Abstract
International Spinal Cord Injury (SCI) Data Sets include core, basic, and extended data sets. To date, 13 data sets have been published on the Web site of the International Spinal Cord Injury Society (ISCoS; www.iscos.org.uk), and several more are forthcoming. The data sets are constituted of data elements, which may be appropriate to use in trials conducted to test novel therapeutic candidates including neuroprotective drugs, various cell types, and rehabilitative strategies and devices. The National Institute of Neurological Disorders and Stroke (NINDS), the National Institutes of Health (NIH), embarked on a Common Data Element (CDE) Project 5 years ago. The mission of the NINDS CDE Project is to develop data standards for clinical research. The NINDS CDE team has since developed variable names and database structures for the International SCI Data Sets (ie, the SCI CDEs; http://www.commondataelements.ninds.nih.gov/SCI.aspx). Dataset variable names and database structure are exemplified with the International SCI Core Data Set and the International SCI Cardiovascular Function Basic Data Set. The consistency of the data sets and the CDE format may improve the ability to transfer critical medical information electronically from one center to another. The goals of the SCI CDE initiative are to increase the efficiency and effectiveness of clinical research studies and clinical treatment, increase data quality, facilitate data sharing, and help educate new clinical investigators. Pilot testing the SCI CDEs is an important step to ensure the SCI CDE effort achieves its goals.
Keywords: common data element, database specifications, data set, international, spinal cord injury, variable names
International Spinal Cord Injury Data Sets: History and Current Status
The International Spinal Cord Injury (SCI) Data Sets are being created to promote the collection of uniform data for various uses. Data sets collected for research purposes using these uniform standards can be applied to broad analyses relating to prevention efforts, quality of care studies, and various multicenter research projects (including trials of novel therapeutics).1 Common data sets for individuals with SCI are needed to facilitate comparisons between injuries, treatments, and outcomes between patients, centers, and countries.
Various basic SCI data sets can be used together to make up the basic medical record used by SCI centers. An extended SCI data set is a more detailed data set, which may be optional for a clinical record but may be recommended for uniform use in specific research studies. To date, International SCI Basic Data Sets have been created in the areas of lower urinary tract function,2 urodynamics,3 urinary tract imaging,4 bowel function,5 pain,6 female sexual and reproductive function,7 male sexual function,8 cardiovascular function,9 pulmonary function, endocrine and metabolic function,10 and quality of life (www.iscos.org.uk). Working groups are developing datasets for nontraumatic lesions, spinal column injury, spinal surgery, urinary tract infection, skin/thermoregulation, musculoskeletal, and activity and participation. Recommendations for translation and reliability testing of International SCI Data Sets are now available.11
The core data set is the recommended minimal data set to be collected for all individuals with a new SCI during their initial inpatient period. It is recommended that these data be included as a descriptive table in most publications including spinal cord injured individuals.12 When specific medical issues are indicated in the core data set, it is then possible to follow-up with one or more specific data set(s) to provide more detailed information on those topics. Databases developed for most clinical trials will include only a subset of the detailed information available in the specific function data sets, however, it is recommended that the core data set be the minimal number of data elements, both for daily clinical practice and research studies addressing a particular topic.
Common Data Elements in SCI Research and Trials
A number of trials have been conducted testing novel therapeutic candidates, including neuroprotective drugs, various cell types, and rehabilitative strategies and devices.13,14 Most of these trials used motor and sensory testing as performed for the ASIA Impairment Scale (AIS) as a primary outcome, including upper and/or lower extremity motor scores, as appropriate for the test population. Other outcomes may include imaging, walking tests, impairment scales, and bladder function. Spasticity and pain may be reported as either a functional or a safety outcome (ie, reduction or exacerbation, respectively). In some trials, evoked potentials or other electrophysiological tests also were reported.
CDE Overview of the Development Process and Collaborative Efforts
The National Institute of Neurological Disorders and Stroke (NINDS), the National Institutes of Health (NIH), embarked on a Common Data Element (CDE) Project 5 years ago. The mission of the NINDS CDE Project is to develop data standards, both generic and disease-specific, for clinical research within the neurological community that will increase the efficiency of the research enterprise, improve data quality, and facilitate data sharing.
