Abstract
Polymer vesicles are being extensively studied to emulate self-assembly in biological systems and also use them in a variety of biological and industrial applications. This study demonstrates a novel strategy to prepare polymer vesicles in a pure aqueous medium by driving the micelle-to-vesicle transition with metallic nanoparticles. We synthesized poly(2-amino-2-hydroxyethyl aspartamide) (PAHA) substituted with octadecyl chains, which could form micelle-like self-aggregates in the aqueous medium and chemically bind with platinum precursors. Then, in situ polymerization of Pt nanoparticles within the PAHA self-aggregates generated polymer vesicles that possess nanoparticles within bilayers, because of the increase of the hydrophilic mass ratio to total mass of PAHA, f (w). This new strategy to prepare polymer vesicles would greatly serve to facilitate the control of self-assembly and ultimately improve the functionality of a wide array of polymer vesicles.
1. Introduction
Molecular self-assembly has been attracting great interest in the fields of chemical biology and material science because of its potential to build nanostructured materials with advanced properties and performance.1–3 In particular, polymer vesicles formed by self-assembly of amphiphilic block copolymers, polypeptides, rod-coil polymers, and dendrimers present a bilayer structure similar to live cells and liposomes.4–9 However, the use of polymers offers more opportunity to control the size, stability, and function of vesicles over a broader range than that of liposomes. Therefore, extensive efforts are being made to use polymer vesicles in a variety of biological and industrial applications including drug delivery, biomedical imaging, and nanoreactors.10–15 Successful use of polymer vesicles in these applications greatly relies on the ability to direct polymeric self-assembly while imparting the desired functionality. In general, however, vesicle-forming polymers should present a higher hydrophobic/hydrophilic ratio than micelle-forming polymers. The multiple hydrophobic units may limit the extent to modification of the polymers with functional epitopes. Besides, conventional methods to prepare polymer vesicles, such as the ‘solvent-switch’ and ‘film hydration’ methods16–17, require the use of organic solvents and additional purification steps. These conventional processes often elevate production costs while decreasing the material performance level, including the molecular loading efficiency.18–19
Therefore, this study presents a novel strategy to prepare a polyaspartamide vesicle by driving the transition of polymer micelles to vesicles using metallic nanoparticles. We hypothesized that incorporation of metallic nanoparticles into the hydrophobic domains of polymer micelles via in situ sol-gel polymerization of metallic precursors would drive the micelle-to-vesicle transition, and ultimately generate stable vesicles (Scheme 1). This hypothesis was examined by conducting sol-gel polymerization of platinum precursors in a micelle of polyaspartamide substituted with varying numbers of alkyl chains and precursor-binding amine groups. The resulting micelle-to-vesicle transition was examined by imaging the polymeric constructs along with metallic nanoparticles. The underlying mechanism was explained by quantifying changes in the hydrophilic mass ratio to total mass of the self-assembled polymer. This new strategy would greatly serve to facilitate the control of self-assembly and ultimately improve the functionality of a wide arrayof polymer vesicles.
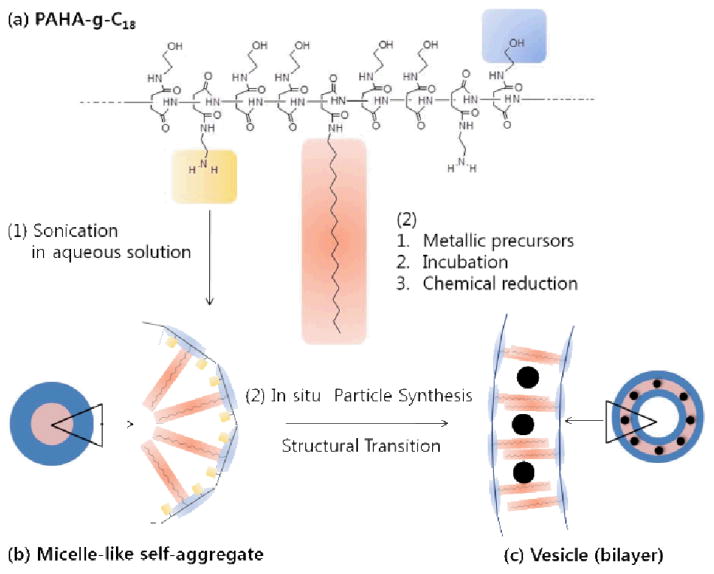
Scheme 1.
