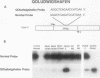
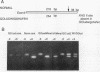
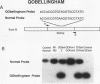
Abstract
The most common deficiency allele of the plasma protease inhibitor alpha 1-antitrypsin (alpha 1AT) is PI*Z. Some rare deficiency alleles of alpha 1AT produce low but detectable amounts of plasma alpha 1AT (1-20% of normal), which can be differentiated by isoelectric focusing. Others, designated null (QO) alleles, produce no alpha 1AT detectable by routine quantitative methods. We have previously described a method using DNA polymorphisms, haplotypes, and polyacrylamide isoelectric focusing gels, to differentiate various deficiency alleles. Based on haplotypes, we previously identified, in eight patients, five different null alleles, four of which had been previously sequenced. We have now analyzed all 12 null alleles in these eight patients, using allele-specific oligonucleotide probes, and have identified six different null alleles. We have cloned and sequenced one of these, PI*QOludwigshafen, which has a base substitution in exon II, replacing isoleucine 92 in the normal sequence with an asparagine. This substitution of a polar for a nonpolar amino acid occurs in one of the alpha-helices and is predicted to disrupt the tertiary structure. A total of 13 different alpha 1AT deficiency alleles, 6 of them null alleles, have been sequenced to date.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud P., Wilson G. B., Koistinen J., Fudenberg H. H. Immunofixation after electrofocusing: improved method for specific detection of serum proteins with determination of isoelectric points. I. Immunofixation print technique for detection of alpha-1-protease inhibitor. J Immunol Methods. 1977;16(3):221–231. doi: 10.1016/0022-1759(77)90200-9. [DOI] [PubMed] [Google Scholar]
- Bao J. J., Reed-Fourquet L., Sifers R. N., Kidd V. J., Woo S. L. Molecular structure and sequence homology of a gene related to alpha 1-antitrypsin in the human genome. Genomics. 1988 Feb;2(2):165–173. doi: 10.1016/0888-7543(88)90099-7. [DOI] [PubMed] [Google Scholar]
- Bates P. F., Swift R. A. Double cos site vectors: simplified cosmid cloning. Gene. 1983 Dec;26(2-3):137–146. doi: 10.1016/0378-1119(83)90183-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantly M., Nukiwa T., Crystal R. G. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988 Jun 24;84(6A):13–31. doi: 10.1016/0002-9343(88)90154-4. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Pemberton P. A., Boswell D. R. The serpins: evolution and adaptation in a family of protease inhibitors. Cold Spring Harb Symp Quant Biol. 1987;52:527–535. doi: 10.1101/sqb.1987.052.01.060. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Dente L., Cortese R. Cell-specific expression of a transfected human alpha 1-antitrypsin gene. Cell. 1985 Jun;41(2):531–540. doi: 10.1016/s0092-8674(85)80026-x. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Billingsley G. D., Callahan J. W. Aggregation of plasma Z type alpha 1-antitrypsin suggests basic defect for the deficiency. FEBS Lett. 1986 Sep 15;205(2):255–260. doi: 10.1016/0014-5793(86)80908-5. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Billingsley G. D., Mansfield T. DNA restriction-site polymorphisms associated with the alpha 1-antitrypsin gene. Am J Hum Genet. 1987 Nov;41(5):891–906. [PMC free article] [PubMed] [Google Scholar]
- Cox D. W., Billingsley G. D. Rare deficiency types of alpha 1-antitrypsin: electrophoretic variation and DNA haplotypes. Am J Hum Genet. 1989 Jun;44(6):844–854. [PMC free article] [PubMed] [Google Scholar]
