Abstract
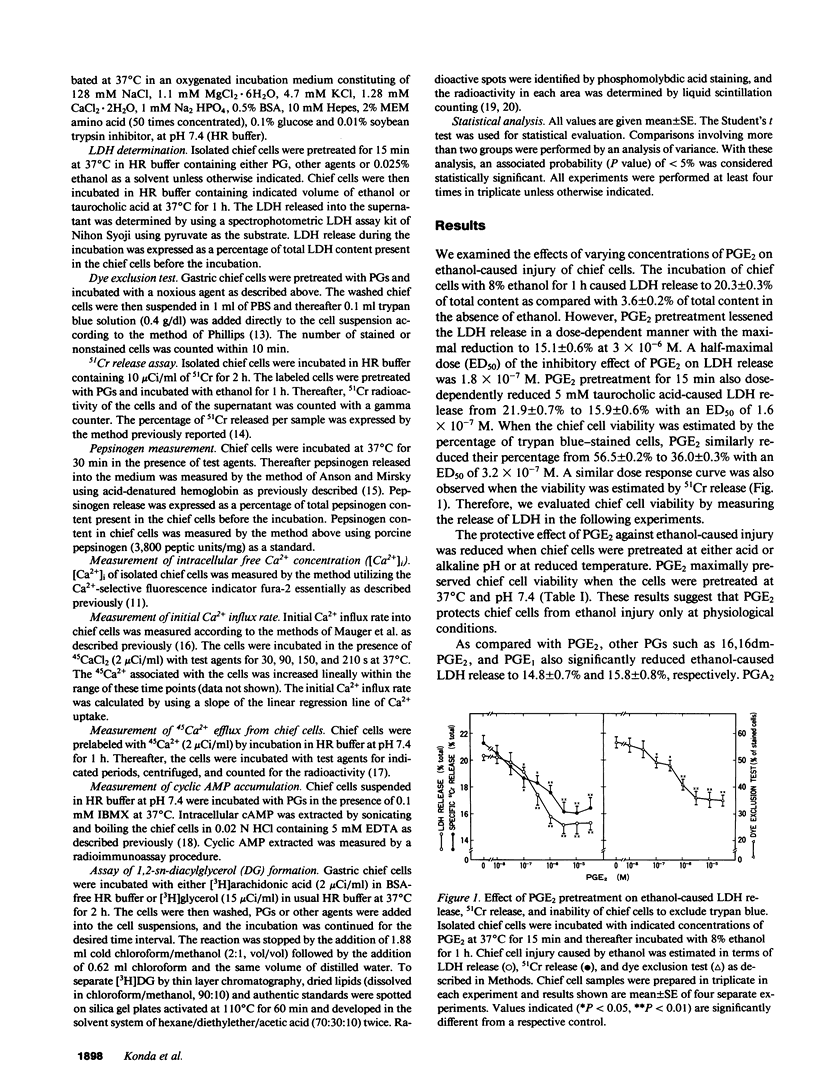
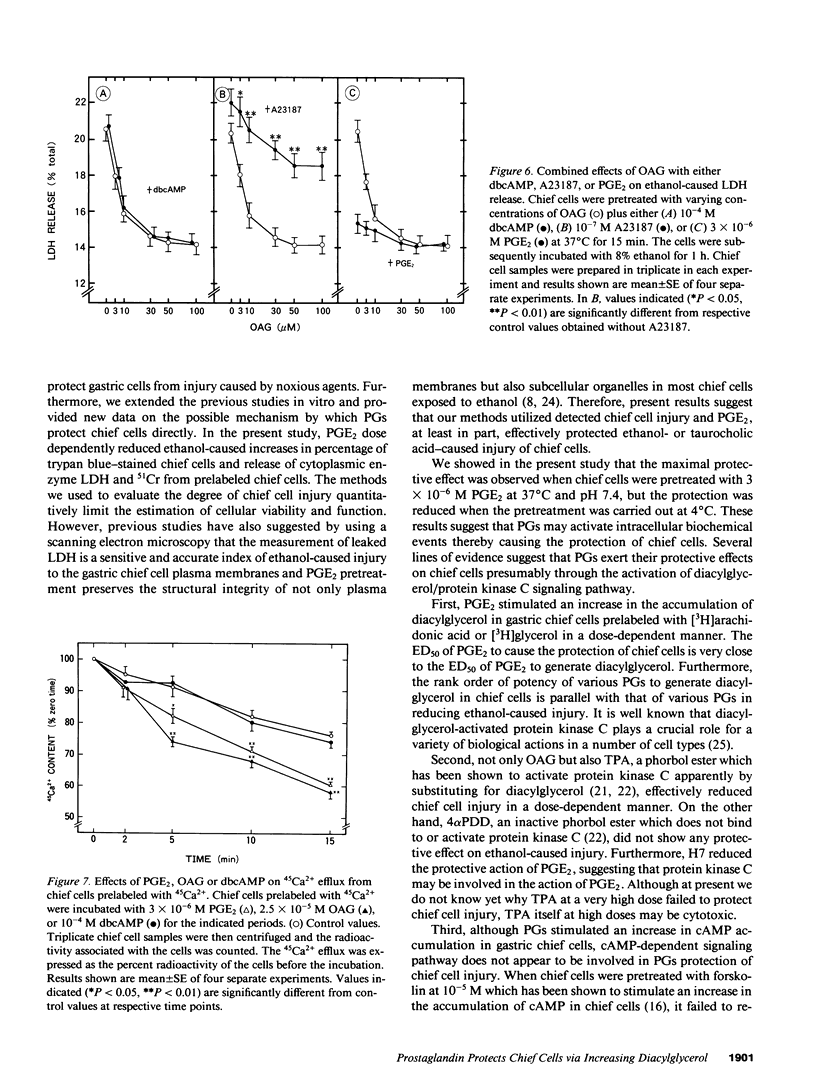
We studied cellular processes activated by prostaglandins (PG) that are involved in the protection of gastric chief cell injury estimated in terms of dye exclusion test, release of lactate dehydrogenase (LDH), or 51Cr from prelabeled chief cells. Pretreatment of chief cells with 3 x 10(-6) M PGE2 or PGE1 at 37 degrees C and pH 7.4 for 15 min maximally reduced not only ethanol- but also taurocholic acid-caused LDH release from chief cells. PGs equipotently stimulated increases in the accumulation of diacylglycerol and cyclic AMP without elevating intracellular Ca2+ concentrations in gastric chief cells. The rank order of the potency was equal to that of PGs to reduce the injury. Pretreatment of chief cells with synthetic 1-oleoyl-2-acetyl-sn-glycerol (OAG) or 12-o-tetradecanoyl phorbol 13-acetate (TPA) reduced the injury of chief cells, while 4 alpha-phorbol 12,13-didecanoate, an inactive phorbol ester, failed to reduce the injury and 1-(5-isouinolinylsulfonyl)-2-methylpiperazine (H7) blocked the protective action of PGE2. On the other hand, forskolin and dbcAMP had no effect on ethanol-caused LDH release and diacylglycerol formation in chief cells. These results suggest that PGE2 and PGE1 possess the direct protective action against ethanol- or taurocholic acid-caused injury in chief cells, presumably through the activation of the diacylglycerol/protein kinase C signaling pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cherner J. A., Naik L., Tarnawski A., Brzozowski T., Stachura J., Singh G. Ability of prostaglandin to reduce ethanol injury to dispersed chief cells from guinea pig stomach. Am J Physiol. 1989 Apr;256(4 Pt 1):G704–G714. doi: 10.1152/ajpgi.1989.256.4.G704. [DOI] [PubMed] [Google Scholar]
- Cheung L. Y. Topical effects of 16,16-dimethyl prostaglandin E2 on gastric blood flow in dogs. Am J Physiol. 1980 Jun;238(6):G514–G519. doi: 10.1152/ajpgi.1980.238.6.G514. [DOI] [PubMed] [Google Scholar]
