Abstract
Background
The flank is commonly used for primary xenografts in mice, but it is rare for these tumors to metastasize. Tail vein injection creates a pattern of metastases, but is artificial. We hypothesized that the liver is a convenient alternative xenograft site and that metastases would gradually proceed spontaneously.
Materials and Methods
Using 15 NOD.CB17-Prkdcscid/NcrCrl (NOD/SCID) mice, 10,000 A549 cells were xenografted into the liver while a second group of five mice were xenografted in the flank with 100,000 A549 cells as a control. Mice were euthanized and grossly dissected at 7 wk. A third group of seven mice received liver xenografts with A549 and a mouse each week was euthanized for 7 wk and evaluated. The liver, lung, and spleen were examined histologically.
Results
At 7 wk, 15/15 liver xenografted mice had gross primary tumor in the liver. Histologic review confirmed multiple microscopic foci of metastatic disease in all mice (15/15) throughout the lungs, mediastinal nodes, and spleen. The control group had primary tumor in the flank (4/5), but none had histologic evidence of metastases. Serially euthanized liver xenografted mice revealed evidence of a gradual spontaneous metastatic model system with the first histologic findings of micrometastases appearing in the lungs by wk 5, which became wide spread by wk 7. Splenic and mediastinal lymph node metastases developed in wk 6 and 7.
Conclusions
Liver xenografting of A549 cells into NOD/SCID mice is a reliable way of developing widespread micrometastases. This model allows the study of a gradually developing solid tumor with subsequent metastatic spread.
Keywords: metastases, xenograft, NOD/SCID, murine, A549, NSCLC, metastatic model
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths not only in the United States but also the world [1, 2]. There were approximately 159,000 deaths in 2007 caused by lung cancer in the United States alone, exceeding the total deaths recorded for breast, prostate, and colon cancer combined [2]. Despite significant advances in medical technology for early detection and pharmacologic therapies for other malignancies, there has been little impact on survival among those diagnosed with lung cancer, especially those who present with metastatic disease [3]. Preclinical mouse models have been recognized as an important way of studying a variety of human malignancies [4–6].
Xenograft models of human non-small-cell lung cancer (NSCLC) cell lines, for example, are usually injected in the subcutaneous tissue of the mouse, often in the flank. Although the flank, as a primary xenograft site, is typically used for the convenience of tumor measurement, rarely do even the most aggressive cell lines develop spontaneous metastases from this site [7]. Tail vein injection has been used as a way of artificially creating a pattern of metastases by spread of the injected cells through the systemic circulation [8]. While this method is appropriate for studying hematologic malignancies, it does not accurately reflect the spontaneous metastases that occur with solid tumors over time. If a human cancer cell line possesses the ability to metastasize spontaneously from a primary tumor [9], knowledge of the timing and extent of metastasis could be utilized as a target for pharmacologic intervention.
In order to study the effect of various medical and surgical treatment strategies on metastasis in a preclinical setting, we developed a mouse model of spontaneous metastases in NSCLC. Using mice with an impaired immune system, NOD.CB17-Prkdcscid/NcrCrl (NOD/SCID), we were able to xenograft a human cancer cell line into the liver of these mice in order to optimize the likelihood of spontaneous metastases compared with the typical lessvascular subcutaneous tissue in the flank. Our hypothesis was that the rich vascular bed of the liver would provide a readily available access for invasive cells to reach the systemic circulation and subsequently metastasize. We speculated that the lungs would be the most likely destination for metastases from the liver parenchyma since it is a direct venous route for metastatic cells via the hepatic veins, the inferior vena cava, the heart and the pulmonary artery before these cells become entrapped in the pulmonary capillary bed. This is in direct contrast to cells xenografted in the flank where there is less access to the systemic circulation. The result is that the flank xenograft model is unreliable for studying the impact of therapy on metastatic disease. With our present model, we are able not only to reproduce reliably wide-spread metastatic disease, but also can predict the timing and location of these spontaneous metastatic deposits before they occur.
While complete surgical resection of any solid tumor before it has spread outside the area of resection is the ultimate goal, this is not often possible for many individuals because of the aggressive nature of many types of cancer. Despite technological advances in screening for the detection of NSCLC at an earlier stage [10], there exists no clinically approved method of detecting micrometastases. Many patients who are surgical candidates will undergo surgical resection of their lung cancer and ultimately recur and die of the disease that was left behind and beyond detection. The purpose of this study was to develop a reliable spontaneous metastatic murine model with human NSCLC cells, and to characterize the model as to the timing and location of the spontaneously occurring metastases.
