Abstract
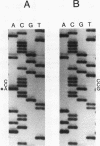
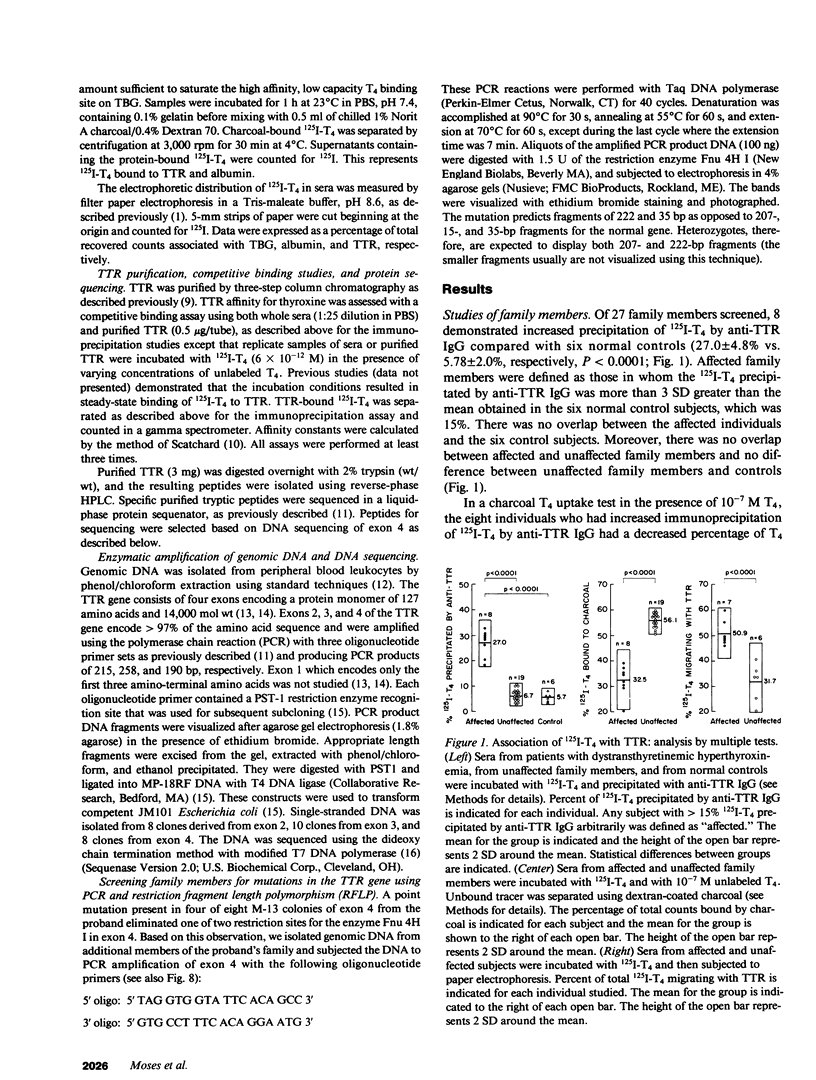
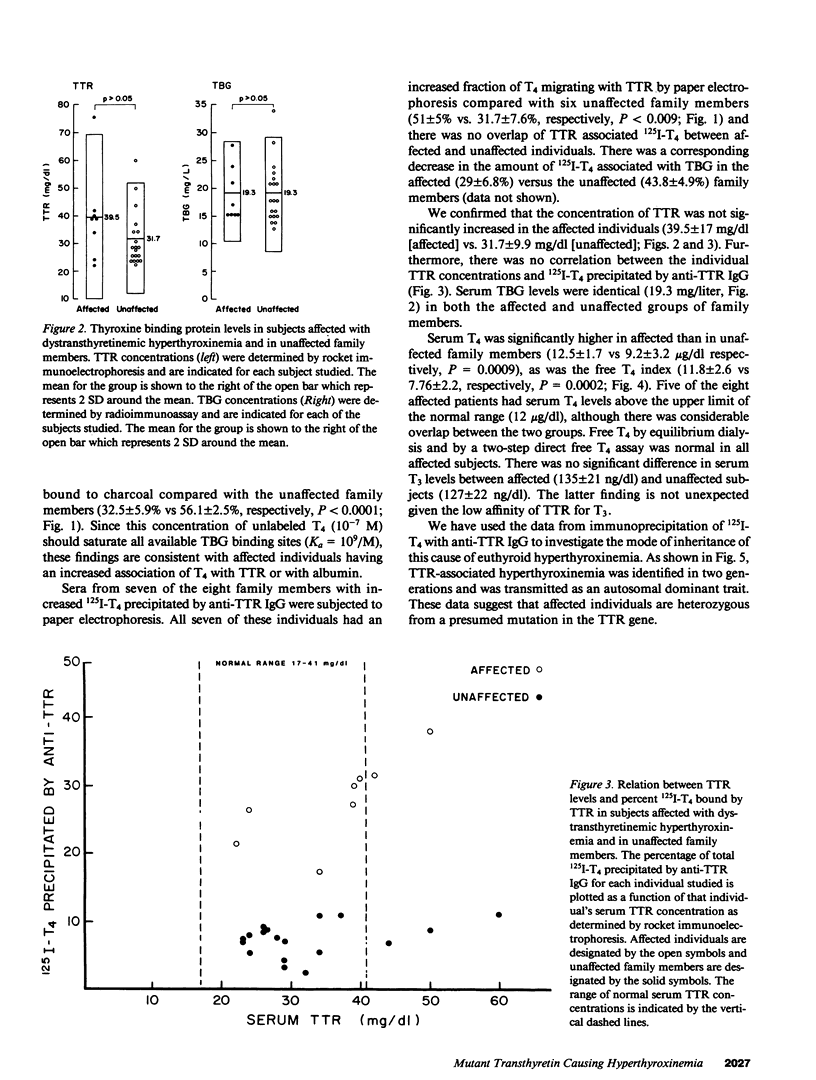
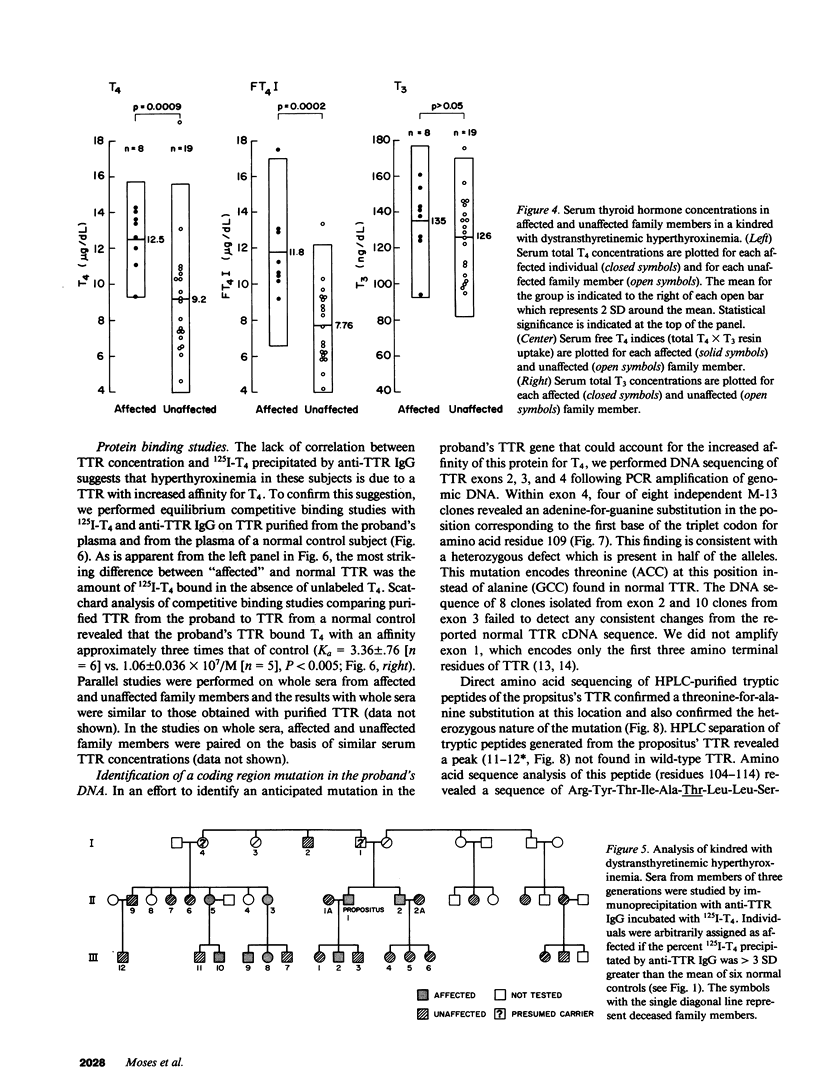
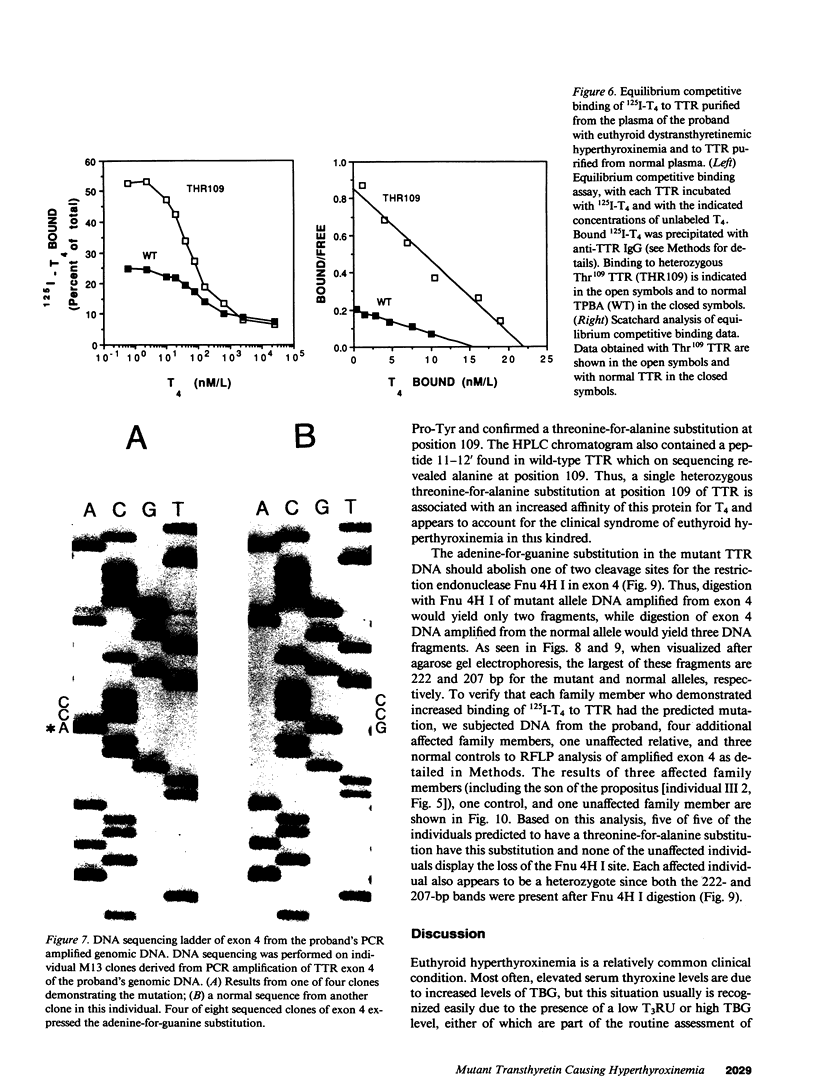
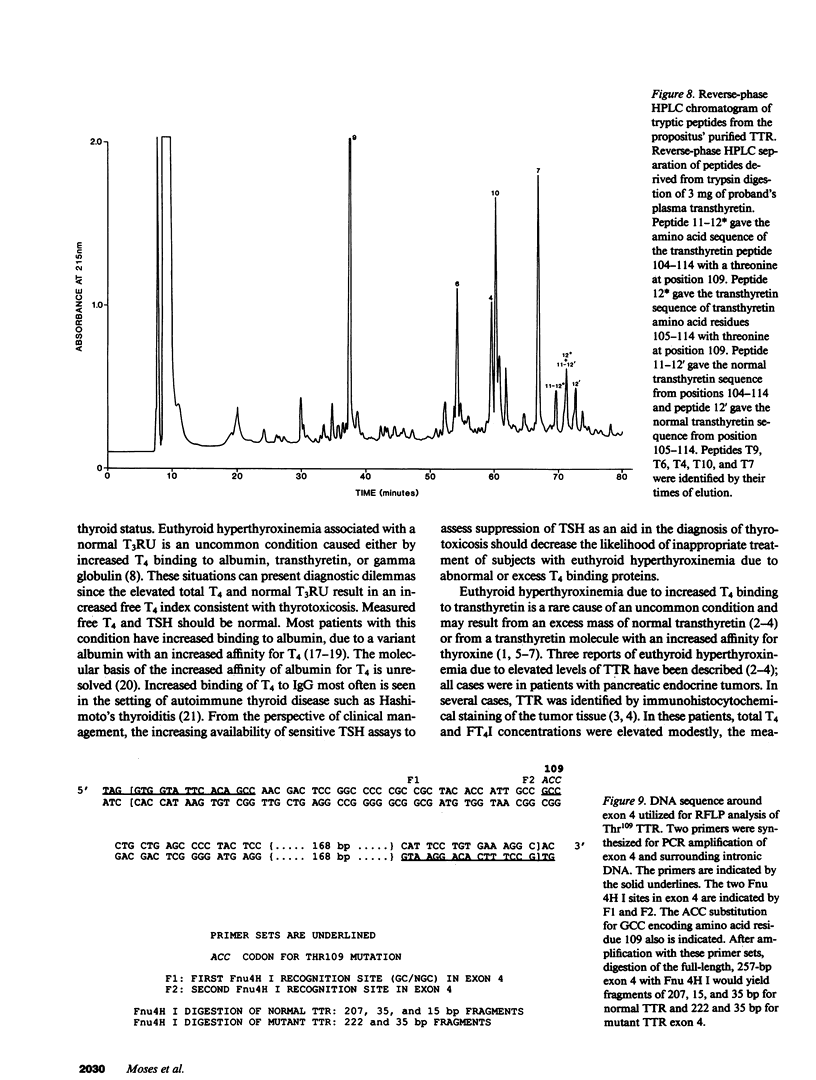
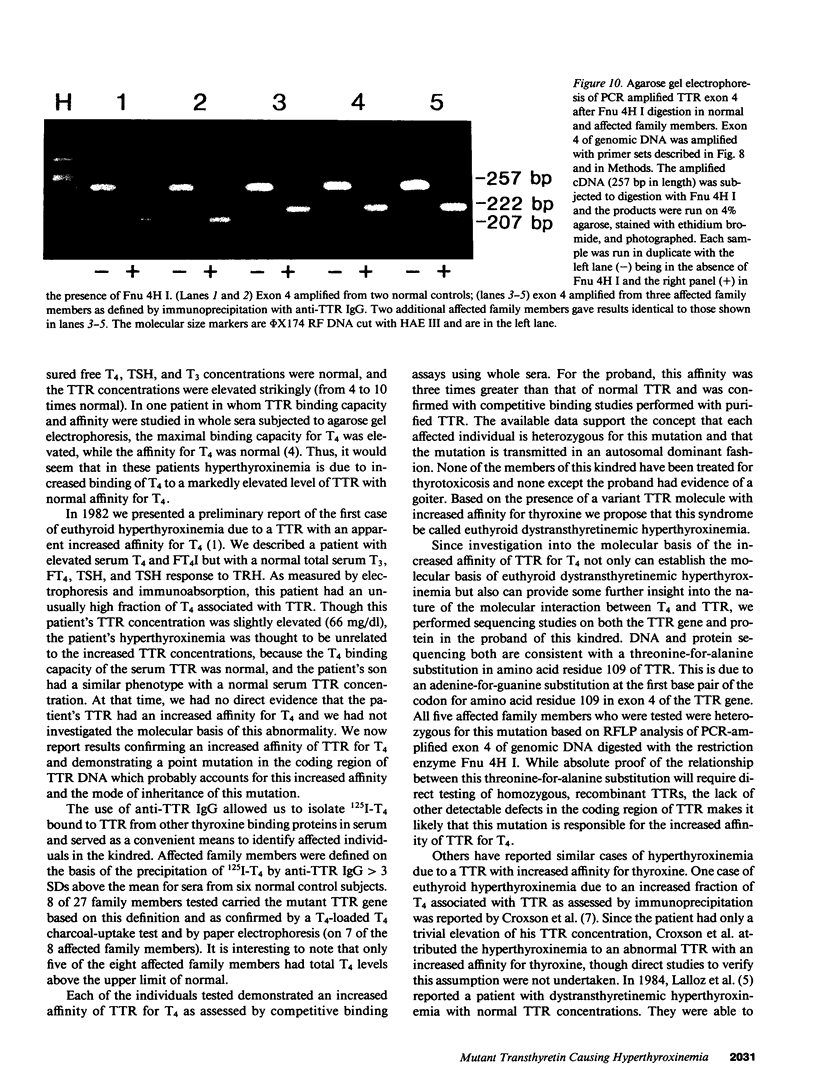
In a family expressing euthyroid hyperthyroxinemia, an increased association of plasma thyroxine (T4) with transthyretin (TTR) is transmitted by autosomal dominant inheritance and is secondary to a mutant TTR molecule with increased affinity for T4. Eight individuals spanning three generations exhibited the abnormality. Although five of eight individuals had elevated total T4 concentrations, all affected individuals were clinically euthyroid and all had normal free T4 levels. Purified TTR from the propositus had an affinity for 125I-T4 three times that of control TTR. Exons 2, 3, and 4 (representing greater than 97% of the coding sequence) of the TTR gene of DNA prepared from the propositus' peripheral blood leukocytes were amplified using the polymerase chain reaction (PCR) and were sequenced after subcloning. Exons 2 and 3 were indistinguishable from normal. In 50% of