- Dormer R. L., Poulsen J. H., Licko V., Williams J. A. Calcium fluxes in isolated pancreatic acini: effects of secretagogues. Am J Physiol. 1981 Jan;240(1):G38–G49. doi: 10.1152/ajpgi.1981.240.1.G38. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Kifor O., Brown E. M. Relationship between diacylglycerol levels and extracellular Ca2+ in dispersed bovine parathyroid cells. Endocrinology. 1988 Dec;123(6):2723–2729. doi: 10.1210/endo-123-6-2723. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Lichtenberger L. M., Graziani L. A., Dial E. J., Butler B. D., Hills B. A. Role of surface-active phospholipids in gastric cytoprotection. Science. 1983 Mar 18;219(4590):1327–1329. doi: 10.1126/science.6828859. [DOI] [PubMed] [Google Scholar]
- Matozaki T., Sakamoto C., Nagao M., Nishizaki H., Konda Y., Baba S. Involvement of Ca2+ influx in F(-)-stimulated pepsinogen release from guinea pig gastric chief cells. Biochem Biophys Res Commun. 1988 Apr 15;152(1):161–168. doi: 10.1016/s0006-291x(88)80694-6. [DOI] [PubMed] [Google Scholar]
- Miller T. A. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am J Physiol. 1983 Nov;245(5 Pt 1):G601–G623. doi: 10.1152/ajpgi.1983.245.5.G601. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohno T., Ohtsuki H., Okabe S. Effects of 16,16-dimethyl prostaglandin E2 on ethanol-induced and aspirin-induced gastric damage in the rat. Scanning electron microscopic study. Gastroenterology. 1985 Jan;88(1 Pt 2):353–361. doi: 10.1016/s0016-5085(85)80189-x. [DOI] [PubMed] [Google Scholar]
- Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979 Oct;77(4 Pt 1):761–767. [PubMed] [Google Scholar]
- Robert A., Nezamis J. E., Lancaster C., Hanchar A. J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979 Sep;77(3):433–443. [PubMed] [Google Scholar]
- Sakamoto C., Matozaki T., Nagao M., Baba S. Coupling of guanine nucleotide inhibitory protein to somatostatin receptors on pancreatic acinar membranes. Am J Physiol. 1987 Sep;253(3 Pt 1):G308–G314. doi: 10.1152/ajpgi.1987.253.3.G308. [DOI] [PubMed] [Google Scholar]
