Abstract
A case of Rickettsia heilongjiangensis infection in Japan was identified in a 35-year-old man who had rash, fever, and eschars. Serum contained R. heilongjiangensis antibodies, and eschars contained R. heilongjiangensis DNA. R. heilongjiangensis was also isolated from ticks in the suspected geographic area of infection.
Keywords: vector-borne infections, Rickettsia heilongjiangensis, Rickettsia japonica, Haemaphysalis concinna, ticks, spotted fever group rickettsiae, bacteria, Japan, dispatch
Spotted fever group (SFG) rickettsiosis is the most prevalent arthropod-borne infectious disease in Japan (1). Before publication of a 1984 report about Japanese spotted fever (JSF) caused by Rickettsia japonica, scrub typhus caused by Orientia tsutsugamushi had been known as the sole rickettsiosis in Japan (1). Although many SFG Rickettsia species (R. japonica, R. helvetica, R. tamurae, R. asiatica, and other related Rickettsia spp.) were known, only R. japonica had been isolated or detected by PCR from Japanese SFG rickettsiosis patients (1–3). R. japonica was found in Dermacentor taiwanensis, Haemaphysalis cornigera, H. flava, H. formonensis, H. hystricis, H. longicornis, and Ixodes ovatus ticks, and R. helvetica in H. japonica, I. columnae, I. monospinosus, I. ovatus, I. pavlovskyi, I. persulcatus, and I. turdus ticks (3,4). Cases of SFG rickettsiosis caused by R. heilongjiangensis, showing mild rash associated with fever and an eschar, have been reported in the Russian Far East and the People’s Republic of China (5–8). In Russia and China, R. heilongjiangensis was isolated from H. concinna and D. sylvarum ticks (6,7). Highly related Rickettsia spp. were detected from H. longicornis ticks by PCR in South Korea (9). In this study, we confirmed a human case of R. heilongjiangensis infection in Japan. We also isolated R. heilongjiangensis from H. concinna ticks, a probable transmission vector, in the suspected geographic area of infection.
The Study
A 35-year-old man had chills and malaise on July 29, 2008 (day 0). On day 3, the patient became febrile (39.3°C). On day 5, a physician recognized the rash and prescribed oral minocycline (200 mg/d). On day 6, the patient was hospitalized because of constant fever and a whole body rash of unknown cause. At that time, laboratory data showed leukocyte count 7.2 × 109 cells/L, thrombocyte count 275 × 109 cells/L, aspartate aminotransferase 129 U/L, alanine aminotransferase 98 IU/L, and C-reactive protein 3.5 mg/dL. Biopsies were performed on eschars 1 and 2 (5–8 mm diameter) with erythema (≈20 mm diameter), above the left scapula and on the right lower back. During hospitalization, the patient received minocycline, 200 mg/day, intravenously. DNA was extracted from skin biopsy specimens by using a commercial kit according to the manufacturer’s instructions (Gentra Puregene; QIAGEN, Valencia, CA, USA). PCR was performed by using primers of 3 rickettsial genes: outer membrane protein A (ompA; primers Rr190.70p and Rr190.602n) (10), citrate synthase (gltA; primers Cs2d and CsEndr) (6), genus Rickettsia–specific outer membrane (17-kDa antigen gene; primers R1 and R2) (11), and primers for O. tsutsugamushi, as reported previously (12).
