Abstract
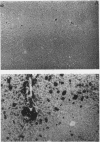
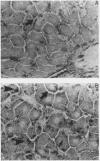
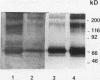
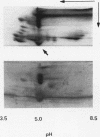
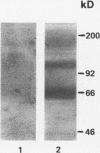
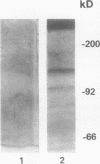
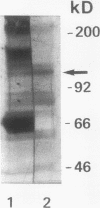
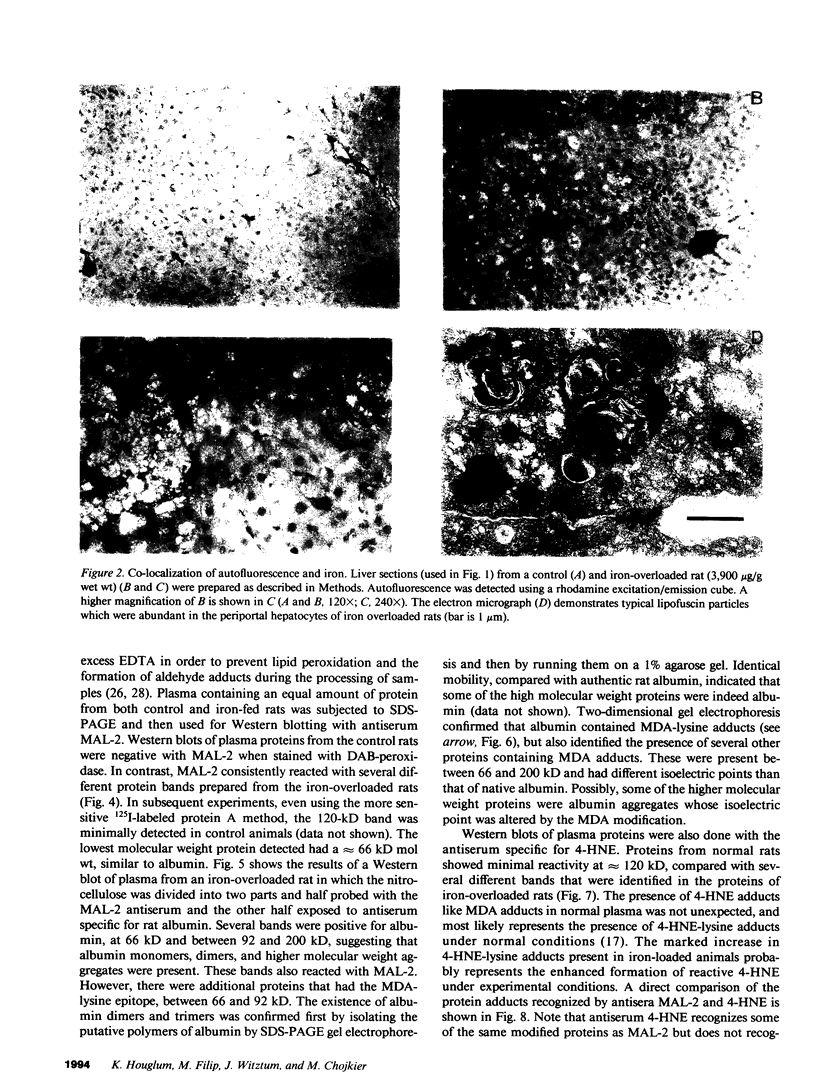
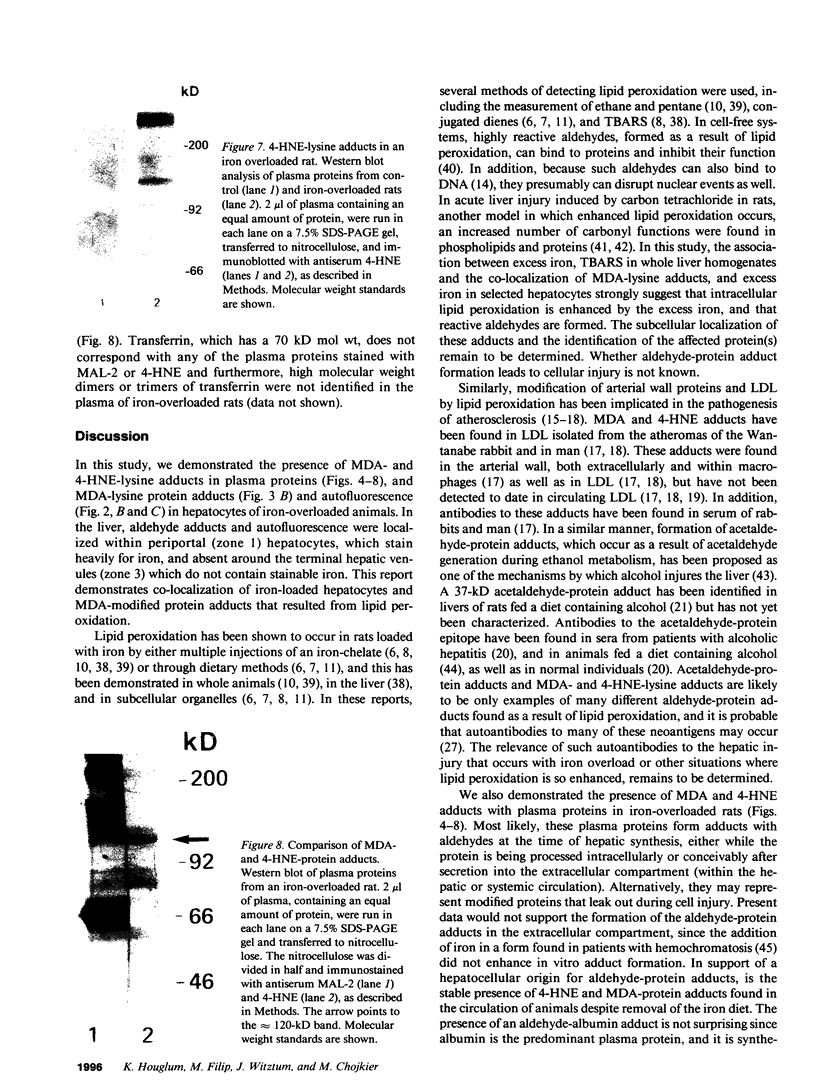
In hepatic iron overload, iron-catalyzed lipid peroxidation has been implicated in the mechanisms of hepatocellular injury. Lipid peroxidation may produce reactive aldehydes such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), which may form aldehyde-protein adducts. We investigated whether lipid peroxidation occurred in rats fed a diet containing 3% carbonyl iron for 5-13 wk, and if this resulted in the formation of MDA- and 4-HNE- protein adducts. Chronic iron feeding resulted in hepatic iron overload (greater than 10-fold) and concomitantly induced a 2-fold increase in hepatic lipid peroxidation. Using an antiserum specific for MDA-lysine protein adducts, we demonstrated by immunohistochemistry the presence of aldehyde-protein adducts in the cytosol of periportal hepatocytes, which co-localized with iron. In addition, MDA- and 4-HNE-lysine adducts were found in plasma proteins of animals with iron overload. Only MDA adducts were detected in albumin, while other plasma proteins including a approximately 120-kD protein had both MDA and 4-HNE adducts. In this animal model of hepatic iron overload, injury occurs primarily in periportal hepatocytes, where MDA-lysine protein adducts and excess iron co-localized.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon B. R., Healey J. F., Brittenham G. M., Park C. H., Nunnari J., Tavill A. S., Bonkovsky H. L. Hepatic microsomal function in rats with chronic dietary iron overload. Gastroenterology. 1986 Jun;90(6):1844–1853. doi: 10.1016/0016-5085(86)90251-9. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Park C. H., Brittenham G. M., O'Neill R., Tavill A. S. Hepatic mitochondrial oxidative metabolism in rats with chronic dietary iron overload. Hepatology. 1985 Sep-Oct;5(5):789–797. doi: 10.1002/hep.1840050514. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Tavill A. S., Brittenham G. M., Park C. H., Recknagel R. O. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest. 1983 Mar;71(3):429–439. doi: 10.1172/JCI110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A., Esterbauer H., Ferrali M., Fulceri R., Comporti M. Evidence for aldehydes bound to liver microsomal protein following CCl4 or BrCCl3 poisoning. Biochim Biophys Acta. 1982 May 13;711(2):345–356. doi: 10.1016/0005-2760(82)90044-3. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Fulceri R., Ferrali M., Ciccoli L., Esterbauer H., Comporti M. Detection of carbonyl functions in phospholipids of liver microsomes in CCl4- and BrCCl3-poisoned rats. Biochim Biophys Acta. 1982 Sep 14;712(3):628–638. doi: 10.1016/0005-2760(82)90292-2. [DOI] [PubMed] [Google Scholar]
- Bomford A., Williams R. Long term results of venesection therapy in idiopathic haemochromatosis. Q J Med. 1976 Oct;45(180):611–623. [PubMed] [Google Scholar]
- Bonkowsky H. L., Healey J. F., Sinclair P. R., Sinclair J. F., Pomeroy J. S. Iron and the liver. Acute and long-term effects of iron-loading on hepatic haem metabolism. Biochem J. 1981 Apr 15;196(1):57–64. doi: 10.1042/bj1960057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. A., Chojkier M. Acetaldehyde increases collagen gene transcription in cultured human fibroblasts. J Biol Chem. 1987 Dec 25;262(36):17690–17695. [PubMed] [Google Scholar]
- Chojkier M., Houglum K., Solis-Herruzo J., Brenner D. A. Stimulation of collagen gene expression by ascorbic acid in cultured human fibroblasts. A role for lipid peroxidation? J Biol Chem. 1989 Oct 5;264(28):16957–16962. [PubMed] [Google Scholar]
- Dillard C. J., Downey J. E., Tappel A. L. Effect of antioxidants on lipid peroxidation in iron-loaded rats. Lipids. 1984 Feb;19(2):127–133. doi: 10.1007/BF02534503. [DOI] [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Fluorescent damage products of lipid peroxidation. Methods Enzymol. 1984;105:337–341. doi: 10.1016/s0076-6879(84)05044-8. [DOI] [PubMed] [Google Scholar]
- Dougherty J. J., Croft W. A., Hoekstra W. G. Effects of ferrous chloride and iron dextran on lipid peroxidation in vivo in vitamin E and selenium adequate and deficient rats. J Nutr. 1981 Oct;111(10):1784–1796. doi: 10.1093/jn/111.10.1784. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Jürgens G., Quehenberger O., Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987 May;28(5):495–509. [PubMed] [Google Scholar]