Schematic illustration of micelle-to-vesicle transition of polyaspartamide self-aggregates during in situ polymerization of Pt nanoparticles. (a) Polyaspartamide grafted with octadecyl chains and amine groups was synthesized by nucleophilic substitution with polysuccinimide (PSI). (b) The polyaspartamide substituted with octadecyl chains and amine groups self-assembled to form a loosely packed micelle-like self-aggregates in aqueous solution. (c) The in situ sol-gel polymerization of Pt precursors within the polyaspartamide micelle drove the micelle-to-vesicle transition while localizing the resulting Pt nanoparticles within its bilayer.
2. Experimental
Synthesis of poly(2-amino-2-hydroxyethyl aspartamide)-g-C18(PAHA-g-C18)
Polysuccinimide (PSI) was synthesized by acid-catalyzed polycondensation of aspartic acid. Briefly, L-aspartic acid (0.188 mol; Sigma Aldrich) and phosphoric acid (9.4 mmol; Fisher Scientific) were refluxed in a solvent consisting of mesitylene and sulfolane at a mass ratio of 7:3, while purging N2. The temperature was kept constant at 180 °C. Water generated during the reaction was continuously removed using a Dean-stark trap with a reflux condenser. After 10 hours, the reaction mixture was precipitated in excess methanol and then washed several times with deionized (DI) water to remove residual acid catalyst until the pH of the mixture became 7.0. The precipitate, PSI, was washed with methanol and dried at 80 °C under vacuum. The molecular structure of the product was confirmed by 1H-NMR analysis. 1H-NMR (DMSO-d6): δ = 2.7 and 3.2 (α, 2H, s); 5.3 (β, 1H, t). The molecular weight of PSI was determined by gel permeation chromatography (Breeze 2 GPC, Waters), with Styragel® HT column (Waters). Dimethylformamide (DMF) containing 20 mM LiBr was used as the eluent. The elution rate was kept constant at 1.0 mL/min. Polystyrene standards were used for calibration.
Synthesized PSI was dissolved in DMF, which is a good solvent for both PSI and octadecyl amine, and the solution was mixed with octadecyl amine at a desired molar ratio to prepare the polyaspartamide-g-C18. The grafting reaction was maintained at 70 °C for 24 hours. Then, 2-ethanol amine (Aldrich) was added to the solution of polyaspartamide-g-C18 to synthesize poly(2-hydroxyethyl aspartamide)-g-C18 (PHEA-g-C18). The aminolysis reaction was continued at 40 °C for 12 hours. Finally, ethylene diamine (Aldrich) was added to the solution of PHEA-g-C18 to prepare the PAHA-g-C18. After stirring for 12 hours at room temperature, the reaction mixture was precipitated in excess methanol twice. The precipitate was filtered and then dried under vacuum at 60 °C. The molecular structures of the PHEA, PAHA, and PAHA-g-C18 were confirmed by 1H-NMR analysis (Fig. S1). The degree of substitution of amine groups to the PAHA-g-C18 was measured with TNBS (trinitrobenzensulfonic acid) assay. Briefly, 5 mg of PAHA-g-C18 was dissolved in phosphate buffered saline (PBS, pH 7.4) and stirred at room temperature for 30 minutes. Then, 0.05 mL of TNBS working solution (0.1 % TNBS, 4 % NaHCO3, pH 8.5) was added to 0.05 mL PAHA-g-C18 solution. The mixture was incubated at 37 °C for two hours. The reacted products were sequentially mixed with 0.05 mL of 10 % sodium dodecyl sulfate and 0.05 mL of 1.0 M HCl. Finally, the ultraviolet (UV) absorbance of the solution at 335 nm was read using a spectrophotometer (Synergy HT, BioTek).
Self-assembly of PHEA-g-C18 and PAHA-g-C18
PHEA-g-C18 or PAHA-g-C18 was suspended in DI water by vortexing the mixture of polymer and water. The resulting suspension of self-assembled polymer was further sonicated for five minutes using a tip type sonifier at room temperature. The resulting suspensions were purified by passing through a 0.45 μm filter (Whatman). The size of the self-assembled particles was measured using a dynamic light scattering (DLS, Brookhaven Instruments Inc.) equipped with a He-Ne Laser at a scattering angle of 90° (by the Stokes-Einstein relationship). The critical aggregation concentration of polymers was measured using a pyrene probe. The aqueous polymer suspension mixed with pyrene (6.0 × 10−7 M, Sigma) was excited at a wavelength of 330 nm, and the resulting emission spectrum was obtained using a fluorometer (Fluoromax-4, Jobin Ivon). The band-width was adjusted to 2.0 nm for both excitation and emission. The morphology of self-assembled polymeric nanoparticles was also analyzed using transmission electron microscopy (TEM). A drop of nanoparticle suspension was placed on a copper grid coated with carbon film. The grid was held horizontally for two minutes to allow the nanoparticles to settle on the grid. The images were captured at 120 kV with JEOL 2100 Cryo TEM.