MATERIALS AND METHODS
Cell Culture
The human adenocarcinoma cell line A549 was obtained from the American Type Culture Collection, (ATCC) (Manassas, VA). These cells were grown in adherent monolayers in a 75 cm2 flask (BD Falcon, Franklin Lakes, NJ, USA) and maintained in ATCC-formulated F-12K Medium mixed with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) at 37°C and atmospheric conditions of 5% CO2. This cell line was initiated in 1972 by Geared, et al. through explant culture of lung adenocarcinoma tissue, obtained from a 58-yr-old Caucasian male [11].
The cells were harvested from the flasks using trypsin (Sigma-Aldrich, St. Louis, MO, USA) after reaching near confluence. They were diluted with the growing media, counted and re-suspended in Hanks balanced salt solution (HBSS) (Invitrogen) after removal of the trypsin and growing media to a final concentration of 10,000 A549 cells/10 μL of HBSS.
Murine Xenografts
Care of all mice was under the supervision of the Director of Laboratory Animal Medicine who oversees the veterinary medical care of animals at the Johns Hopkins University. The mouse experiment protocol was approved by the Institutional Animal Care and Use Committee whose responsibilities are federally mandated by the Animal Welfare Act regulations and the Public Health Service Policy.
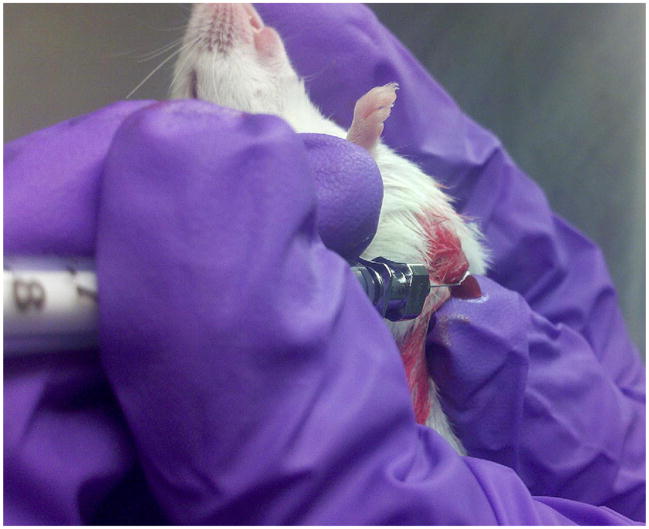
Group 1 consisted of 15 NOD/SCID mice anesthetized with tribromoethanol (Avertin, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) in which we xenografted 10,000 A549 cells suspended in 10 μL of HBSS into the liver with a 32 gauge needle. The liver was injected under direct vision by making a 1 cm subcostal incision just to the left of the midline through which the left lobe of the liver was partially eviscerated (Fig. 1).
FIG. 1.
Technique for liver xenograft using a 32 gauge needle to inject 10,000 A549 cells/10 μL of HBSS.
Confirmation of a successful xenograft was observed by witnessing a 1–2 s blanching of the liver parenchyma at time of injection. A sterile cotton tipped swab was held at the site for 10–15 s immediately after extracting the needle from the liver for hemostasis. The liver was then reduced back into the abdomen and the fascia and skin were both closed with 4-0 Vicryl absorbable suture (Ethicon Inc., Somerville, NJ, USA). Mice were monitored weekly and were euthanized after 7 wk from the time of the liver xenograft because of labored breathing and the development of moderate to severe ascites.
As a control, Group 2 was established which consisted of 5 NOD/SCID mice xenografted in the subcutaneous tissue of the right flank with 100,000 A549 cells suspended in 100 μL of HBSS. Tumors were measured weekly and mice in Group 2 were euthanized after tumors reached 2 cm in any dimension, which occurred at 7 wk.
Immediately after euthanization of both groups of mice at 7 wk, the mice were perfused with formalin, and grossly dissected. The liver, lung, mediastinal lymph nodes, and spleen were processed, embedded in paraffin and sectioned every 10 μ throughout the entirety of all organs and stained with hematoxylin and eosin(H&E)every tenth slide to evaluate for histologic evidence of metastatic disease. A total of 1600 H&E slides (80/mouse) were carefully reviewed (JH) and significant positive and negative findings were confirmed by an animal pathologist (CB).