- Sakamoto C., Matozaki T., Nagao M., Nishizaki H., Baba S. Regulation of free cytosolic Ca2+ in the isolated guinea pig gastric chief cells. Biochem Biophys Res Commun. 1987 Feb 13;142(3):865–871. doi: 10.1016/0006-291x(87)91493-8. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Sherline P., Fox J. A. Insulin-stimulated diacylglycerol production results from the hydrolysis of a novel phosphatidylinositol glycan. J Biol Chem. 1987 Jan 25;262(3):1116–1121. [PubMed] [Google Scholar]
- Tarnawski A., Brzozowski T., Sarfeh I. J., Krause W. J., Ulich T. R., Gergely H., Hollander D. Prostaglandin protection of human isolated gastric glands against indomethacin and ethanol injury. Evidence for direct cellular action of prostaglandin. J Clin Invest. 1988 Apr;81(4):1081–1089. doi: 10.1172/JCI113420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnawski A., Hollander D., Stachura J., Klimczyk B., Mach T., Bogdal J. Prostaglandin protection of the human gastric mucosa against alcohol-induced injury. Endoscopic, histologic, and functional assessment. Scand J Gastroenterol Suppl. 1986;125:165–169. doi: 10.3109/00365528609093833. [DOI] [PubMed] [Google Scholar]
- Terano A., Mach T., Stachura J., Tarnawski A., Ivey K. J. Effect of 16,16 dimethyl prostaglandin E2 on aspirin induced damage to rat gastric epithelial cells in tissue culture. Gut. 1984 Jan;25(1):19–25. doi: 10.1136/gut.25.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terano A., Ota S., Mach T., Hiraishi H., Stachura J., Tarnawski A., Ivey K. J. Prostaglandin protects against taurocholate-induced damage to rat gastric mucosal cell culture. Gastroenterology. 1987 Mar;92(3):669–677. doi: 10.1016/0016-5085(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Truett A. P., 3rd, Snyderman R., Murray J. J. Stimulation of phosphorylcholine turnover and diacylglycerol production in human polymorphonuclear leukocytes. Novel assay for phosphorylcholine. Biochem J. 1989 Jun 15;260(3):909–913. doi: 10.1042/bj2600909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle B. J., Steel G. Evaluation of the protection of rat gastric mucosa by a prostaglandin analogue using cellular enzyme marker and histologic techniques. Gastroenterology. 1985 Jan;88(1 Pt 2):315–327. doi: 10.1016/s0016-5085(85)80186-4. [DOI] [PubMed] [Google Scholar]