In situ synthesis of Pt nanoparticles within a self-assembled PAHA-g-C18 aggregates
6 mg of PAHA-g-C18 was suspended in 2 mL of deionized water and sonicated for five minutes to prepare a nanotemplate in an aqueous solution. The predetermined amount of Pt precursor, potassium tetrachloroplatinate (K2PtCl4, Sigma), was preloaded into the self-assembled polymeric micelle. Then, the mixture was incubated for 24 hours to ensure the complete ligand-exchange reaction between Pt precursors and amine groups of the PAHA-g-C18. The binding between PAHA-g-C18 and K2PtCl4 was examined with a shift of the UV absorbance peak from 215 to 245 nm (Fig. 2b). The 0.5 M sodium tetraborohydrate (NaBH4, Sigma) solution was added to the suspension of PAHA-g-C18 micelles bound with K2PtCl4 to activate the reduction of Pt precursors. The resulting reduction of Pt precursors was monitored with the intensity change of the UV absorbance peak at 245 nm. Following reaction for 24 hours, the mixture was dialyzed for 24 hours against distilled water using a dialysis membrane (MWCO = 8,000 – 12,000 g/mol). TEM images of Pt nanoparticles in micelles or remodeled vesicles were acquired on a JEOL-2010 high-resolution transmission electron microscope operated at 200 kV with a point resolution of 0.23 nm. X-ray diffraction (XRD) analysis was carried out using a Rigaku D/Max-b (Rigaku Americas) with 2θ angles from 30° to 90° at a scan rate of 2.0° mm−1.
Fig. 2.
(a) Schematic diagram of in situ synthesis of Pt nanoparticles within a self-assembled PAHA-g-C18 aggregates. (b) UV spectra of PAHA-g-C18 solutions before (●) and after (■) incubation with K2PtCl4. The binding between PAHA-g-C18 and K2PtCl4 was confirmed with a shift of the UV absorbance peak from 215 to 245 nm. (c) The sol-gel polymerization of Pt increased the absorbance of the solution. (d) The sol-gel polymerization changed the color of the solution from transparent light brownish-yellow to brown and then finally to opaque black. (e) Pt nanoparticles prepared without PAHA-g-C18 dispersed into the oil phase (chloroform) due to their hydrophobic surface (e-1). In contrast, Pt nanoparticles prepared with PAHA-g-C18 with DSC18 of 4 mol % dispersed more preferably into the water phase (e-2). In (e-2), the DS of amine groups was kept constant at 32 mol %.
3. Result and Discussion
3.1 Synthesis and self-assembly of PHEA-g-C18
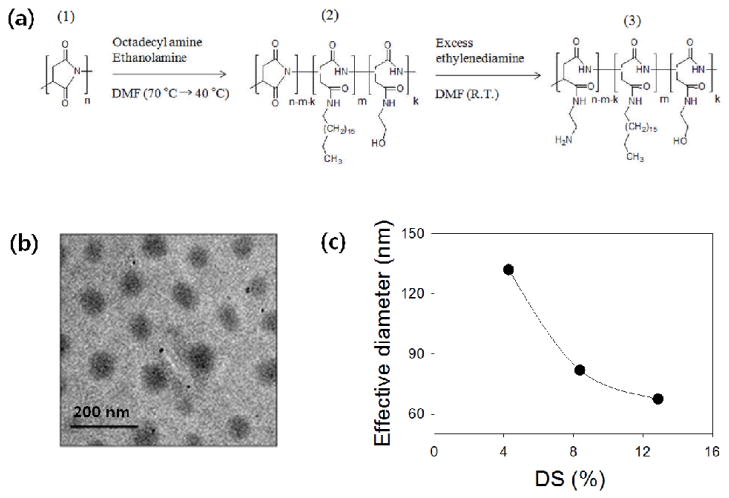
First, we synthesized poly(2-hydroxyethyl aspartamide) substituted with octadecyl chains, termed PHEA-g-C18, by modifying polysuccinimide (PSI) in a top-down manner. PSI was prepared via the acid-catalyzed polycondensation of L-aspartic acid, as previously reported (Fig. 1a-1).20–22 The number average molecular weight (Mn) and weight average molecular weight (Mw) of PSI, measured with gel permeation chromatography, were 57,000 and 73,500 g/mol, respectively. Therefore, the polydispersity index (PDI) of PSI, a ratio between Mw and Mn was 1.3. Then, designated amounts of octadecylamine and 2-ethanol amine were sequentially added to the PSI solution to prepare PHEA-g-C18 through nucleophilic substitution to PSI (Fig. 1a-2). The presence of octadecyl chains in the PHEA-g-C18 was confirmed by a 1H NMR spectrum (Table 1 and Fig. S1). The degree of substitution (DS) of octadecyl chains to PHEA (DSC18), defined as the percentage of succinimide units substituted with octadecyl chains, was varied from 4 to 13 mol % by altering the molar ratio between the octadecylamine and the succinimide units of PSI. There was no significant difference between the predicted and measured values of DSC18, as confirmed by 1H NMR analysis, likely because of the use of DMF as the reaction medium. DMF is a good solvent for both PSI and octadecyl amine. It has been reported that the substitution pattern depends on the solvent quality as well as on the molar ratio between alkyl chains and backbone polymer. Therefore, we assumed that octadecyl chains are uniformly distributed on PHEA with regular spacing, controlled by DSC18.