Group 3 consisted of 7 NOD/SCID mice that were also xenografted in the liver with 10,000 A549 cells, but this time, one mouse was euthanized each week for 7 wk and evaluated by the same protocol as Group 1.
RESULTS
In Group 1 at 7 wk, all 15 liver xenografted mice had gross primary tumor in the liver as well as grossly evident splenic (10/15) and mediastinal metastases (5/15). All 15 mice had ascites and 5/15 had gross evidence of carcinomatosis at wk 7. There were no gross pulmonary metastases observed (0/15). Histologic review, however, confirmed multiple microscopic foci of metastatic disease in all mice (15/15) throughout the lungs bilaterally, the mediastinal lymph nodes and spleen.
The control, Group 2, had primary tumor development in 4/5 mice with the maximum size of each tumor reaching or exceeding 2 cm at 7 wk. There was no evidence of any gross or histologic metastases to the liver, lung, mediastinal lymph nodes, or spleen in any of the five control mice. There was evidence of nodal metastases to the axilla on the ipsilateral side of the tumor in 1/5 mice.
The serially euthanized mice of Group 3 revealed histologic evidence of a gradual spontaneous metastatic model system. Histologically evident primary tumor was apparent by wk 1 (Fig. 2) with gross primary tumor development in the liver by wk 2. Gross and histologic progression of primary tumor size was seen in wk 3 and 4 (Fig. 3) with no apparent evidence of microscopic distal metastases (Fig. 4). By wk 5, the first histologic findings of micrometastases appeared in the lungs (Fig. 5), which progressed further in wk 6 and became widespread in all the pulmonary parenchyma by wk 7 (Fig. 6). Ascites did not develop until wk 6. Splenic and mediastinal lymph node involvement were observed histologically in both wk 6 and 7 mice. None of the mice in Group 3 developed carcinomatosis.
FIG. 2.
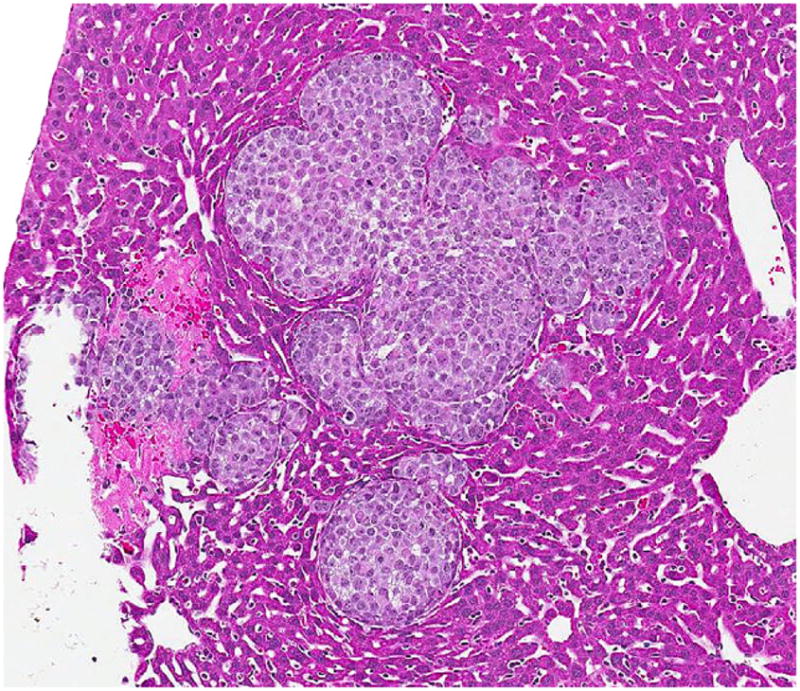
Microscopic appearance (×350) of the primary A549 liver tumor on H&E slide, 1 wk from the time of injection with 10,000 cells.
FIG. 3.
