Abstract
Agitation is common in the medical and psychiatric emergency department, and appropriate management of agitation is a core competency for emergency clinicians. In this article, the authors review the use of a variety of first-generation antipsychotic drugs, second-generation antipsychotic drugs, and benzodiazepines for treatment of acute agitation, and propose specific guidelines for treatment of agitation associated with a variety of conditions, including acute intoxication, psychiatric illness, delirium, and multiple or idiopathic causes. Pharmacologic treatment of agitation should be based on an assessment of the most likely cause of the agitation. If agitation results from a delirium or other medical condition, clinicians should first attempt to treat the underlying cause instead of simply medicating with antipsychotics or benzodiazepines.
INTRODUCTION
The proper management of an agitated patient is essential to keep staff safe and ensure appropriate treatment for the patient. Most emergency physicians think of agitation as one of the simplest cases to treat, with haloperidol being a common approach in many emergency departments.1–4 In most circumstances, nonpharmacologic methods of behavior control, such as a verbal intervention, de-escalation, or even nicotine replacement therapy, may be helpful initially to manage agitated patients.5,6 When medications are required, second-generation antipsychotics, preferred by many psychiatrists over first-generation antipsychotics for long-term management of psychiatric illnesses, have also become increasingly used in the acute setting for management of agitation.7 This paper represents consensus recommendations from a workgroup of the American Association for Emergency Psychiatry. This workgroup convened in 2010–2011 to recommend best practices in the use of medication to manage agitated patients in the emergency setting.8
THE RATIONALE FOR USING MEDICATION
Agitation is prevalent in the emergency setting. The National Emergency Department Safety Study, for instance, documented that at least 25% of emergency department staff felt safe at work “sometimes,” “rarely,” or “never.”9 In addition, the 2010 Emergency Nurses Association study on violence in the workplace reported that more than half of emergency nurses had been verbally or physically threatened at work within the preceding 7 days.10 Agitation can also have effects on patients as well, with case reports of death due to untreated excited delirium.11,12
Calming of agitated patients therefore is of primary importance. When initial verbal methods have failed to calm the patient, medications may become necessary. One of the first crucial steps in prescribing medication is the establishment of a provisional diagnosis as to its cause. It is often not possible to make a definitive diagnosis but clinicians should attempt a diagnosis of the most likely cause, since this can guide the choice of medication following guidelines discussed later. The timing of the administration of medication can be crucial to the outcome of successfully managing an agitated patient. If an agitated patient is medicated too aggressively or too early, it may hinder psychiatric evaluation. If the patient is medicated too late, it places the patient, staff, and others at increased risk for harm. In addition, the agitation may also become more pronounced, and greater doses or repeated medication administration may be required to abort the agitation.
THE GOALS OF USING MEDICATION
The goal of using medication is to calm the patient so that he or she can be more accurately assessed by clinicians. Medication used in this manner is consistent with current guidelines on medication administration, which state that the proper endpoint of medication administration is calming without inducing sleep.7,13 In the acute setting, this more easily permits a diagnosis of the underlying cause of the agitation and allows patients to have some participation in their own care. More practically, however, patients who are not asleep are easier to discharge from the emergency department. Whether this matters for waiting times is controversial, with recent research indicating that the longest length of time that patients with psychiatric illnesses spend in the emergency department actually occurs between consultant disposition and discharge.14 However, between emergency departments, the most variable length of time that patients spend is between triage and contacting of psychiatry consultants, thus potentially allowing for improvements in these times by careful use of medication.
TYPES OF MEDICATION
There is no type of medication considered to be “best” in all cases of agitation but 3 general classes of medication have been studied and used most frequently for agitation, including first-generation antipsychotics, second-generation antipsychotics, and benzodiazepines. Three routes of administration are possible (though not for each medication): oral/oral fast-dissolving tablets, intramuscular, or intravenous. The workgroup believes that patients should be involved, if possible, in both the selection of the type and the route of any medication.
Although antipsychotics and benzodiazepines may manage the level of agitation a patient exhibits, this does not imply that these medications are doing so by directly addressing the underlying etiology of the agitation. For example, a large number of physiologic (eg, hypoxia) and metabolic (eg, hypoglycemia) perturbations that compromise brain function can produce delirium that is associated with agitation. Treatment to correct the specific underlying medical disturbance is the definitive and preferred treatment of agitation in such cases, but this article will not attempt to address the optimal treatments for such medical disturbances or the other varied etiologies of agitation. Rather, this article will discuss best-practice pharmacologic approaches to use when agitation requires emergent management before stabilization of the underlying etiology.