clones amplified from exon 4, a substitution of adenine (ACC) for guanine (GCC) in codon 109 resulted in the replacement of threonine-for-alanine, a mutation confirmed by amino acid sequencing of tryptic peptides derived from purified plasma TTR. The adenine-for-guanine substitution abolishes one of two Fnu 4H I restriction sites in exon 4. PCR amplification of exon 4 of TTR and restriction digestion with Fnu 4H I confirmed that five affected family members with increased binding of 125I-T4 to TTR are heterozygous for the threonine 109 substitution that increases the affinity of this abnormal TTR for T4.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake C. C., Geisow M. J., Oatley S. J., Rérat B., Rérat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol. 1978 May 25;121(3):339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Oatley S. J. Protein-DNA and protein-hormone interactions in prealbumin: a model of the thyroid hormone nuclear receptor? Nature. 1977 Jul 14;268(5616):115–120. doi: 10.1038/268115a0. [DOI] [PubMed] [Google Scholar]
- Croxson M. S., Palmer B. N., Holdaway I. M., Frengley P. A., Evans M. C. Detection of familial dysalbuminaemic hyperthyroxinaemia. Br Med J (Clin Res Ed) 1985 Apr 13;290(6475):1099–1102. doi: 10.1136/bmj.290.6475.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Polymorphism of human plasma thyroxine binding prealbumin. Biochem Biophys Res Commun. 1983 Jul 29;114(2):657–662. doi: 10.1016/0006-291x(83)90831-8. [DOI] [PubMed] [Google Scholar]
- Kanda Y., Goodman D. S., Canfield R. E., Morgan F. J. The amino acid sequence of human plasma prealbumin. J Biol Chem. 1974 Nov 10;249(21):6796–6805. [PubMed] [Google Scholar]
- Lalloz M. R., Byfield P. G., Goel K. M., Loudon M. M., Thomson J. A., Himsworth R. L. Hyperthyroxinemia due to the coexistence of two raised affinity thyroxine-binding proteins (albumin and prealbumin) in one family. J Clin Endocrinol Metab. 1987 Feb;64(2):346–352. doi: 10.1210/jcem-64-2-346. [DOI] [PubMed] [Google Scholar]
- Lalloz M. R., Byfield P. G., Himsworth R. L. A prealbumin variant with an increased affinity for T4 and reverse-T3. Clin Endocrinol (Oxf) 1984 Oct;21(4):331–338. doi: 10.1111/j.1365-2265.1984.tb03219.x. [DOI] [PubMed] [Google Scholar]