- Cox D. W., Johnson A. M., Fagerhol M. K. Report of Nomenclature Meeting for alpha 1-antitrypsin, INSERM, Rouen/Bois-Guillaume-1978. Hum Genet. 1980;53(3):429–433. doi: 10.1007/BF00287070. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Levison H. Emphysema of early onset associated with a complete deficiency of alpha-1-antitrypsin (null homozygotes). Am Rev Respir Dis. 1988 Feb;137(2):371–375. doi: 10.1164/ajrccm/137.2.371. [DOI] [PubMed] [Google Scholar]
- Cox D. W. New variants of alpha 1-antitrypsin: comparison of Pi typing techniques. Am J Hum Genet. 1981 May;33(3):354–365. [PMC free article] [PubMed] [Google Scholar]
- Cox D. W., Woo S. L., Mansfield T. DNA restriction fragments associated with alpha 1-antitrypsin indicate a single origin for deficiency allele PI Z. Nature. 1985 Jul 4;316(6023):79–81. doi: 10.1038/316079a0. [DOI] [PubMed] [Google Scholar]
- Curiel D. T., Vogelmeier C., Hubbard R. C., Stier L. E., Crystal R. G. Molecular basis of alpha 1-antitrypsin deficiency and emphysema associated with the alpha 1-antitrypsin Mmineral springs allele. Mol Cell Biol. 1990 Jan;10(1):47–56. doi: 10.1128/mcb.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel D., Brantly M., Curiel E., Stier L., Crystal R. G. Alpha 1-antitrypsin deficiency caused by the alpha 1-antitrypsin Nullmattawa gene. An insertion mutation rendering the alpha 1-antitrypsin gene incapable of producing alpha 1-antitrypsin. J Clin Invest. 1989 Apr;83(4):1144–1152. doi: 10.1172/JCI113994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLella A. G., Marvit J., Brayton K., Woo S. L. An amino-acid substitution involved in phenylketonuria is in linkage disequilibrium with DNA haplotype 2. 1987 May 28-Jun 3Nature. 327(6120):333–336. doi: 10.1038/327333a0. [DOI] [PubMed] [Google Scholar]
- Faber J. P., Weidinger S., Goedde H. W., Ole K. The deficient alpha-I-antitrypsin phenotype PI P is associated with an A-to-T transversion in exon III of the gene. Am J Hum Genet. 1989 Jul;45(1):161–163. [PMC free article] [PubMed] [Google Scholar]
- Fraizer G. C., Harrold T. R., Hofker M. H., Cox D. W. In-frame single codon deletion in the Mmalton deficiency allele of alpha 1-antitrypsin. Am J Hum Genet. 1989 Jun;44(6):894–902. [PMC free article] [PubMed] [Google Scholar]
- Fraizer G. C., Siewertsen M., Harrold T. R., Cox D. W. Deletion/frameshift mutation in the alpha 1-antitrypsin null allele, PI*QObolton. Hum Genet. 1989 Nov;83(4):377–382. doi: 10.1007/BF00291385. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Kalsheker N. A., Newton C. R., Bamforth F. J., Powell S. J., Markham A. F. Molecular characterisation of three alpha-1-antitrypsin deficiency variants: proteinase inhibitor (Pi) nullcardiff (Asp256----Val); PiMmalton (Phe51----deletion) and PiI (Arg39----Cys). Hum Genet. 1989 Dec;84(1):55–58. doi: 10.1007/BF00210671. [DOI] [PubMed] [Google Scholar]
- Green P. M., Bentley D. R., Mibashan R. S., Nilsson I. M., Giannelli F. Molecular pathology of haemophilia B. EMBO J. 1989 Apr;8(4):1067–1072. doi: 10.1002/j.1460-2075.1989.tb03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hofker M. H., Nelen M., Klasen E. C., Nukiwa T., Curiel D., Crystal R. G., Frants R. R. Cloning and characterization of an alpha 1-antitrypsin like gene 12 KB downstream of the genuine alpha 1-antitrypsin gene. Biochem Biophys Res Commun. 1988 Sep 15;155(2):634–642. doi: 10.1016/s0006-291x(88)80542-4. [DOI] [PubMed] [Google Scholar]
- Hofker M. H., Nukiwa T., van Paassen H. M., Nelen M., Kramps J. A., Klasen E. C., Frants R. R., Crystal R. G. A Pro----Leu substitution in codon 369 of the alpha-1-antitrypsin deficiency variant PI MHeerlen. Hum Genet. 1989 Feb;81(3):264–268. doi: 10.1007/BF00279001. [DOI] [PubMed] [Google Scholar]
- Hofker M. H., Wapenaar M. C., Goor N., Bakker E., van Ommen G. J., Pearson P. L. Isolation of probes detecting restriction fragment length polymorphisms from X chromosome-specific libraries: potential use for diagnosis of Duchenne muscular dystrophy. Hum Genet. 1985;70(2):148–156. doi: 10.1007/BF00273073. [DOI] [PubMed] [Google Scholar]