The Use of First-Generation Antipsychotics
Typical or first-generation antipsychotics (FGA) have a long history of use for treatment of agitation. The exact mechanism of calming with FGAs is unknown but most likely due to their inhibition of dopamine transmission in the human brain, which reduces the underlying psychotic symptoms causing the agitation. In addition, some FGAs are structurally similar to the human inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and interact with the human GABA receptor at high doses.15
The phenothiazines, a class of medication that includes low-potency antipsychotics such as chlorpromazine (Thorazine), the first FGA approved and marketed by the US Food and Drug Administration (FDA), have a propensity to cause more hypotension, more anticholinergic side effects, and lower the seizure threshold, compared to FGAs such as haloperidol.16 Thus, phenothiazines are not preferred for the treatment of acute agitation.
Haloperidol, an FGA belonging to the butyrophenone class, is a highly potent and selective antagonist of the dopamine-2 (D2) receptor. Haloperidol, which is FDA approved for oral or intramuscular use in schizophrenia, has a long track record of effective and safe use for the treatment of agitation in the acute setting. This drug is by far the most common FGA currently used to treat acute agitation.3,17 Droperidol, another butyrophenone with D2 receptor–blocking effects, has not been approved for psychiatric use but is approved as a preanesthetic to reduce nausea and vomiting associated with anesthesia. It has also been used widely in acute settings to treat agitation.
Both haloperidol and droperidol have minimal effects on vital signs, negligible anticholinergic activity, and minimal interactions with other nonpsychiatric medications. Unfortunately, both medications have important side effects. Notably, droperidol and haloperidol have a propensity to lengthen QTc intervals. Cases of torsades de pointes (TdP) have been reported with both drugs. There is much controversy regarding the degree and clinical significance of this QTc prolongation, and much research has indicated that clinically adverse cardiac effects are rare occurrences. Nevertheless, both drugs carry warnings about QTc prolongation in labeling of which physicians should be aware. The haloperidol label warning, for instance, indicates that “Higher doses and intravenous administration of haloperidol appear to be associated with a higher risk of QT prolongation and TdP.”18 This has led to an increasing number of hospitals implementing restrictive guidelines on the use of intravenous haloperidol, typically requiring electrocardiogram (ECG) monitoring during administration. Regardless of the true clinical risk, however, it seems prudent for physicians to avoid intravenous administration of haloperidol (which is not an FDA-approved route of administration for this medication), especially for patients who are taking other medication that can prolong QTc, who have a preexisting long QTc, or who have other conditions predisposing to TdP or QTc prolongation, such as underlying cardiac abnormalities, electrolyte imbalances (particularly hypokalemia and hypomagnesemia), or hypothyroidism. When haloperidol must be administered intravenously, the dose should be limited to 5 to 10 mg/day and administered in conjunction with continuous ECG monitoring.
Droperidol carries even more stringent warnings from the FDA about QTc prolongation and TdP. In 2007, this warning was upgraded to a black box, the most serious warning the FDA can require. This warning has subsequently proved highly controversial, as many reviews of the FDA data have claimed that it was based upon a very limited number of adverse events, mostly involving doses of droperidol much higher than those typically used to treat agitation (for an excellent review of the data see Jackson et al19). Other evidence also suggests that droperidol does not warrant such strong safety concerns. For instance, Isbister et al20 found that doses of up to 10 mg of droperidol had fewer adverse events than the use of midazolam in agitated emergency department patients, and Shale et al21 did not find a single case of a clinically significant adverse cardiac event in more than a decade of treating psychiatric emergencies with droperidol (typically 5 mg) in a busy emergency psychiatry unit. Emergency department–based studies, such as by Martel and colleagues,22 have even indicated that droperidol may have better efficacy and fewer side effects than ziprasidone, a second-generation antipsychotic approved for agitation. The FDA has indicated that it would revisit the evidence for droperidol's black-box warning either through an internal review or through review of an external study.19 Until such time as the FDA warning is modified or removed, however, it is prudent for clinicians to avoid using droperidol for agitation, especially because it is not FDA approved for psychiatric use.
In addition to cardiac effects, haloperidol and droperidol carry a risk of inducing acute extrapyramidal side effects (EPS) such as dystonia or neuroleptic malignant syndrome. High doses of these drugs can also cause catatonic reactions due to excessive central dopamine blockade. Although the true incidence of such EPS events is not clear, 1 study noted that EPS symptoms occurred in 20% of agitated patients treated with haloperidol alone but in only 6% of agitated patients treated with a combination of haloperidol and lorazepam.23 This combination treatment was also found to produce more rapid reduction in agitation. Other studies have found that adding promethazine to haloperidol can similarly reduce the incidence of extrapyramidal side effects.24,25 In part because of these studies, haloperidol is frequently administered in combination with another medication such as lorazepam, promethazine, or diphenhydramine.3 However, using multiple medications to control agitation may increase the risk both of oversedation and interactions with other medications. In addition, studies on patient preference have indicated that FGAs sometimes cause dysphoria after use.26,27 Given that most second-generation antipsychotics have demonstrated good efficacy in treating acute agitation, have low rates of extrapyramidal side effects (see upcoming text), and are subjectively preferred by patients over FGAs,26,27 the workgroup considers haloperidol to be less preferred than second-generation antipsychotics when an antipsychotic is indicated.