The NINDS CDE Team contacted the Executive Committee of the International SCI Standards and Data Sets committees (ECSCI) after it became aware of the work performed by the various SCI data set working groups. In subsequent discussions between the committee and the NINDS CDE Team, a decision was made to cooperate in developing variable names and database structures for the International SCI Data Sets (ie, the SCI CDEs).15 The 2 teams have thus far collaborated on 13 different data sets and the International Standards for Neurological Classification of SCI. The results of their work can be found on 2 Web sites:
NINDS CDE Web site (http://www.commondataelements.ninds.nih.gov/SCI.aspx)
ISCoS Web site (http://www.iscos.org.uk/page.php?content=20)
International SCI Core Data Set
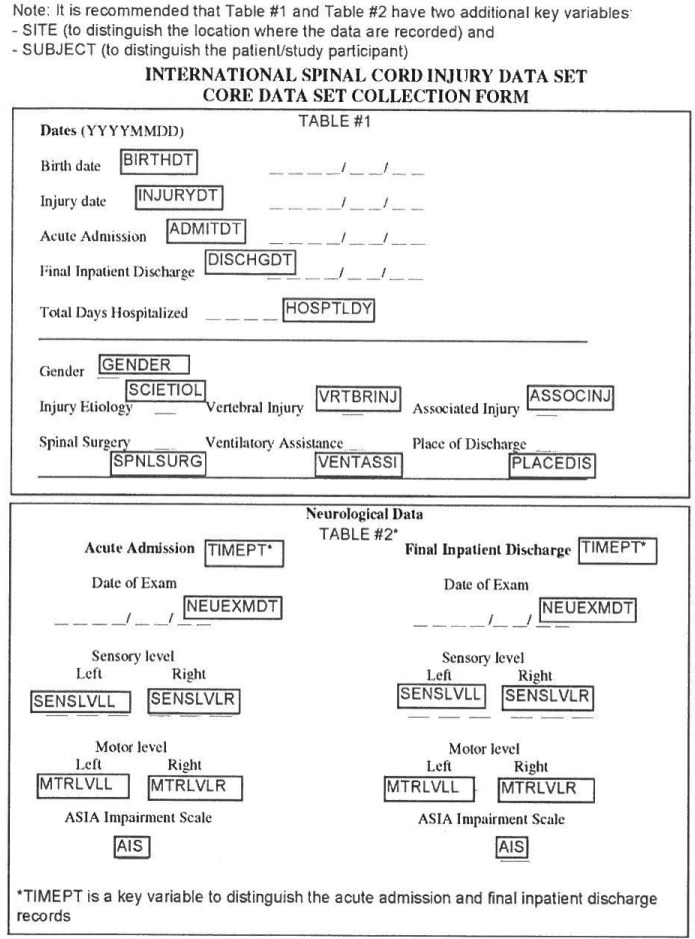
The International SCI Core Data Set variables can be captured on a 1-page data collection form.12 A detailed data collection syllabus was developed with clear unambiguous definitions of the included variables, which should have international applicability and have demonstrated validity/reliability in previous experience. The International SCI Core Data Set variables and the developed variable names are seen in Figure 1.15
Figure 1.
Data collection form for the International Spinal Cord Injury Core Data Set, including variable names in capital letters within boxes. Reprinted, with permission, from Biering-Sørensen F, et al. Incorporation of the International Spinal Cord Injury Data Set Elements into the National Institute of Neurological Disorders and Stroke Common Data Elements. Spinal Cord . 2011;49:60-64. Copyright © 2011 Nature Publishing Group.
The dates included make it possible to calculate age at injury, current age, and time postinjury, together with the calculation of age-adjusted (agestandardized) rates and outcomes.
The variable injury etiology is adapted from the International Classification of External Causes of Injury (ICECI) and shall be coded in priority as Sports, Assault, Transport, Fall, Other traumatic cause, and Nontraumatic spinal cord dysfunction.
The variables vertebral injury and associated injury are coded as yes/no. The associated injuries include the following: Glasgow Coma Scale < 13 at discharge, surgery for nonvertebral fracture, severe facial injury affecting sense organ, traumatic or surgical amputation of a limb, severe hemorrhage, and surgery for damage to internal organ.