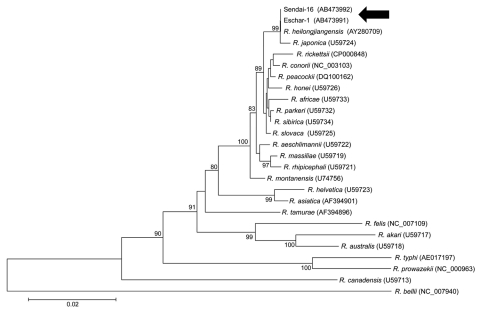
Although many cases of R. heilongjiangensis infection show a single eschar as a result of a tick bite, ompA, gltA, and 17-kDa antigen genes were detected by PCR (but not with O. tsutsugamushi–specific primers) in both eschar specimens. Amplicons were sequenced and analyzed phylogenetically (Figure 1). The 491-bp fragment of ompA from eschar 1 (GenBank accession no. AB473995) demonstrated 99.8% and 97.1% nucleotide homology with R. heilongjiangensis strain HLJ-054 and R. japonica strain YM, respectively. The 1,250-bp fragment of gltA of eschar 1 (accession no. AB473991) demonstrated 99.9%, 99.8%, and 96.8% nucleotide homology with the R. heilongjiangensis strain HLJ-054, R. japonica strain YM, and R. helvetica strain C9P9, respectively. The 392-bp fragment of the 17-kDa antigen gene of eschar 1 (accession no. AB473987) demonstrated 100.0% and 99.2% nucleotide homology with R. heilongjiangensis strain HLJ-054 and R. japonica strain YM, respectively. Blood specimens were negative for rickettsial antigens by PCR, possibly because they were collected after minocycline treatment. Three serial blood samples were tested serologically by immunoperoxidase assays against rickettsial antigens: R. japonica strain YH; O. tsutsugamushi Karp, Kato, Gilliam, Kawasaki, Kuroki, and Shimokoshi strains; and R. heilongjiangensis strain CH8–1 (13). The R. heilongjiangensis strain CH8-1 used in our analysis was isolated from H. concinna ticks collected in Inner Mongolia, China, as an unknown SFG Rickettsia species (3). Strain CH8–1 was identified as R. heilongjiangensis by DNA analysis in this study (ompA, accession no. AB473813; gltA, AB473812; and 17-kDa antigen genes, AB473811). R. japonica and R. heilongjiangensis antibody titers were substantially elevated from day 6 to day 16. Titers against R. heilongjiangensis were 2–4 times higher than titers against R. japonica on day 16 (Table).
Figure 1.
Phylogenetic analysis of citrate synthase (gltA) sequences of Rickettsia spp. Sequences were aligned by using MEGA4 software (www.megasoftware.net). Neighbor-joining phylogenetic tree construction and bootstrap analyses were performed according to the Kimura 2-parameter distances method. Pairwise alignments and multiple alignments were performed with an open gap penalty of 15 and a gap extension penalty of 6.66. The percentage of replicate trees in which the associated taxa were clustered together in the bootstrap test (1,000 replicates) was calculated. Phylogenetic branches were supported by bootstrap values of >80%. All positions containing alignment gaps and missing data were eliminated in pairwise sequence comparisons (pairwise deletion). Scale bar indicates the percentage of sequence divergence. Arrows indicate eschar specimens.
Table. Antibody titers to spotted fever group rickettsiae in patient’s serum samples, Sendai, Japan, 2008*.
| Days after symptom onset | Antibody titers (IgG/IgM) |
||
|---|---|---|---|
| Rickettsia japonica | R. heilongiangensis | Orientia tsutsugamushi | |
| 6 | <10/<10 | <10/<10 | <10/<10 |
| 16 | 40/160 | 160/320 | <10/<10 |
| 23 | 320/640 | 320/640 | <10/<10 |
*Ig, immunoglobulin.