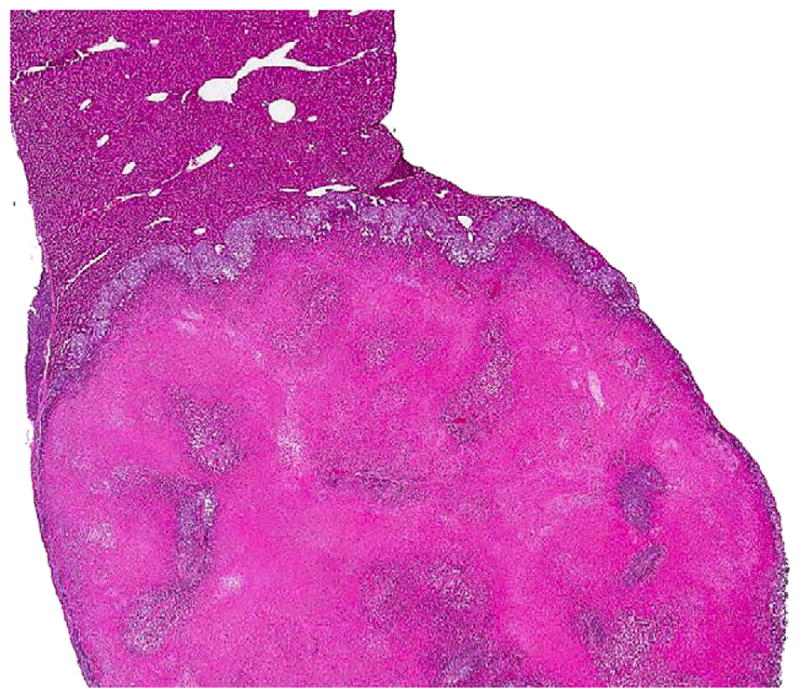
Microscopic appearance (×50) of the primary A549 liver tumor on H&E slide, 3 wk from the time of injection with 10,000 cells.
FIG. 4.
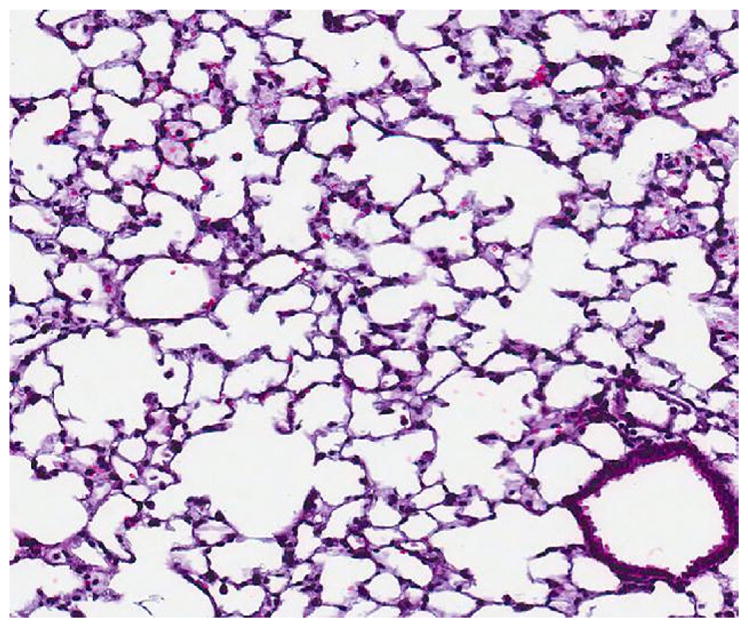
Microscopic appearance (×600) of normal lungs (as seen in wk 1 through 4 and in all control mice) on H&E slide, 4 wk from the time of liver injection with 10,000 A549 cells.
FIG. 5.