One common clinical scenario where haloperidol may still be the medication of choice is agitation in the context of acute alcohol intoxication. In agitation secondary to alcohol intoxication, medications to manage agitation should be generally avoided if possible, with nonpharmacologic methods, such as reduced environmental stimulation, being the preferred method of treatment.4,7 If medication is required, previous expert consensus documents have recommended benzodiazepines, given the possibility that a component of withdrawal may be contributing to the agitation.7 However, alcohol intoxication and withdrawal are distinct nonoverlapping presentations, which clinicians are generally able to differentiate. In addition, although there is no clear scientific evidence of respiratory depression with benzodiazepine use, there is a potential for clinically significant respiratory depression when benzodiazepines are administered to alcohol-intoxicated patients, as both agents are central nervous system (CNS) depressants.22 As such, this workgroup recommends the use of antipsychotics instead of benzodiazepines to treat agitation in the context of alcohol intoxication but the opposite in alcohol withdrawal (see upcoming text). There are both widespread clinical experience and published literature on the safe and effective use of haloperidol in intoxicated patients. Second-generation antipsychotics, however, have not been well studied in this situation. Thus, haloperidol remains preferred by the workgroup in this clinical scenario, although further study is needed.
THE USE OF SECOND-GENERATION ANTIPSYCHOTICS
Atypical antipsychotics, also called second-generation antipsychotics (SGA), were mostly developed in the 1990s and beyond. Several of these medications are commonly used in the acute setting. Olanzapine (Zyprexa), ziprasidone (Geodon), and aripiprazole (Abilify) come in both intramuscular and oral preparations. Risperidone (Risperdal) and quetiapine (Seroquel) are available in an oral formulation only.
As a class, these medications act as antagonists at the D2 receptor, as do FGAs, but also have comparable or stronger antagonism of other receptor subtypes, particularly serotonin-2A (5-HT2A) receptors. In addition, this class of medication has actions at other receptor types, such as histamine, norepinephrine, and α-2 receptors. Ziprasidone, for instance, has a high affinity for serotonin receptors compared to D2 receptors,28,29 while olanzapine and quetiapine have relatively higher affinities for the histamine receptor. In general, when compared with older drugs, SGAs have a reduced risk of near-term side effects such as dystonia or akathisia,30–32 with reported rates of less than 1%.30–32 This is lower than that reported with haloperidol alone23 and is some 10 times lower than even the combination of haloperidol + lorazepam.23,33
With the exception of risperidone, most randomized controlled trials of second-generation antipsychotics have been conducted in a psychiatric emergency department or inpatient ward, and not typical acute adult/pediatric emergency departments. Most of this research has generally indicated that most members of the class are effective in reducing agitation when compared to placebo, and are at least as calming as haloperidol.34–40 This is true of oral and oral rapid-dissolving formulations as well. In the limited number of studies that have compared oral antipsychotics, the combination of oral risperidone + lorazepam is as efficacious as intramuscular haloperidol + lorazepam, and oral risperidone alone is as efficacious as intramuscular haloperidol alone.37–40 Although there are no comparisons of oral olanzapine or oral ziprasidone with intramuscular haloperidol + lorazepam, oral olanzapine is as efficacious as oral risperidone alone.39 With the exception of risperidone, however, none of the SGAs have been compared against the more common regimen of haloperidol + lorazepam.37,38 Further, many of the published SGA investigations were industry-sponsored studies.
Although there have been no head-to-head trials of SGAs in the acute setting, published reviews have attempted to compare the effectiveness of different drugs in the class on a common scale, such as number-needed-to-treat.36 These reviews have generally indicated that most SGAs are equally effective at reducing agitation, with 3 possible exceptions. First, aripiprazole, the only partial D2 agonist approved for agitation, appears slightly less efficacious than other SGAs.36 Second, research on quetiapine has indicated that while this medication is useful in inpatient settings, it has an unacceptably high risk of orthostatic hypotension in the emergency department where patients are often volume depleted.41 Third, clozapine is only FDA approved for treatment-resistant schizophrenia and is not generally a first-line agent. Thus, although more study is needed, the use of aripiprazole, quetiapine, or clozapine cannot be recommended as first-line agents in the acute control of agitation. Other agents, such as lurasidone, iloperidone, and asenapine, are promising but have not yet been tested for acute agitation.