- GOLBERG L., MARTIN L. E., BATCHELOR A. Biochemical changes in the tissues of animals injected with iron. 3. Lipid peroxidation. Biochem J. 1962 May;83:291–298. doi: 10.1042/bj0830291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootveld M., Bell J. D., Halliwell B., Aruoma O. I., Bomford A., Sadler P. J. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989 Mar 15;264(8):4417–4422. [PubMed] [Google Scholar]
- Gupta R. C., Earley K., Becker F. F. Analysis of DNA adducts in putative premalignant hepatic nodules and nontarget tissues of rats during 2-acetylaminofluorene carcinogenesis. Cancer Res. 1988 Sep 15;48(18):5270–5274. [PubMed] [Google Scholar]
- Gutteridge J. M., Quinlan G. J. Malondialdehyde formation from lipid peroxides in the thiobarbituric acid test: the role of lipid radicals, iron salts, and metal chelators. J Appl Biochem. 1983 Aug-Oct;5(4-5):293–299. [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M. The role of altered lipoproteins in the pathogenesis of atherosclerosis. Am Heart J. 1987 Feb;113(2 Pt 2):573–577. doi: 10.1016/0002-8703(87)90635-1. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Horak E., Hohnadel D. C., Sunderman F. W., Jr Modified method for analysis of serum iron. Ann Clin Lab Sci. 1975 Jul-Aug;5(4):303–307. [PubMed] [Google Scholar]
- Israel Y., Hurwitz E., Niemelä O., Arnon R. Monoclonal and polyclonal antibodies against acetaldehyde-containing epitopes in acetaldehyde-protein adducts. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7923–7927. doi: 10.1073/pnas.83.20.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin R. C., Smith R. S., Lumeng L. Detection of a protein-acetaldehyde adduct in the liver of rats fed alcohol chronically. J Clin Invest. 1988 Feb;81(2):615–619. doi: 10.1172/JCI113362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini A., Trenti T., Ventura E., Ceccarelli-Stanzani D., Muscatello U. Functional efficiency of mitochondrial membrane of rats with hepatic chronic iron overload. Biochem Biophys Res Commun. 1984 Oct 30;124(2):462–469. doi: 10.1016/0006-291x(84)91576-6. [DOI] [PubMed] [Google Scholar]
- Miyagishi T., Takahata N., Iizuka R. Electron microscopic studies on the lipo-pigments in the cerebral cortex nerve cells of senile and vitamin E deficient rats. Acta Neuropathol. 1967 Aug 2;9(1):7–17. doi: 10.1007/BF00688154. [DOI] [PubMed] [Google Scholar]