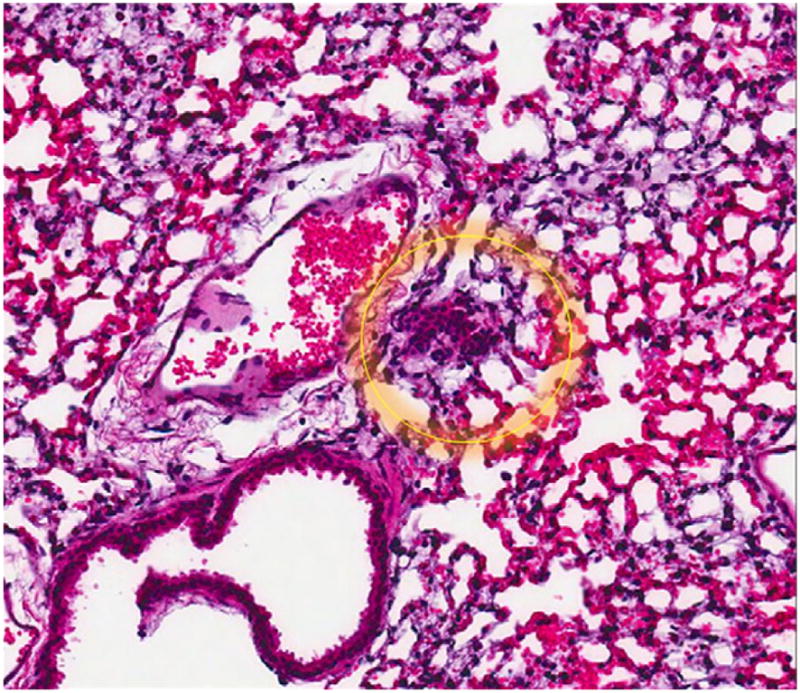
Microscopic appearance (×600) showing first appearance of lung micrometastases on H&E slide, 5 wk from the time of liver injection with 10,000 A549 cells.
FIG. 6.
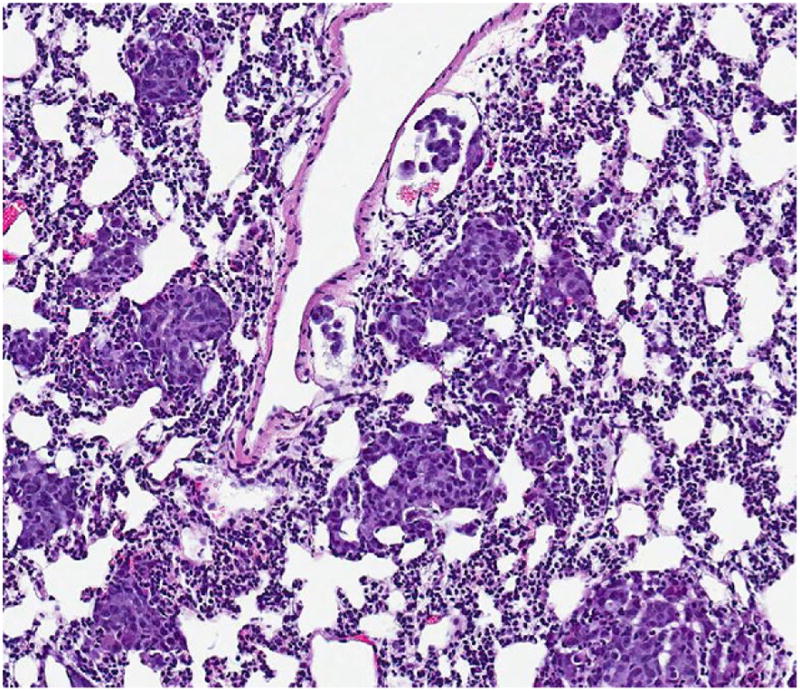
Microscopic appearance (×00) showing development of widespread lung micrometastases (seen in the wk #7 mouse and 15/15 Group 1 mice) on H&E slide, 7 wk from the time of liver injection with 10,000 A549 cells.
DISCUSSION
Liver xenografting of the human lung adenocarcinoma cell line A549 into NOD/SCID mice is a reliable way of developing widespread micrometastases to the lungs, mediastinal lymph nodes, and spleen over a period of 7 wk. As seen with cancer cell lines, which are injected into the spleen that subsequently metastasize to the liver through the portal vein due to anatomical proximity [12], our rationale for injecting lung cancer cell lines into the liver was that the probable target organ of metastases from the liver would be the lungs. This was based on mouse anatomy since the lungs are the first major organs reached after the cells enter the systemic circulation, and the pulmonary capillary bed would be a likely soil for such metastatic cells. Patterns of metastasis have also been established for various malignancies by molecular targets, which influence cancer cell tropism for specific organs [13, 14]. Our A549 cells, being derived from explanted lung carcinomatous tissue, could have a molecular predilection for the pulmonary parenchyma.
This model is clinically relevant since, unlike tail vein injection, it allows the study of the biology and treatment of a gradually developing primary tumor with subsequent metastatic spread. This model also opens the opportunity for resection of the primary tumor from the liver as a surgically relevant preclinical model. Post-resection, the model could then even be used to study the efficacy of subsequent, adjuvant treatment of micrometastases using in-vivo chemotherapy.