Most published studies of second-generation antipsychotics in agitated patients have not investigated their use either with benzodiazepines or in alcohol-intoxicated patients. Marder et al42 described a number of adverse events in patients who were administered the combination of olanzapine with benzodiazepines, and this combination is not currently recommended by the manufacturer. In 2 small retrospective studies, Wilson and colleagues43,44 noted that the combination of olanzapine + benzodiazepines did not cause vital sign abnormalities in patients who had not ingested alcohol. In some patients who had ingested alcohol, however, intramuscular olanzapine + benzodiazepines were associated with decreased oxygen saturations. These studies were too small, however, to provide conclusive evidence for the safety of olanzapine + benzodiazepines in nonintoxicated patients; thus, this combination should be avoided. Similarly, as little research has been conducted on other second-generation antipsychotics in alcohol-intoxicated patients, a first-generation antipsychotic may be a safer choice, especially if clinicians anticipate using a benzodiazepine as well.
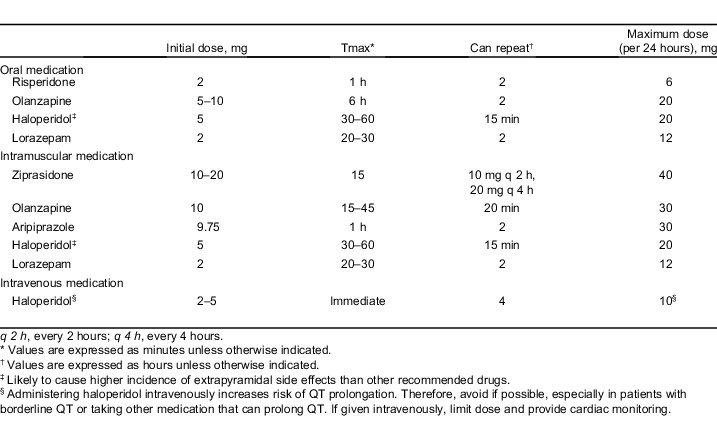
A summary of dosing for medications recommended in the treatment of agitation is provided in the Table.
Table.
Medications recommended in the treatment of agitation.
BENZODIAZEPINES
Benzodiazepines such as diazepam, lorazepam, and clonazepam act on the GABA receptor, the main inhibitory neurotransmitter in the human brain. These medications have a long record of efficacy for agitation, and are often preferred by clinicians when the patient is known to be suffering from stimulant intoxication, ethanol withdrawal, or when the etiology of agitation is undetermined. However, in agitation involving psychosis, benzodiazepines alone may only sedate a patient while not addressing the underlying disease that is producing the agitation. In addition, these medications may be oversedating and have the potential for respiratory depression or hypotension when used parenterally in patients with underlying respiratory conditions or in combination with other CNS depressants such as alcohol. In a minority of patients who chronically abuse stimulants, particularly amphetamines, psychotic symptoms develop as a result of their amphetamine use. In these patients, a first- or second-generation antipsychotic is often useful in addition to, or in place of, a benzodiazepine.45
SPECIFIC GUIDELINES FOR MEDICATION USE
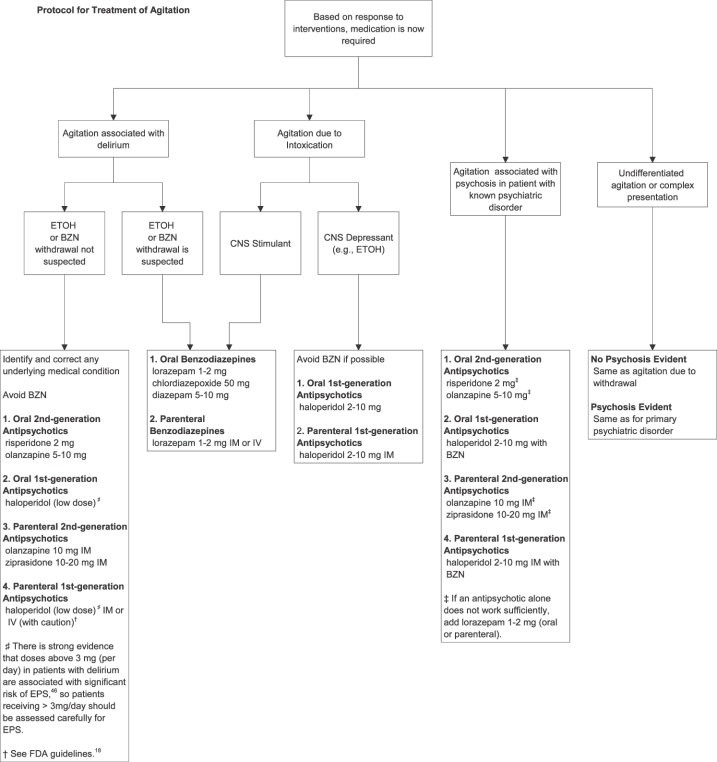
A recommended protocol for the treatment of agitation is shown in an algorithm in the Figure.