Fig. 1.
Synthesis of PAHA-g-C18 and characterization of the self-assembled morphology. (a) The overall synthetic scheme of poly(2-amino-2-hydroxyethyl aspartamide)-g-C18 (PAHA-g-C18). (b) TEM image of micelle-like self-aggregates formed from self-assembly of PHEA-g-C18 with DSC18 of 4 %. (c) The effective diameter of self-assembled PAHA-g-C18 was reduced with increasing DSC18.
Table 1.
Molecular characterization of PAHA-g-C18
| Sample | Feed (mol %)a | DSC18b | DSNH2c | f (w)d | Size (nm) | CAC (mg/ml) | Morphology |
|---|---|---|---|---|---|---|---|
| PAHA | 100/0 | ||||||
| C18-D5 | 95/5 | 4 | 32 | 91 % | 132 | 5.6 × 10−2 | Spherical shape |
| C18-D10 | 90/10 | 9 | 31 | 81 % | 82 | 5.4 × 10−3 | Spherical shape |
| C18-D15 | 85/15 | 13 | 33 | 74 % | 67 | 8.0 × 10−4 | Spherical shape |
Feed mole ratio (succinimide unit/octadecyl amine).
Determined based on 1H-NMR of graft copolymers.
Determined based on TNBS assay.
Hydrophilic mass ratio to total mass, f (w)
Increasing DSC18 exponentially decreased the critical aggregation concentration (CAC) of PHEA-g-C18 in aqueous media, marked by a significant increase in the ratio (I3/I1) of the pyrene emission intensity at a wavelength of 385 nm (I3) to that at 373 nm (I1)23 (Fig. S2). According to this result, the PHEA-g-C18 was incorporated into aqueous media at a concentration higher than CAC in order to induce self-assembly between the polymers. The polymers self-assembled into micelle-like forms at a DSC18 of both 4 and 13 mol %, as shown in the transmission electron microscope (TEM) images (Fig. 1b). The hydrodynamic diameter (2RH) of self-aggregates measured by the dynamic light scattering (DLS), decreased with increasing DSC18 (Fig. 1c). This inverse dependency between micelle size and DSC18 indicates that increasing DSC18 elevates the degree of hydrophobic association between alkyl chains to generate more compact micelles with the lower aggregation number.
3.2 Synthesis and Self-assembly of PAHA-g-C18
Next, the PHEA-g-C18 was modified to present amine groups that strongly interact with the platinum ions of the platinum precursor, K2PtCl4 (Fig. 1a-3). The amine groups were conjugated to PHEA-g-C18 by nucleophilic substitution of ethylene diamine to prepare poly(2-amino-2-hydroxyethyl aspartamide)-g-C18, termed PAHA-g-C18. The nucleophilic substitution of the excess ethylene diamine was conducted in dilute PHEA-g-C18 solution in order to circumvent undesirable cross-linking reactions between the polymers. The resulting degree of substitution of amine groups (DSNH2) was kept constant at 32 % as measured with TNBS assay (Table 1).
