotti P. Helper inducer T cells in the lungs of sarcoidosis patients. Analysis of their pathogenic and clinical significance. Chest. 1989 Apr;95(4):811–816. doi: 10.1378/chest.95.4.811. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hara T., Fu S. M. Human T cell activation. I. Monocyte-independent activation and proliferation induced by anti-T3 monoclonal antibodies in the presence of tumor promoter 12-o-tetradecanoyl phorbol-13 acetate. J Exp Med. 1985 Apr 1;161(4):641–656. doi: 10.1084/jem.161.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraoui B., Wilder R. L., Malone D. G., Allen J. B., Katona I. M., Wahl S. M. Immune function in severe, active rheumatoid arthritis: a relationship between peripheral blood mononuclear cell proliferation to soluble antigens and mononuclear cell subset profiles. J Immunol. 1984 Aug;133(2):697–701. [PubMed] [Google Scholar]
- Hollander N., Pillemer E., Weissman I. L. Effects of Lyt antibodies on T-cell functions: augmentation by anti-Lyt-1 as opposed to inhibition by anti-Lyt-2. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1148–1151. doi: 10.1073/pnas.78.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitas G. D., Salmon M., Farr M., Gaston J. S., Bacon P. A. Deficient interleukin 2 production in rheumatoid arthritis: association with active disease and systemic complications. Clin Exp Immunol. 1988 Aug;73(2):242–249. [PMC free article] [PubMed] [Google Scholar]
- Kurata N., Tan E. M. Identification of antibodies to nuclear acidic antigens by counterimmunoelectrophoresis. Arthritis Rheum. 1976 May-Jun;19(3):574–580. doi: 10.1002/art.1780190309. [DOI] [PubMed] [Google Scholar]
- Lögdberg L., Shevach E. M. Role of the Ly 1 antigen in interleukin 1-induced thymocyte activation. Eur J Immunol. 1985 Oct;15(10):1007–1013. doi: 10.1002/eji.1830151009. [DOI] [PubMed] [Google Scholar]
- Malone D. G., Wahl S. M., Tsokos M., Cattell H., Decker J. L., Wilder R. L. Immune function in severe, active rheumatoid arthritis. A relationship between peripheral blood mononuclear cell proliferation to soluble antigens and synovial tissue immunohistologic characteristics. J Clin Invest. 1984 Oct;74(4):1173–1185. doi: 10.1172/JCI111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe R., Oxholm P., Prause J. U., Schiødt M. The Copenhagen criteria for Sjögren's syndrome. Scand J Rheumatol Suppl. 1986;61:19–21. [PubMed] [Google Scholar]
- Matsuyama T., Anderson P., Daley J. F., Schlossman S., Morimoto C. CD4+CD45R+ cells are preferentially activated through the CD2 pathway. Eur J Immunol. 1988 Sep;18(9):1473–1476. doi: 10.1002/eji.1830180926. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos H. M., Chused T. M., Mann D. L., Klippel J. H., Fauci A. S., Frank M. M., Lawley T. J., Hamburger M. I. Sjögren's syndrome (Sicca syndrome): current issues. Ann Intern Med. 1980 Feb;92(2 Pt 1):212–226. doi: 10.7326/0003-4819-92-2-212. [DOI] [PubMed] [Google Scholar]
- Nel A. E., Wooten M. W., Galbraith R. M. Molecular signaling mechanisms in T-lymphocyte activation pathways: a review and future prospects. Clin Immunol Immunopathol. 1987 Aug;44(2):167–186. doi: 10.1016/0090-1229(87)90064-x. [DOI] [PubMed] [Google Scholar]
- Pease C. T., Shattles W., Charles P. J., Venables P. J., Maini R. N. Clinical, serological, and HLA phenotype subsets in Sjögren's syndrome. Clin Exp Rheumatol. 1989 Mar-Apr;7(2):185–190. [PubMed] [Google Scholar]
- Rudd C. E., Anderson P., Morimoto C., Streuli M., Schlossman S. F. Molecular interactions, T-cell subsets and a role of the CD4/CD8:p56lck complex in human T-cell activation. Immunol Rev. 1989 Oct;111:225–266. doi: 10.1111/j.1600-065x.1989.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Sato K., Miyasaka N., Yamaoka K., Okuda M., Yata J., Nishioka K. Quantitative defect of CD4+2H4+ cells in systemic lupus erythematosus and Sjögren's syndrome. Arthritis Rheum. 1987 Dec;30(12):1407–1411. doi: 10.1002/art.1780301212. [DOI] [PubMed] [Google Scholar]
- Slade J. D., Hepburn B. Prednisone-induced alterations of circulating human lymphocyte subsets. J Lab Clin Med. 1983 Mar;101(3):479–487. [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]