Figure.
Protocol for treatment of agitation. BZN, benzodiazepine; EPS, extrapyramidal side effects; ETOH, alcohol; IM, intramuscular.
General Recommendations
-
1
The use of medication as a restraint (ie, to restrict movement) should be discouraged. Rather, clinicians should, to whatever extent possible, attempt a provisional diagnosis of the most likely cause of the agitation and target medication to the most likely disease.
-
2
Nonpharmacologic approaches, such as verbal de-escalation and reducing environmental stimulation (quiet room, low lighting), should be attempted, if possible, before medications are administered.
-
3
Medication should be used to calm patients, not to induce sleep.
-
4
Patients should be involved in the process of selecting medication to whatever extent possible (eg, oral vs intramuscular).
-
5
If the patient is able to cooperate with taking oral medications, these are preferred over intramuscular preparations.
Agitation Due to Intoxication
-
1
Drugs: For intoxication with most recreational drugs, especially stimulants, benzodiazepines are generally considered first-line agents.47 A minority of chronic amphetamine users develop psychotic symptoms from their amphetamine use.45 In these patients, a second-generation antipsychotic may be useful in addition to a benzodiazepine.
-
2
Alcohol: Medication to treat agitation associated with alcohol intoxication should be used sparingly if at all. If medication is required, benzodiazepines should be avoided because of the potential to compound the risk of respiratory depression. Thus, antipsychotics are preferred. Haloperidol has the longest track record of safety and efficacy and has minimal effects on respiration. Second-generation antipsychotics, such as olanzapine and risperidone, have not been well studied for alcohol intoxication but may be a reasonable alternative to haloperidol for agitation in the context of alcohol intoxication. Of note, it is important to distinguish agitation secondary to alcohol intoxication versus agitation secondary to alcohol withdrawal, as benzodiazepines are preferred over antipsychotics in alcohol withdrawal (see the “Agitation Associated with Delirium” section). Agitation in a chronic alcohol user who exhibits features of delirium, such as tachycardia, diaphoresis, tremors, and a low or undetectable alcohol blood level, should be presumed to be due to withdrawal and treated accordingly.
Agitation Due to a Psychiatric Illness
-
1
For psychosis-driven agitation in a patient with a known psychiatric disorder (eg, schizophrenia, schizoaffective disorder, bipolar disorder), antipsychotics are preferred over benzodiazepines because they address the underlying psychosis.
-
2
Second-generation antipsychotics with supportive data for their use in acute agitation are preferred over haloperidol either alone or with an adjunctive medication. If the patient is willing to accept oral medication, oral risperidone has the strongest evidence for safety and efficacy, with a smaller number of studies supporting the use of oral antipsychotics such as olanzapine. If the patient cannot cooperate with oral medications, intramuscular ziprasidone or intramuscular olanzapine is preferred for acute control of agitation.
-
3
If an initial dose of antipsychotic is insufficient to control agitation, the addition of a benzodiazepine such as lorazepam is preferred to additional doses of the same antipsychotic or to a second antipsychotic.
Agitation Associated with Delirium
-
1
Delirium is a distinct clinical syndrome that frequently is associated with psychosis and agitation. It is important for clinicians to be able to recognize agitation associated with delirium for 2 reasons. First, the presence of delirium signals an underlying medical perturbation affecting brain function or a rapid change in the established environment of the brain. This can occur with sudden withdrawal from a chronically ingested agent (eg, alcohol or medication) or recent ingestion of a drug or medication, such as an anticholinergic agent in an elderly patient. Thus, the presence of delirium should impel the treating physician to identify the cause and correct it. Second, the symptomatic control of agitation secondary to delirium necessitates different choices of calming agents than agitation from other causes.
-
2
Hallmarks of delirium include a decreased level of awareness and disturbances in attention and cognition (eg, memory) that develop over an acute time course (hours to days). The disturbances in cognition and awareness typically fluctuate over the course of hours (ie, wax and wane). Prominence of visual hallucinations or visual perceptual disturbances is a particularly characteristic feature of delirium.