The variable spinal surgery is likewise coded yes/no and includes laminectomy, neural canal restoration, reduction, spinal fusion, and internal fixation.
Neurological status at admission and discharge or enrollment and termination of study is provided according to the International Standards for Neurological Classification of SCI. 16
International SCI Cardiovascular Function Basic Data Set
The International SCI Cardiovascular Function Basic Data Set was created to standardize collection and reporting of a minimal amount of information on cardiovascular function in SCI patients, considering that cardiovascular abnormalities and disorders are well documented following SCI.9 In their work to develop variable names and a proposed database structure for this data set and others like it, the NINDS CDE Team and the ECSCI needed to decide whether to adopt a more horizontal (short and wide) data structure or a more vertical (tall and narrow) data structure. The teams considered the pros and cons of each approach and decided to go with a more horizontal data structure. The Cardiovascular Function Basic Data Set also illustrates 2 other aspects of the proposed database structure for the SCI CDEs: (1) The number of database tables proposed for a given data set is determined by whether groups of data elements can be collected at disparate time points; and (2) there is not necessarily a one-toone correspondence between the data collection form and the database structure.
Results of the International SCI Data Sets/CDE Effort
Collaboration between the NINDS CDE and the International SCI Data Sets projects has proven beneficial in many ways. Common wording in the data sets and recommended database structure are now available, enabling researchers from around the world to design databases in a way that enhances data sharing and multisite research. From the standpoint of clinical practice, the consistency of the data sets and the CDE formats may improve the ability to transfer critical medical information electronically from one center to another. The data sets and CDEs may also improve consistency in the standard parameters for carrying out various components of the physical examination. In addition, this collaboration has the potential to facilitate studies on topics that may be common to various diagnoses, such as quality of life or sexual function in SCI, brain injury, or other neurologic conditions.
Next Steps and Future Plans
The goals of the SCI CDE initiative are the same as those for the overall NINDS CDE Project, namely to increase the efficiency and effectiveness of clinical research studies and clinical treatment, increase data quality, facilitate data sharing, and help educate new clinical investigators. Pilot testing the SCI CDEs is an important step to ensure the SCI CDE effort achieves its goals. Feedback from pilot testers will enable the NINDS CDE Team and the ECSCI to improve the standard definitions and codes, variable names, and proposed database structures of the SCI CDEs. There are a variety of ways to submit feedback about the SCI CDEs including emailing the contact person for the particular International SCI Data Set and/or the NINDS CDE Team or filling out a feedback form on the NINDS CDE Web site (http://www.commondataelements.ninds.nih.gov/Feedback.aspx?page=sci).
Research or clinical teams can incorporate the SCI CDEs into their clinical studies and practice in a number of ways. A research or clinical team without their own data system can use the SCI CDEs “as is” to create a simple database to store data about their research participants or patients. A research or clinical team, without an existing database but whose institution already uses a particular database/statistical software, can use the SCI CDE standard definitions and codes and make some modifications to the variable names and/or data table structures depending on the requirements of their software. Finally, a research or clinical team that is already collecting data from SCI research participants or clinical patients can harmonize the data they are collecting with the SCI CDEs and map their database variables to the SCI CDE variables and data table structures.
The SCI CDE Project will continue to create variable names and database structures for each published International SCI Data Set. In addition, it is the intention to integrate existing International SCI Data Sets into a complete medical record for SCI individuals. This may require revisions to SCI CDEs, in particular the database structures and potentially variable names. There is also a wish to harmonize SCI CDEs with other CDEs developed through NINDS CDE Project where there is overlap, for example, traumatic brain injury and stroke CDEs.
The SCI CDEs can serve as building blocks for additional standards, including data sharing and aggregation tools. Such data sharing and aggregation standards may necessitate an adjustment in database structure to allow a more vertical approach and the addition of variables that describe research design, study population, and data quality procedures. Data sharing standards will make meta-analysis and sharing of existing SCI clinical research data and/or clinical data sets even easier.
Acknowledgments
The views expressed here are those of the authors and do not represent those of the National Institutes of Health (NIH), the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Disability and Rehabilitation Research (NIDRR), or the US Government.