- Holmes M., Curiel D., Brantly M., Crystal R. G. Characterization of the intracellular mechanism causing the alpha-1-antitrypsin Nullgranite falls deficiency state. Am Rev Respir Dis. 1989 Dec;140(6):1662–1667. doi: 10.1164/ajrccm/140.6.1662. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O. Amino acid substitution Glu leads to Lys alpha1-antitrypsin PiZ. FEBS Lett. 1976 Jun 1;65(2):195–197. doi: 10.1016/0014-5793(76)80478-4. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Fagerhol M. Properties of isolated human alpha1-antitrypsins of Pi types M, S and Z. Eur J Biochem. 1978 Feb 1;83(1):143–153. doi: 10.1111/j.1432-1033.1978.tb12078.x. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., Brouwers J. W., Maesen F., Dijkman J. H. PiMheerlen, alpha PiM allele resulting in very low alpha 1-antitrypsin serum levels. Hum Genet. 1981;59(2):104–107. doi: 10.1007/BF00293055. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Matsunaga E., Shiokawa S., Nakamura H., Maruyama T., Tsuda K., Fukumaki Y. Molecular analysis of the gene of the alpha 1-antitrypsin deficiency variant, Mnichinan. Am J Hum Genet. 1990 Mar;46(3):602–612. [PMC free article] [PubMed] [Google Scholar]
- Nukiwa T., Brantly M. L., Ogushi F., Fells G. A., Crystal R. G. Characterization of the gene and protein of the common alpha 1-antitrypsin normal M2 allele. Am J Hum Genet. 1988 Sep;43(3):322–330. [PMC free article] [PubMed] [Google Scholar]
- Nukiwa T., Takahashi H., Brantly M., Courtney M., Crystal R. G. alpha 1-Antitrypsin nullGranite Falls, a nonexpressing alpha 1-antitrypsin gene associated with a frameshift to stop mutation in a coding exon. J Biol Chem. 1987 Sep 5;262(25):11999–12004. [PubMed] [Google Scholar]
- Reid C. L., Wiener G. J., Cox D. W., Richter J. E., Geisinger K. R. Diffuse hepatocellular dysplasia and carcinoma associated with the Mmalton variant of alpha 1-antitrypsin. Gastroenterology. 1987 Jul;93(1):181–187. doi: 10.1016/0016-5085(87)90332-5. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Satoh K., Nukiwa T., Brantly M., Garver R. I., Jr, Hofker M., Courtney M., Crystal R. G. Emphysema associated with complete absence of alpha 1- antitrypsin in serum and the homozygous inheritance [corrected] of a stop codon in an alpha 1-antitrypsin-coding exon. Am J Hum Genet. 1988 Jan;42(1):77–83. [PMC free article] [PubMed] [Google Scholar]
- Sharp H. L., Bridges R. A., Krivit W., Freier E. F. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognized inherited disorder. J Lab Clin Med. 1969 Jun;73(6):934–939. [PubMed] [Google Scholar]
- Sifers R. N., Brashears-Macatee S., Kidd V. J., Muensch H., Woo S. L. A frameshift mutation results in a truncated alpha 1-antitrypsin that is retained within the rough endoplasmic reticulum. J Biol Chem. 1988 May 25;263(15):7330–7335. [PubMed] [Google Scholar]
- Takahashi H., Nukiwa T., Satoh K., Ogushi F., Brantly M., Fells G., Stier L., Courtney M., Crystal R. G. Characterization of the gene and protein of the alpha 1-antitrypsin "deficiency" allele Mprocida. J Biol Chem. 1988 Oct 25;263(30):15528–15534. [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Whitehouse D. B., Abbott C. M., Lovegrove J. U., McIntosh I., McMahon C. J., Mieli-Vergani G., Mowat A. P., Hopkinson D. A. Genetic studies on a new deficiency gene (PI*Ztun) at the PI locus. J Med Genet. 1989 Dec;26(12):744–749. doi: 10.1136/jmg.26.12.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Lieberman J., Gaidulis L., Ewing C. Molecular abnormality of human alpha1-antitrypsin variant (Pi-ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1324–1328. doi: 10.1073/pnas.73.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]