-
3
If alcohol or benzodiazepine withdrawal is the suspected cause of delirium, then a benzodiazepine is the agent of choice,48 since rapid loss of chronic GABA receptor inhibition is implicated in the delirium produced in these circumstances. Clonidine can also be helpful in reducing the sympathetic overdrive of alcohol or benzodiazepine withdrawal, thereby easing delirium and agitation.49
-
4
If withdrawal from another agent is suspected, replacement of the agent with another that has similar pharmacologic properties should be attempted if safe and appropriate (eg, nicotine for nicotine withdrawal).
-
5
If the recent ingestion of a new agent (or an increased dose of a chronically ingested agent) is the suspected cause of the delirium, then the delirium will be self-limiting. However, agitation may require temporary pharmacologic management (see No. 7).
-
6
When an underlying medical abnormality (eg, hypoglycemia, electrolyte imbalance, hypoxia) is the likely cause of delirium, the definitive treatment of the delirium and its associated agitation is correction of the underlying medical condition.
-
7
If immediate pharmacologic control of agitation is needed in a patient with delirium that is not due to alcohol, benzodiazepine withdrawal, or sleep deprivation, second-generation antipsychotics are the preferred agents. Haloperidol is also acceptable in low doses.46 Benzodiazepines should be generally avoided because they can exacerbate the delirium.50
Agitation from Unknown or Complex (More Than 1 Cause) Reasons
If medication is needed to control agitation in a nondelirious patient for whom the underlying etiology of the agitation is not clear, there is little in the way of formal evidence to guide the decision of which agent to use. In patients who do not display psychosis (hallucinations, delusional thinking, paranoia), a benzodiazepine is recommended as first-line treatment. An antipsychotic is recommended in patients who are displaying psychotic features. See the Table for additional dosing information.
CONCLUSIONS
After reviewing available evidence, the workgroup makes the following recommendations. Best practices for treating agitation include the following (please see specific recommendations for detailed recommendations in different clinical scenarios):
-
1
Pharmacologic treatment of agitation should be based on an assessment of the most likely cause for the agitation. If the agitation is from a medical condition or delirium, clinicians should first attempt to treat this underlying cause instead of simply medicating with antipsychotics or benzodiazepines.
-
2
Oral medications should be offered over intramuscular injections if the patient is cooperative and no medical contraindications to their use exist.
-
3
Antipsychotics are indicated as first-line management of acute agitation with psychosis of psychiatric origin.
-
4
When an antipsychotic is indicated for treatment of agitation, certain SGAs (such as olanzapine, risperidone, or ziprasodone), with good evidence to support their efficacy and lack of adverse events, are preferred over haloperidol or other FGAs. Agitation secondary to intoxication with a CNS depressant, such as alcohol, may be an exception in which haloperidol is preferred owing to few data on second-generation antipsychotics in this specific clinical scenario.
-
5
If haloperidol is used, clinicians should consider administering it with a benzodiazepine to reduce extrapyramidal side effects unless contraindications to use of this medication exist.
ACKNOWLEDGMENT
The authors would like to especially acknowledge Dr Scott Zeller who provided invaluable help on earlier versions of these recommendations.
Footnotes
Supervising Section Editor: Leslie Zun, MD
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding, sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
REFERENCES
- 1.Battaglia J. Pharmacological management of acute agitation. Drugs. 2005;65:1207–1222. doi: 10.2165/00003495-200565090-00003. [DOI] [PubMed] [Google Scholar]
- 2.Currier GW, Trenton A. Pharmacological treatment of psychotic agitation. CNS Drugs. 2002;16:219–228. doi: 10.2165/00023210-200216040-00002. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald K, Wilson MP, Minassian A, et al. A retrospective analysis of intramuscular haloperidol and olanzapine in the treatment of agitation in drug- and alcohol-using patients. Gen Hosp Psychiatry. 2010;32:443–445. doi: 10.1016/j.genhosppsych.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Vilke GM, Wilson MP. Agitation: what every emergency physician should know. Emerg Med Rep. 2009;30:233–244. [Google Scholar]
- 5.Hill S, Petit J. The violent patient. Emerg Med Clin North Am. 2000;18:301–315. doi: 10.1016/s0733-8627(05)70126-7. [DOI] [PubMed] [Google Scholar]
- 6.Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry. 2006;67((suppl 10)):13–21. [PubMed] [Google Scholar]
- 7.Allen MH, Currier GW, Carpenter D, et al. Expert Consensus Panel for Behavioral Emergencies 2005. The Expert Consensus Guideline Series: treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005. 5 pp.108 pp. [DOI] [PubMed]
- 8.Holloman GH, Jr, Zeller SL. Overview of Project BETA: best practices in evaluation and treatment of agitation. West J Emerg Med. 2011;13:1–2. doi: 10.5811/westjem.2011.9.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kansagra SM, Rao SR, Sullivan AF, et al. A survey of workplace violence across 65 U.S. emergency departments. Acad Emerg Med. 2008;15:1268–1274. doi: 10.1111/j.1553-2712.2008.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emergency Nurses Association Institute for Emergency Nursing Research. Emergency department violence surveillance study. Available at: http://www.ena.org/IENR/Documents/ENAEVSSReportAugust2010.pdf. Accessed February 24, 2011.