- Niemelä O., Klajner F., Orrego H., Vidins E., Blendis L., Israel Y. Antibodies against acetaldehyde-modified protein epitopes in human alcoholics. Hepatology. 1987 Nov-Dec;7(6):1210–1214. doi: 10.1002/hep.1840070607. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W., Ylä-Herttuala S., Rosenfeld M. E., Butler S. W., Socher S. A., Parthasarathy S., Curtiss L. K., Witztum J. L. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990 May-Jun;10(3):325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- Park C. H., Bacon B. R., Brittenham G. M., Tavill A. S. Pathology of dietary carbonyl iron overload in rats. Lab Invest. 1987 Nov;57(5):555–563. [PubMed] [Google Scholar]
- Powell L. W., Bassett M. L., Halliday J. W. Hemochromatosis: 1980 update. Gastroenterology. 1980 Feb;78(2):374–381. [PubMed] [Google Scholar]
- Risdon R. A., Barry M., Flynn D. M. Transfusional iron overload: the relationship between tissue iron concentration and hepatic fibrosis in thalassaemia. J Pathol. 1975 Jun;116(2):83–95. doi: 10.1002/path.1711160204. [DOI] [PubMed] [Google Scholar]
- Shedlofsky S. I., Bonkowsky H. L., Sinclair P. R., Sinclair J. F., Bement W. J., Pomeroy J. S. Iron loading of cultured hepatocytes. Effect of iron on 5-aminolaevulinate synthase is independent of lipid peroxidation. Biochem J. 1983 May 15;212(2):321–330. doi: 10.1042/bj2120321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodum R. S., Chung F. L. 1,N2-ethenodeoxyguanosine as a potential marker for DNA adduct formation by trans-4-hydroxy-2-nonenal. Cancer Res. 1988 Jan 15;48(2):320–323. [PubMed] [Google Scholar]
- Sorrell M. F., Tuma D. J. Hypothesis: alcoholic liver injury and the covalent binding of acetaldehyde. Alcohol Clin Exp Res. 1985 Jul-Aug;9(4):306–309. doi: 10.1111/j.1530-0277.1985.tb05549.x. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Fisher M., Witztum J. L., Curtiss L. K. Immunogenicity of homologous low density lipoprotein after methylation, ethylation, acetylation, or carbamylation: generation of antibodies specific for derivatized lysine. J Lipid Res. 1984 Oct;25(10):1109–1116. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir M. P., Gibson J. F., Peters T. J. Biochemical studies on the isolation and characterization of human spleen haemosiderin. Biochem J. 1984 Oct 1;223(1):31–38. doi: 10.1042/bj2230031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]