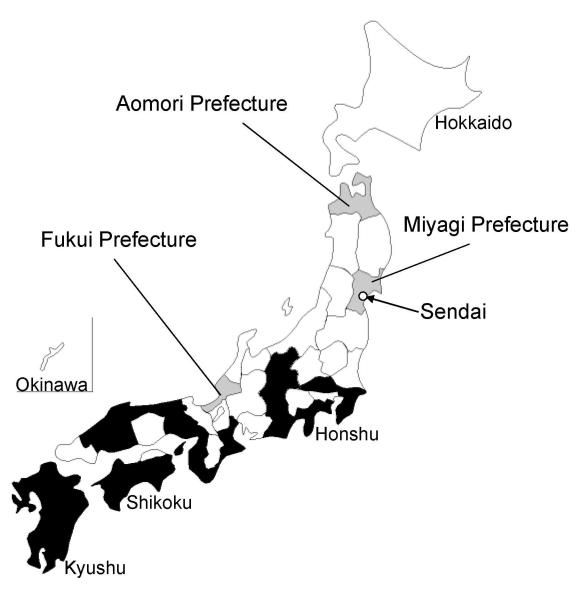
An interview with the patient after the laboratory diagnosis of R. heilongjiangensis infection revealed more information about the context of the infection. He resided in an urban area of Sendai, Miyagi Prefecture, Japan (Figure 2). For 2 weeks before onset of symptoms, his outdoor activity was limited to daily walking with a companion dog along a river near his residence. The suspected area where he may have become infected through a tick bite was investigated in September 2008. We captured and examined 72 Haemaphysalis spp. ticks (52 H. longicornis, 15 H. concinna, 4 H. flava, and 1 H. megaspinosa) and 7 rodents (4 Rattus norvegicus and 3 Microtus montebelli) for investigation of SFG Rickettsia spp. Tick and rodent spleens were homogenized and subjected to isolation studies with L929 cells in shell vial (3), and detection of Rickettsia DNA by PCR was performed in parallel as previously described. Of the 72 tick samples, 3 H. concinna nymphs yielded Rickettsia isolates and a DNA fragment of Rickettsia, which was detected by PCR. Sequences of 3 isolates and amplicons were identical to those from the patient’s specimens (Figure 1, tick-derived isolates assigned Sendai-16, 29, 32; Sendai-16: ompA, gltA, and 17-kDa antigen gene accession nos. AB473996, AB473992, and AB473988, respectively; Sendai-29: ompA, gltA, and 17-kDa antigen gene accession nos. AB473997, AB473993, and AB473989, respectively; and Sendai-32: ompA, gltA, and 17-kDa antigen gene accession nos. AB473998, AB473994, and AB473990, respectively). PCR-detectable rickettsial agents were not isolated from the rodents; however, 3 of the 4 R. norvegicus specimens had high antibody titers to R. heilongjiangensis.
Figure 2.
Distribution of reported Japanese spotted fever cases in Japan (≈2008). Prefectures in which Japanese spotted fever cases were reported up to 2008 are shown in black; Fukui, Aomori, and Miyagi prefectures are shown in gray. The map was drawn by using data on reported infectious diseases in Japan (http://idsc.nih.go.jp/idwr/pdf-j.html).
To date, most cases of SFG rickettsiosis have been reported as JSF in the western regions of Honshu Island, Japan (1,2). R. japonica was isolated from ixodids in the area where JSF is endemic, and R. helvetica from the entire country of Japan (3). Moreover, only R. japonica has been isolated from patients with SFG rickettsiosis (1,2). A case-patient with JSF demonstrated serologic evidence of SFG rickettsiosis caused by agents other than R. japonica; however, those agents have not been defined (R. helvetica in Fukui) (14). In 2007, another case of JSF was detected serologically by using only R. japonica antigen in Aomori Prefecture, the northernmost prefecture of Honshu Island (15). However, R. japonica has not been detected in this area (3). These results suggest that some cases of SFG rickettsiosis in Japan may have been caused by SFG Rickettsia species other than R. japonica.
The case reported in this article occurred in an urban area of Sendai in the northern section of Honshu Island (Figure 2). Scrub typhus caused by O. tsutsugamushi occurs in this area with 2 seasonal peaks: from early spring to early summer, and from early fall to early winter (1). Serologic and microbiologic data ruled out scrub typhus in the present case. R. heilongjiangensis infection has been reported in the summer in the disease-endemic area of the Eurasian continent. Notably, the present case occurred in midsummer.
Conclusions
R. japonica has been the only known causative agent of SFG rickettsiosis in Japan, possibly because of limited availability of laboratory test systems. Further studies are needed to define the prevalence of SFG rickettsiosis caused by Rickettsia species other than R. japonica.
Acknowledgment
We thank the staff of the Sendai City Municipal Government.
This study was supported by a grant for Research on Emerging and Reemerging Infectious Diseases (H18-Shinkou-Ippan-014 and H21-Shinkou-Ippan-06) from the Ministry of Health, Labor and Welfare, Japan.