The PAHA-g-C18 and the K2PtCl4 were incubated in aqueous media to form complexes, observed with a shift in the ultraviolet absorbance peak from 215 to 245 nm (Fig. 2b). Note that the absorption peak at 215 nm represents the ligand-to-metal charge transfer transition.25 The peak shift appeared with PAHAs substituted with octadecyl chains at DSC18 of both 4 and 13 %. In contrast, incubation of K2PtCl4 with amine-free PHEA-g-C18 only minimally shifted the absorbance peak of Pt, which indicates the absence of association between K2PtCl4 and the amine-free polymer (Fig. 2a and b). The presence of K2PtCl4 made minimal changes in the morphology and size of PAHA-g-C18 self-aggregates compared to self-aggregates formed without K2PtCl4 (Fig. S4).
Subsequently, tetraborohydrate (BH4−) was added into the aqueous suspension of K2PtCl4-encapulated PAHA-g-C18 micelles to activate the sol-gel polymerization of K2PtCl4 via chemical reduction (Fig. 2a). This process gradually decreased the transparency of the mixture of K2PtCl4 and PAHA-g-C18 and increased the height of the UV absorbance peak at 245 nm, both of which represent the growth of Pt nanoparticles in the media (Fig. 2c and d). The resulting Pt nanoparticles were added to the mixture of water and chloroform in order to test which medium better facilitated particle dispersion. Interestingly, Pt nanoparticles prepared within the PAHA-g-C18 construct were more preferably dispersed into the water phase, while those prepared without PAHA-g-C18 were mostly dispersed into the chloroform phase (Fig. 2e). This result implies that the Pt nanoparticles were packaged within the self-assembled PAHA-g-C18 construct with hydrophilic hydroxyl groups on the surface.
3.3. Polyaspartamide Micelle-to-Vesicle Transition
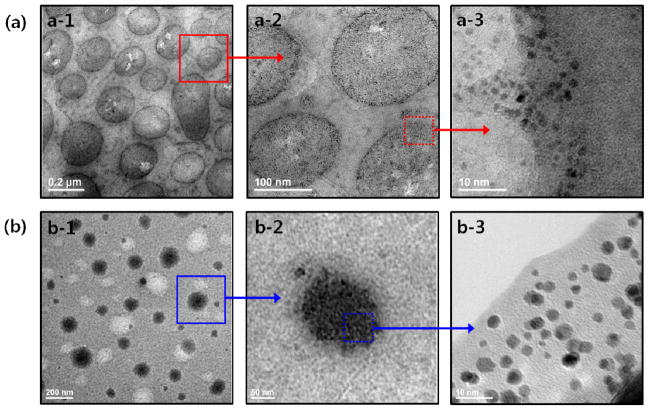
The morphology, diameter, and spacing of Pt nanoparticles associated with the self-assembled PAHA-g-C18 constructs were further examined using TEM. Strikingly, the polymerization of K2PtCl4 within the micelles of PAHA-g-C18 with DSC18 at 4 mol % generated Pt nanoparticles embedded in the bilayer of PAHA-g-C18 vesicles with an average diameter of 200 nm (Fig. 3a-1). This result demonstrates that Pt nanoparticles resulting from the sol-gel polymerization process drive the micelle-to-vesicle transition of PAHA-g-C18. The reorganized vesicular morphology of PAHA-g-C18 with DSC18 at 4 mol % was similar to that of the pure PHEA-g-C18 with DSC18 at 35 mol %, prepared by the precipitation and dialysis method.21–22 The vesicles formed via the micelle-to-vesicle transition remained stable in phosphate buffer saline (PBS), as confirmed with a minimal change in the vesicular diameter (Fig. S3). In addition, the average diameter of Pt nanoparticles synthesized within the vesicular construct was approximately 2.2 nm (Fig. 3a-3). More interestingly, the diameter of Pt nanoparticles was equivalent to the length of fully stretched octadecyl chains linked to PAHA. This result implicates that the growth of Pt nanoparticles was confined by the octadecyl chains. Furthermore, these Pt nanoparticles formed within the vesicular bilayer displayed a crystallinity similar to a face-centered cubic (fcc) lattice, as characterized with high-resolution TEM (HRTEM) and the X-ray diffraction (XRD) pattern (Fig. S5). In contrast, polymerization of K2PtCl4 encapsulated within PAHA-g-C18 with a DSC18 of 13 mol % did not drive the micelle-to-vesicle transition. Instead, nanoparticles with an average diameter of 3.7 nm were tightly packed within the hydrophobic cores, similar to nanoparticles formed with conventional templates (Fig. 3b). In addition, both the Pt particles prepared without PAHA-g-C18 and those prepared with amine-free PHEA-g-C18 were highly agglomerated. Sonication at high frequency failed to disperse these particles in aqueous media (Fig. S6).