While this mouse model has proven to be reliable for the development of spontaneous metastases using the human adenocarcinoma cell line A549 in NOD/SCID mice, it may not work with some less aggressive cell lines that lack metastatic potential or in other mice with a normal immune system. Since the mice used in this model have a compromised immune system, it does not allow the study of interaction of the host immune system to the tumor, making this model of little benefit for studying some kinds of immunotherapy.
Although carcinomatosis was present in 5/15 mice at 7 wk, there was no carcinomatosis seen in any of the mice in Group 3, which were serially euthanized each week, suggesting that the carcinomatosis seen in the 5/15 mice of the first group was not a result of tumor spillage, but progression of disease from the overwhelming tumor burden by wk 7. Finally, A549 is known to grow in aggregate sizes ranging from 100 to 400μ, but since the actual size of a single A549 cell is 10–15 μ, it is possible that some cells could have been missed in the screening process, since H&E slides were screened every 100 μ. Further confirmation of the timeline of metastases could be ascertained by more frequent screening of H&E slides at a greater cost of both time and capital than expended for this experiment.
This model is simple to perform and can be, in theory, applied to the human or murine cancer cell line of any solid tumor that has the potential to develop spontaneous metastases in NOD/SCID mice. By choosing the liver as our primary xenograft site, we allow the injected cancer cells the best opportunity to form a primary tumor with later widespread, spontaneous metastases.
Acknowledgments
CMH and MVB were supported in part by the Commonwealth Funds. JEH is supported by a Johns Hopkins University NIH T32 Grant entitled, “Clinical and Laboratory Research Training in Surgical Oncology” (5T32CA126607-03).
Footnotes
Presented as a Poster at the 102nd Annual Meeting of the American Association for Cancer Research, Orlando, Fl. April 2–6, 2011 and as an oral presentation at the 21st Annual Meeting of the Society of Black Academic Surgeons, Boston, MA, April 28–30, 2011.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet On-col. 2001;2:533. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2010. [Accessed April 10, 2011]. Available at: http://www.cdc.gov/uscs. [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuveson DA, Zhu L, Gopinathan A, et al. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 2006;66:242. doi: 10.1158/0008-5472.CAN-05-2305. [DOI] [PubMed] [Google Scholar]
- 5.Hruban RH, Rustgi AK, Brentnall TA, et al. Pancreatic cancer in mice and man: The Penn workshop, 2005. Cancer Res. 2005;66:14. doi: 10.1158/0008-5472.CAN-05-3914. [DOI] [PubMed] [Google Scholar]
- 6.Nikitin AY, Alcaraz A, Anver MR, et al. Classification of proliferative pulmonary lesions of the mouse: Recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64:2307. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]
- 7.Doré JF, Bailly M, Bertrand S. Metastases of human tumors in experimental animals. Anticancer Res. 1987;7:997. [PubMed] [Google Scholar]
- 8.Dingemans KP. Behavior of intravenously injected malignant lymphoma cells. A morphologic study. J Natl Cancer Inst. 1973;51:1883. doi: 10.1093/jnci/51.6.1883. [DOI] [PubMed] [Google Scholar]
- 9.Ramaswamy S, Ross KN, Lander ES, et al. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49. doi: 10.1038/ng1060. Epub 2002 Dec 9. [DOI] [PubMed] [Google Scholar]
- 10.Patz EF, Jr, Caporaso NE, Dubinett SM, et al. National Lung Cancer Screening Trial American College of Radiology Imaging Network Specimen Biorepository originating from the Contemporary Screening for the Detection of Lung Cancer Trial (NLST, ACRIN 6654): Design, intent, and availability of specimens for validation of lung cancer biomarkers. J Thorac Oncol. 2010;5:1502. doi: 10.1097/JTO.0b013e3181f1c634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giard DJ, Aaronson SA, Todaro GJ, et al. in vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 12.Bruns CJ, Liu W, Davis DW, et al. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89:488. [PubMed] [Google Scholar]
- 13.Andy J, Minn Yibin Kang, Serganova Inna, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115:44. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, McRoberts KS, Theodorescu D. The role of PTEN in prostate cancer cell tropism to the bone micro-environment. Carcinogenesis. 2007;28:1393. doi: 10.1093/carcin/bgm050. Epub 2007 Mar 7. [DOI] [PubMed] [Google Scholar]