- 11.Vilke GM, Chan TC. Agitated delirium and sudden death. Prehosp Emerg Care. 2002;6:259. [PubMed] [Google Scholar]
- 12.Vilke GM, Debard ML, Chan TC, et al. Excited Delirium Syndrome (ExDS): defining based on a review of the literature. J Emerg Med. [published online ahead of print March, 24, 2011] [DOI] [PubMed]
- 13.Battaglia J, Lindborg SR, Alaka K, et al. Calming versus sedative effects of intramuscular olanzapine in agitated patients. Am J Emerg Med. 2003;21:192–198. doi: 10.1016/s0735-6757(02)42249-8. [DOI] [PubMed] [Google Scholar]
- 14.Chang G, Weiss AP, Orav EJ, et al. Hospital variability in emergency department length of stay for adult patients receiving psychiatric consultation: a prospective study. Ann Emerg Med. 2011;58:127–136. doi: 10.1016/j.annemergmed.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Richards JR, Schneir AB. Droperidol in the emergency department: is it safe? J Emerg Med. 2003;24:441–447. doi: 10.1016/s0736-4679(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 16.Citrome L, Volavka J. Violent patients in the emergency setting. Psychiatr Clin North Am. 1999;22:789–800. doi: 10.1016/s0193-953x(05)70126-x. [DOI] [PubMed] [Google Scholar]
- 17.Clinton JE, Sterner S, Stelmachers Z, et al. Haloperidol for sedation of disruptive emergency patients. Ann Emerg Med. 1987;16:319–322. doi: 10.1016/s0196-0644(87)80179-8. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. Information for healthcare professionals: haloperidol (marketed as Haldol, Haldol Decanoate and Haldol Lactate) Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm085203.htm. Accessed July 24, 2011.
- 19.Jackson CW, Sheehan AH, Reddan JG. Evidence-based review of the black-box warning for droperidol. Am J Health Syst Pharm. 2007;64:1174–1186. doi: 10.2146/ajhp060505. [DOI] [PubMed] [Google Scholar]
- 20.Isbister GK, Calver LA, Page CB, et al. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56:392–401. doi: 10.1016/j.annemergmed.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 21.Shale JH, Shale CM, Mastin WD. A review of the safety and efficacy of droperidol for the rapid sedation of severely agitated and violent patients. J Clin Psychiatry. 2003;64:500–505. doi: 10.4088/jcp.v64n0502. [DOI] [PubMed] [Google Scholar]
- 22.Martel M, Sterzinger A, Miner J, et al. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12:1167–1172. doi: 10.1197/j.aem.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation: a multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997;15:335–340. doi: 10.1016/s0735-6757(97)90119-4. [DOI] [PubMed] [Google Scholar]
- 24.Raveendran NS, Tharyan P, Alexander J, et al. TREC-India II Collaborative Group. Rapid tranquillisation in psychiatric emergency settings in India: pragmatic randomised controlled trial of intramuscular olanzapine versus intramuscular haloperidol plus promethazine. BMJ. 2007;335:865–873. doi: 10.1136/bmj.39341.608519.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huf G, Alexander J, Allen MH, et al. Haloperidol plus promethazine for psychosis-induced aggression. Cochrane Database Syst Rev. 2009. 3:CD005146. [DOI] [PubMed]
- 26.Lambert M, Schimmelmann BG, Karow A, et al. Subjective well-being and initial dysphoric reaction under antipsychotic drugs—concepts, measurement and clinical relevance. Pharmacopsychiatry. 2003;36((suppl 3)):S181–S190. doi: 10.1055/s-2003-45128. [DOI] [PubMed] [Google Scholar]
- 27.Karow A, Schnedler D, Naber D. What would the patient choose: subjective comparison of atypical and typical neuroleptics. Pharmacopsychiatry. 2006;39:47–51. doi: 10.1055/s-2006-931541. [DOI] [PubMed] [Google Scholar]
- 28.Mendelowitz AJ. The utility of intramuscular ziprasidone in the management of acute psychotic agitation. Ann Clin Psychiatry. 2004;16:145–154. doi: 10.1080/10401230490487025. [DOI] [PubMed] [Google Scholar]
- 29.Warrington L, Lombardo I, Loebel A, et al. Ziprasidone for the treatment of acute manic or mixed episodes associated with bipolar disorder. CNS Drugs. 2007;21:835–849. doi: 10.2165/00023210-200721100-00004. [DOI] [PubMed] [Google Scholar]