Fig. 3.
TEM images of Pt nanoparticles formed within self-assembled aggregates of PAHA-g-C18 with DSC18 of 4 (a) and 13 mol % (b). (a) TEM image shows that Pt nanoparticles are organized within a bilayer of a polymeric vesicle with an average diameter of 200 nm (a-1). The magnified image of the vesicular bilayer (a-2) exhibits the Pt nanoparticles with monodispersed size and shape distribution (a-3). (b) TEM image that shows Pt nanoparticles aggregated within the hydrophobic core of a micelle of PAHA-g-C18 with DSC18 of 13 mol %.
3.4. The role of nanoparticles in molecular assembly
In order to address the mechanism by which nanoparticles drive this interesting micelle-to-vesicle transition, we examined the role of nanoparticles in the hydrophilic mass ratio to total mass f (w), which has been used to predict the morphology of self-assembly between block-copolymers.26 Assuming that octadecyl chains of PAHA-g-C18 present regular spacing and the same level of interaction energy at a given DSC18, the f (w) of PAHA-g-C18 was calculated following the geometric relation for a graft copolymer (Eq. (1)),26
| (1) |
where aw is the mass of hydrophilic backbone units occupied by one octadecyl chain at a given DSC18 of PAHA-g-C18 and bw is the mass of one octadecyl chain (Fig. 4a). Therefore, aw should decrease with increasing DSC18. In the absence of Pt nanoparticles, the f (w) of PAHA-g-C18 exponentially decreased with increasing DSC18, because of the decrease in aw.
Fig. 4.
Calculation of hydrophilic mass ratio to total mass, f (w), of PAHA-g-C18 in the absence and presence of Pt nanoparticles. (a) Scheme used to estimate the f (w) for a graft-polymer system. In (a), a represents the hydrophilic backbone units occupied with one octadecyl chain, and b represents a hydrophobic unit. (b) Estimation of the f (w) of PAHA-g-C18. The solid curve represents a calibration curve to relate f (w) to the self-assembled morphology of the Pt particle-free PAHA-g-C18 with varying DSC18. In (b), the green, pink, and blue zones represent the range of f (w) generating micelle-like self-aggregates [f (w) > 45%], polymer vesicles [f (w) ≈ 35% ± 10%], and inverted structures [f (w) < 25 %], respectively. The * represents the hydrophilic mass ratio of PAHA-g-C18 with DSC18 of 4 mol %, associated with a Pt nanoparticle.
We then related f (w) to the self-assembled morphology of PHEA-g-C18, controlled by increasing DSC18 up to 70 %. According to predictions proposed by Discher and Eisenberg,26 the critical f (w) required to form the polymer vesicles ranges from 25 to 45 %. In addition, this calibration shows that the critical f (w) to drive the micelle-to-vesicle transition is 45 % (Fig. 4b). We separately evaluated the f (w) of the PAHA-g-C18 associated with Pt nanoparticles using Eq. (1). We proposed that, at DS of amine groups of 32 mol %, the mass of the hydrophobic moiety of PAHA-g-C18 with a DSC18 of 4 mol % should become equal to the sum of the mass of an octadecyl chain and that of Pt nanoparticles containing Pt atoms bound with amine groups. Accordingly, the f (w) of the PAHA-g-C18 associated with the Pt nanoparticles was calculated to be 37 %. This value is lower than the critical value at which the micelle-to-vesicle transition of pure PAHA-g-C18 occurs, but higher than the critical f (w) to drive the formation of an inverted structure, which is 25 % (Fig. 4b). In contrast, Pt nanoparticles synthesized using the PAHA-g-C18 with a DSC18 of 13 mol % were mostly confined within the highly packed hydrophobic cores of self-aggregates. We interpret that the PAHA-g-C18 with a DSC18 of 13 mol % was more strongly aggregated than that with a DSC18 of 4 mol % and also did not allow the localization of Pt precursors in the space between octadecyl chains.
Taken together, our results demonstrate that in situ polymerization of Pt precursors chemically bound with PAHA-g-C18 triggers the micelle-to-vesicle transition. Therefore, we can prepare polymer vesicles with a defined size and shape in a purely aqueous medium, without needing to increase the number of alkyl chains linked to the polymer. This interesting morphological transition could not be achieved by simply incorporating pre-made nanoparticles into a micelle according to previous studies that encapsulated various magnetic nanoparticles into a micells.28–29 Rather, such pre-made nanoparticles disrupts the structural integrity of self-assembled constructs.