- Maye P., Bisetti A., Burger A., Docter R., Gaillard R., Griessen M., Pelte M. F. Hyperprealbuminemia, euthyroid hyperthyroxinemia, Zollinger-Ellison-like syndrome and hypercorticism in a pancreatic endocrine tumour. Acta Endocrinol (Copenh) 1989 Jan;120(1):87–91. doi: 10.1530/acta.0.1200087. [DOI] [PubMed] [Google Scholar]
- Moller D. E., Yokota A., Flier J. S. Normal insulin-receptor cDNA sequence in Pima Indians with NIDDM. Diabetes. 1989 Nov;38(11):1496–1500. doi: 10.2337/diab.38.11.1496. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Lawlor J., Haddow J., Jackson I. M. Familial euthyroid hyperthyroxinemia resulting from increased thyroxine binding to thyroxine-binding prealbumin. N Engl J Med. 1982 Apr 22;306(16):966–969. doi: 10.1056/NEJM198204223061605. [DOI] [PubMed] [Google Scholar]
- Nichols W. C., Liepnieks J. J., McKusick V. A., Benson M. D. Direct sequencing of the gene for Maryland/German familial amyloidotic polyneuropathy type II and genotyping by allele-specific enzymatic amplification. Genomics. 1989 Oct;5(3):535–540. doi: 10.1016/0888-7543(89)90020-7. [DOI] [PubMed] [Google Scholar]
- Premachandra B. N., Ginsberg J., Walfish P. G. Binding of reverse triiodothyronine to serum immunoglobulins in man and the rabbit. J Clin Endocrinol Metab. 1980 Apr;50(4):802–805. doi: 10.1210/jcem-50-4-802. [DOI] [PubMed] [Google Scholar]