References
- 1.Biering-Sørensen F, Charlifue S, DeVivo M, Noonan V, Post M, Stripling T, Wing P.International Spinal Cord Injury Data Sets. Spinal Cord. 2006;44(9): 530-534 [DOI] [PubMed] [Google Scholar]
- 2.Biering-Sørensen F, Craggs M, Kennelly M, Schick E, Wyndaele JJ.International Lower Urinary Tract Function Basic Spinal Cord Injury Data Set. Spinal Cord. 2008;46(5): 325-330 [DOI] [PubMed] [Google Scholar]
- 3.Biering-Sørensen F, Craggs M, Kennelly M, Schick E, Wyndaele JJ.International Urodynamic Basic Spinal Cord Injury Data Set. Spinal Cord. 2008;46(7): 513-516 [DOI] [PubMed] [Google Scholar]
- 4.Biering-Sørensen F, Craggs M, Kennelly M, Schick E, Wyndaele J-J.International Urinary Tract Imaging Basic Spinal Cord Injury Data Set. Spinal Cord. 2009;47: 379-383 [DOI] [PubMed] [Google Scholar]
- 5.Krogh K, Perkash I, Stiens SA, Biering-Sørensen F.International Bowel Function Basic Spinal Cord Injury Data Set. Spinal Cord. 2009;47(3): 230-234 [DOI] [PubMed] [Google Scholar]
- 6.Widerström-Noga E, Biering-Sørensen F, Bryce T, et al. The International Spinal Cord Injury Pain Basic Data Set. Spinal Cord. 2008;46: 818-823 [DOI] [PubMed] [Google Scholar]
- 7.Alexander MS, Biering-Sørensen F, Elliott S, Kreuter M, Sønksen J.International Spinal Cord Injury Male Sexual Function Basic Data Set. Spinal Cord. 2011July;49(7): 795-798 [DOI] [PubMed] [Google Scholar]
- 8.Alexander MS, Biering-Sørensen F, Elliott S, Kreuter M, Sønksen J.International Spinal Cord Injury Female Sexual and Reproductive Function Basic Data Set. Spinal Cord. 2011July;49(7): 787-790 [DOI] [PubMed] [Google Scholar]
- 9.Krassioukov A, Alexander MS, Karlsson AK, Donovan W, Mathias CJ, Biering-Sørensen F.International Spinal Cord Injury Cardiovascular Function Basic Data Set. Spinal Cord. 2010;48(8): 586-590 [DOI] [PubMed] [Google Scholar]
- 10.Bauman WA, Biering-Sørensen F, Krassioukov A. The International Spinal Cord Injury Endocrine and Metabolic Function Basic Data Set 2011;49(10): 1068-1072 Spinal Cord. [DOI] [PubMed] [Google Scholar]
- 11.Biering-Sørensen F, Alexander MS, Burns S, et al. Recommendations for translation and reliability testing of International Spinal Cord Injury Data Sets. Spinal Cord. 2011;49(3): 357-360 [DOI] [PubMed] [Google Scholar]
- 12.DeVivo M, Biering-Sørensen F, Charlifue S, Noonan V, Post M, Stripling T, Wing P.International Spinal Cord Injury Core Data Set. Spinal Cord. 2006;44(9): 535-540 [DOI] [PubMed] [Google Scholar]
- 13.Bauchet L, Lonjon N, Perrin F-E, Gilbert C, Privat A, Fattal C.Strategies for spinal cord repair after injury: a review of the literature and information. Ann Phys Rehabil Med. 2009;52: 330-351 [DOI] [PubMed] [Google Scholar]
- 14.Hawryluk GW, Rowland J, Kwon BK, Fehlings MG.Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25(5): E14. [DOI] [PubMed] [Google Scholar]
- 15.Biering-Sørensen F, Charlifue S, DeVivo MJ, Grinnon ST, Kleitman N, Lu Y, Odenkirchen J.Incorporation of the International Spinal Cord Injury Data Set Elements into the National Institute of Neurological Disorders and Stroke Common Data Elements. Spinal Cord. 2011;49: 60-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marino RJ, Barros T, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26 (suppl1): S50-S56 [DOI] [PubMed] [Google Scholar]