We suggest that the metallic nanoparticle-driven micelle-to-vesicle transition of PAHA-g-C18 is related to significant changes in the hydrophilic mass ratio to total mass, f (w) and subsequent intermolecular energy. Octadecyl chains grafted to PAHA associate with each other in aqueous environments and further reduce the rotational and translational degrees of freedom of the PAHA backbone. We therefore envision that the f (w) decreased with DSC18 elevates the energy level to drive self-assembly due to the increase of exothermic intermolecular forces (i.e., enthalpy) and the decrease of the entropy of the PAHA backbone. Subsequently, the intermolecular energy rises to a level sufficient to cause morphological changes in self-assembly, such as the micelle-to-vesicle transition. Similarly, Pt nanoparticles formed within a loosely packed micelle of PAHA-g-C18 at a DSC18 of 4 mol % likely led to the decrease in the f (w), which thereby increased the enthalpy of hydrophobic association and decreased the entropy of PAHA. Therefore, we propose that the nanoparticles elevate the intermolecular association energy of PAHA-g-C18 to a level at which vesicle formation is thermodynamically favored. In contrast, the Pt nanoparticles formed within the compact micelle of PAHA-g-C18 at a DSC18 of 13 mol % do not associate with octadecyl chains, so the nanoparticles aggregate within the hydrophobic core of the spherical self-aggregates. The resulting minimal change in the molecular hydrophilic-to-hydrophobic ratio would not push the intermolecular association energy towards vesicle formation.
Conversely, the remodeling of self-assembled PAHA-g-C18 to a vesicular form likely served to deactivate the sol-gel polymerization process. In addition, octadecyl chains might act as a physical barrier to prevent interparticle aggregation as the Pt particles were uniformly distributed without aggregation. We propose that amine groups of PAHA localize the Pt precursors in a space confined by octadecyl chains, so the hydrophobic alkyl chains control the growth of and interaction between nanoparticles. This interpretation is supported by the particle aggregates formed within the self-aggregates of PAHA-g-C18 with a DSC18 of 13 mol %, which do not undergo micelle-to-vesicle transition during the sol-gel polymerization process. We interpret that the PAHA-g-C18 with a DSC18 of 13 mol % was more strongly aggregated than PAHA-g-C18 with a DSC18 of 4 mol %, and did not allow the localization of Pt precursors in the space between octadecyl chains.
The polyaspartamide vesicle can be further modified with functional epitopes via the stepwise nucleophilic substitution of bioactive peptides and proteins to the polyaspartamide. This stepwise modification of polyaspartamide was recently developed to create a multivalent polymeric linker which can link various types of protein molecules with target substrates including hydrogels and gold-coated silicon substrates.30 We envisage that this synthesis strategy would allow us to readily prepare the functionalized polyaspartamide vesicles useful in a broad array of applications. In addition, the potential toxicity of the Pt nanoparticles in the polymer vesicle should be examined thoroughly in future studies.
Conclusions
Overall, with this study, we have created a novel method to prepare polymer vesicles in pure aqueous medium by driving the micelle-to-vesicle transition with in situ polymerization of nanoparticles. These nanoparticles-mediated vesicles remained stable in the aqueous medium without any agglomeration. It is suggested that this nanoparticle-driven micelle-to-vesicle transition can be achieved by decrease in the hydrophilic mass ratio of PAHA-g-C18, so its remodeling to a vesicle is thermodynamically favored. Reciprocally, this remodeling process enables the polymer’s alkyl chains to control the aggregation of Pt nanoparticles. We propose that this new strategy of molecular assembly hybridized with nanoparticles will be highly useful to direct other forms of self-assembly in a wide array of self-assembling molecules and to create functional vesicles for use in a variety of biological applications.
Supplementary Material
Acknowledgments
The authors thank the US Army Research Laboratory (W911NF-09-2) and the National Institutes of Health (1R01 HL109192) for financial support of their research.
Footnotes
Supporting Information. Detailed experimental procedures, 1H-NMR, Fluorescence emission spectra, UV/vis spectra, High resolution TEM image, and X-ray diffraction (XRD) pattern.