- Rajatanavin R., Liberman C., Lawrence G. D., D'Arcangues C. M., Young R. A., Emerson C. H. Euthyroid hyperthyroxinemia and thyroxine-binding prealbumin excess in islet cell carcinoma. J Clin Endocrinol Metab. 1985 Jul;61(1):17–21. doi: 10.1210/jcem-61-1-17. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Dwulet F. E., Benson M. D. Reduced affinity for thyroxine in two of three structural thyroxine-binding prealbumin variants associated with familial amyloidotic polyneuropathy. J Clin Endocrinol Metab. 1986 Dec;63(6):1432–1437. doi: 10.1210/jcem-63-6-1432. [DOI] [PubMed] [Google Scholar]
- Ruiz M., Rajatanavin R., Young R. A., Taylor C., Brown R., Braverman L. E., Ingbar S. H. Familial dysalbuminemic hyperthyroxinemia: a syndrome that can be confused with thyrotoxicosis. N Engl J Med. 1982 Mar 18;306(11):635–639. doi: 10.1056/NEJM198203183061103. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockigt J. R., Dyer S. A., Mohr V. S., White E. L., Barlow J. W. Specific methods to identify plasma binding abnormalities in euthyroid hyperthyroxinemia. J Clin Endocrinol Metab. 1986 Jan;62(1):230–233. doi: 10.1210/jcem-62-1-230. [DOI] [PubMed] [Google Scholar]