- 30.Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21:151–156. doi: 10.1097/YCO.0b013e3282f53132. [DOI] [PubMed] [Google Scholar]
- 31.Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53:1142–1145. doi: 10.1016/s0006-3223(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 32.Kane JM. Tardive dyskinesia rates with atypical antipsychotics in adults: prevalence and incidence. J Clin Psychiatry. 2004;65((suppl 9)):16–20. [PubMed] [Google Scholar]
- 33.Gillies D, Beck A, McCloud A, et al. Benzodiazepines alone or in combination with antipsychotic drugs for acute psychosis. Cochrane Database Syst Rev. 2005. 4:CD003079. [DOI] [PubMed]
- 34.Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry. 2002;59:441–448. doi: 10.1001/archpsyc.59.5.441. [DOI] [PubMed] [Google Scholar]
- 35.Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis: Ziprasidone IM Study Group. J Clin Psychiatry. 2000;61:933–941. doi: 10.4088/jcp.v61n1208. [DOI] [PubMed] [Google Scholar]
- 36.Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68:1876–1885. doi: 10.4088/jcp.v68n1207. [DOI] [PubMed] [Google Scholar]
- 37.Currier GW, Simpson GM. Risperidone liquid concentrate and oral lorazepam versus intramuscular haloperidol and intramuscular lorazepam for treatment of psychotic agitation. J Clin Psychiatry. 2001;62:153–157. doi: 10.4088/jcp.v62n0303. [DOI] [PubMed] [Google Scholar]
- 38.Currier GW, Chou JCY, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65:386–394. [PubMed] [Google Scholar]
- 39.Hsu W-Y, Huang S-S, Lee B-S, et al. Comparison of intramuscular olanzapine, orally disintegrating olanzapine tablets, oral risperidone solution, and intramuscular haloperidol in the management of acute agitation in an acute care psychiatric ward in Taiwan. J Clin Psychopharmacol. 2010;30:230–234. doi: 10.1097/JCP.0b013e3181db8715. [DOI] [PubMed] [Google Scholar]
- 40.Lim HK, Kim JJ, Pae CU, et al. Comparison of risperidone orodispersible tablet and intramuscular haloperidol in the treatment of acute psychotic agitation: a randomized open, prospective study. Neuropsychobiology. 2010;62:81–86. doi: 10.1159/000315437. [DOI] [PubMed] [Google Scholar]
- 41.Currier GW, Trenton AJ, Walsh PG, et al. A pilot, open-label study of quetiapine for treatment of moderate psychotic agitation in the emergency setting. J Psychiatr Pract. 2006;12:223–228. doi: 10.1097/00131746-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Marder SR, Sorsaburu S, Dunayevich E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatry. 2010;71:433–441. doi: 10.4088/JCP.08m04411gry. [DOI] [PubMed] [Google Scholar]
- 43.Wilson MP, MacDonald K, Vilke GM, et al. Potential complications of combining intramuscular olanzapine with benzodiazepines in agitated emergency department patients. J Emerg Med. [published online ahead of print June 12, 2010] [DOI] [PubMed]
- 44.Wilson MP, MacDonald K, Vilke GM, et al. A comparison of the safety of olanzapine and haloperidol in combination with benzodiazepines in emergency department patients with acute agitation. J Emerg Med. [published online ahead of print May 19, 2011] [DOI] [PubMed]
- 45.Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009. 1:CD003026. [DOI] [PMC free article] [PubMed]
- 46.Lonergan E, Britton AM, Luxenberg J. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007. 2:CD005594. [DOI] [PubMed]
- 47.Ricuarte GA, McCann UD. Recognition and management of complications of new recreational drug use. Lancet. 2005;365:2137–2145. doi: 10.1016/S0140-6736(05)66737-2. [DOI] [PubMed] [Google Scholar]
- 48.Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010. 3:CD005063. [DOI] [PubMed]
- 49.Muzyk AJ, Fowler JA, Norwood DK, et al. Role of α2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother. 2011;45:649–657. doi: 10.1345/aph.1P575. [DOI] [PubMed] [Google Scholar]
- 50.Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40:23–29. doi: 10.1093/ageing/afq140. [DOI] [PubMed] [Google Scholar]