Biography
Dr Ando is chief of the Laboratory of Rickettsia and Chlamydia, Department of Virology I, National Institute of Infectious Diseases, Japan. His primary research interests are ecology and epidemiology of rickettsial and chlamydial diseases and the development of new diagnostic methods for vector-borne diseases.
Footnotes
Suggested citation for this article: Ando S, Kurosawa M, Sakata A, Fujita H, Sakai K, Sekine M, et al. Human Rickettsia heilongjiangensis infection, Japan. Emerg Infect Dis [serial on the Internet]. 2010 Aug [date cited]. http://dx.doi.org/10.3201/eid1608.100049
References
- 1.National Institute of Infectious Diseases and Tubetculosis and Infectious Diseases Control Division, Ministry of Health, Labour and Welfare. Scrub typhus and Japanese spotted fever in Japan, as of December 2005. Infectious Agents Surveillance Report. 2006;27:27–8. [Google Scholar]
- 2.Mahara F. Japanese spotted fever: report of 31 cases and review of the literature. Emerg Infect Dis. 1997;3:105–11. 10.3201/eid0302.970203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujita H. Cell culture system for isolation of disease agents: 15 years of experience in Ohara Research Laboratory. Annual Report of Ohara General Hospital. Fukshima (Japan); Ohara General Hospital; 2008. p. 21–42. [Google Scholar]
- 4.Fournier PE, Fujita H, Takada N, Raoult D. Genetic identification of rickettsiae isolated from ticks in Japan. J Clin Microbiol. 2002;40:2176–81. 10.1128/JCM.40.6.2176-2181.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence–based criteria for identification of new Rickettsia isolates and description on Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–65. 10.1128/JCM.41.12.5456-5465.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mediannikov OY, Sidelnikov Y, Ivanov L, Morkretsova E, Fournier PE, Tarasevich I, et al. Acute tick-borne rickettsiosis caused by Rickettsia heilongjiangensis in Russian Far East. Emerg Infect Dis. 2004;10:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shpynov SN, Fournier PE, Rudakov NV, Samoilenko IE, Reshetinokova TA, Yastrebov VK, et al. Molecular identification of a collection of spotted fever group rickettsiae obtained from patients and ticks from Russia. Am J Trop Med Hyg. 2006;74:440–3. [PubMed] [Google Scholar]
- 8.Zhang JZ, Fan MY, Wu YM, Fournier PE, Roux V, Raoult D. Genetic classification of “Rickettsia heilongjiangensii” and “Rickettsia hulinii,” two Chinese spotted fever group rickettsiae. J Clin Microbiol. 2000;38:3498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Park HS, Jung KD, Jang WJ, Koh SE, Kang SS, et al. Identification of the spotted fever group rickettsiae detected from Haemaphysalis longicornis in Korea. Microbiol Immunol. 2003;47:301–4. [DOI] [PubMed] [Google Scholar]
- 10.Noda H, Munderloh UG, Kurtti TJ. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl Environ Microbiol. 1997;63:3926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson BE. Comparative sequence analysis of a genus-common rickettsial antigen gene. J Bacteriol. 1989;171:5199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuya Y, Yoshida Y, Katayama T, Kawamori F, Yamamoto S, Ohashi N, et al. Specific amplification of Rickettsia tsutsugamushi DNA from clinical specimens by polymerase chain reaction. J Clin Microbiol. 1991;29:2628–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suto T. Evidence of spotted fever rickettsial infection in Japan as demonstrated by the indirect immunoperoxide test. Microbiol Immunol. 1985;29:1243–6. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguro F, Takada N, Fujita H, Noji Y, Yano Y, Iwasaki H. Survey of the vectorial competence of ticks in an endemic area of spotted fever group rickettsioses in Fukui Prefecture, Japan. Microbiol Immunol. 2008;52:305–9. 10.1111/j.1348-0421.2008.00042.x [DOI] [PubMed] [Google Scholar]
- 15.Mori S, Akasaka T. A case of Japanese spotted fever [in Japanese]. Jpn J Clin Dermatol. 2008;62:443–5. [Google Scholar]