- Stockigt J. R., Topliss D. J., Barlow J. W., White E. L., Hurley D. M., Taft P. Familial euthyroid thyroxine excess: an appropriate response to abnormal thyroxine binding associated with albumin. J Clin Endocrinol Metab. 1981 Aug;53(2):353–359. doi: 10.1210/jcem-53-2-353. [DOI] [PubMed] [Google Scholar]
- Tsuzuki T., Mita S., Maeda S., Araki S., Shimada K. Structure of the human prealbumin gene. J Biol Chem. 1985 Oct 5;260(22):12224–12227. [PubMed] [Google Scholar]
- Yabu Y., Amir S. M., Ruiz M., Braverman L. E., Ingbar S. H. Heterogeneity of thyroxine binding by serum albumins in normal subjects and patients with familial dysalbuminemic hyperthyroxinemia. J Clin Endocrinol Metab. 1985 Mar;60(3):451–459. doi: 10.1210/jcem-60-3-451. [DOI] [PubMed] [Google Scholar]
- Yeo P. P., Yabu Y., Etzkorn J. R., Rajatanavin R., Braverman L. E., Ingbar S. H. A four generation study of familial dysalbuminemic hyperthyroxinemia: diagnosis in the presence of an acquired excess of thyroxine-binding globulin. J Endocrinol Invest. 1987 Feb;10(1):33–38. doi: 10.1007/BF03347147. [DOI] [PubMed] [Google Scholar]