Notes and references
- 1.Zhang L, Eisenberg A. Science. 1995;268:1728. doi: 10.1126/science.268.5218.1728. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo EG, Wyrsta MD, Pakstis L, Pochan DJ, Deming TJ. Nat Mater. 2004;3:244. doi: 10.1038/nmat1093. [DOI] [PubMed] [Google Scholar]
- 3.Byun M, Han W, Qiu F, Bowden NB, Lin Z. Small. 2010;6:2250. doi: 10.1002/smll.201000816. [DOI] [PubMed] [Google Scholar]
- 4.Discher BM, Won YY, Ege DS, Lee JCM, Bates FS, Discher DE, Hammer DA. Science. 1999;284:1143. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 5.Yang P, Lipowsky R, Dimova R. Small. 2009;5:2033. doi: 10.1002/smll.200900560. [DOI] [PubMed] [Google Scholar]
- 6.Shum HC, Zhao YJ, Kim SH, Weitz DA. Angew Chem Int Ed. 2011;50:1648. doi: 10.1002/anie.201006023. [DOI] [PubMed] [Google Scholar]
- 7.Wu D, Abezgauz L, Danino D, Ho CC, Co CC. Soft Matter. 2008;4:1066. doi: 10.1039/b715608a. [DOI] [PubMed] [Google Scholar]
- 8.Hordyjewicz-Baran Z, You L, Smarsly B, Sigel R, Schlaad H. Macromolecules. 2007;40:3901. [Google Scholar]
- 9.Savariar EN, Anthimanikandan SV, Thayumanavan S. J Am Chem Soc. 2006;128:16224. doi: 10.1021/ja065213o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nardin C, Widmer J, Winterhalter M, Meier W. Eur Phys J E. 2001;4:403. [Google Scholar]
- 11.Antonietti M, Forster S. Adv Mater. 2003;15:1323. [Google Scholar]
- 12.Discher DE, Eisenberg A. Science. 2002;297:967. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 13.Kim JM, Ji EK, Woo SM, Lee H, Ahn DJ. Adv Mater. 2003;15:1118. [Google Scholar]
- 14.Meng F, Zhong A, Feijen J. Biomacromolecules. 2009;10:197. doi: 10.1021/bm801127d. [DOI] [PubMed] [Google Scholar]
- 15.Litvinchuk S, Lu Z, Rigler P, Hirt TD, Meier W. Pharmaceutical Research. 2009;26:1711. doi: 10.1007/s11095-009-9881-7. [DOI] [PubMed] [Google Scholar]
- 16.Soo PL, Eisenberg A. J Polym Sci: Polym Phys. 2004;42:923. [Google Scholar]
- 17.Reinecke AA, Doebereiner HG. Langmuir. 2003;19:605. [Google Scholar]
- 18.Egli S, Nussbaumer MG, Balasubramanian V, Chami M, Bruns N, Palivan C, Meier W. J Am Chem Soc. 2011;133:4476. doi: 10.1021/ja110275f. [DOI] [PubMed] [Google Scholar]
- 19.Du J, O’Reilly RK. Soft Matter. 2009;5:3455. [Google Scholar]
- 20.Kang HS, Yang SR, Kim JD, Han SH, Chang IS. Langmuir. 2001;17:7501. [Google Scholar]
- 21.Jeong JH, Kang HS, Yang SR, Kim JD. Polymer. 2003;44:583. [Google Scholar]
- 22.Lee HJ, Yang SR, An EJ, Kim JD. Macromolecules. 2006;39:4938. [Google Scholar]
- 23.Kalyanasundaram K, Thomas JK. J Am Chem Soc. 1977;99:2039. [Google Scholar]
- 24.Porcel E, Liehn S, Remita H, Usami N, Kobayashi K, Furusawa Y, Sech CL, Lacombe S. Nanotechnology. 2010;21:85103. doi: 10.1088/0957-4484/21/8/085103. [DOI] [PubMed] [Google Scholar]
- 25.Gerloch M, Constable EC. Transition Metal Chemistry: The Valence Shell in d-Block Chemistry. VCH; Weinheim: 1994. [Google Scholar]
- 26.Discher DE, Eisenberg A. Science. 2002;297:967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 27.Creighton TE. Proteins; structure and molecular principles. W.H. Freeman; New York: 1983. [Google Scholar]
- 28.Ginzburg VV, Balijepailli S. Nano Lett. 2007;7:3716. doi: 10.1021/nl072053l. [DOI] [PubMed] [Google Scholar]
- 29.Wi HS, Lee K, Pak HK. J Phys: Condens Matter. 2008;20:494211. [Google Scholar]
- 30.Cha C, Jeong JH, Xin T, Andrew Z, Prakash Y, Zimmerman S, Saif T, Kong HJ. Bioconjugate Chem. doi: 10.1021/bc200339s. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.