Abstract
Generation of induced pluripotent stem (iPS) cells using defined factors has been considered a ground-breaking step towards establishing patient-specific pluripotent stem cells for various applications. The isolation of human embryonic stem (ES) cells set the standard that pluripotent stem cells are attainable as potentially immortal cells for regeneration of many types of tissues. Different approaches have been tested to obtain pluripotent stem cells by circumventing the need for embryos. iPS cells appear to be an ideal substitute for ES cells. Since the first demonstration of creating iPS cells in 2006, tremendous efforts have been made into improving iPS cell generation methods and understanding the reprogramming mechanism as well as the nature of iPS cells. To improve iPS cell generation, several approaches have been taken: (1) eliminate the viral vector integration after delivering the defined factors; (2) select different cell types that more effectively give rise to iPS cells; (3) use of chemicals to facilitate reprogramming; (4) use of protein factors to reprogram cells. The iPS cells are also being rigorously characterized in comparison to ES cells. All these efforts are made for the purpose of making iPS cells closer to clinical applications. This article will give an overview of the following areas: (1) mechanisms of iPS cell derivation; (2) characterization of iPS cells; (3) iPS cells for cell-based therapy; and (4) iPS cells for studying disease mechanism. Questions as to what aspects of iPS cells require further understanding before they may be put to clinical use are also discussed.
Keywords: cancer-iPS cells, embryonic stem (ES) cells, genome-wide epigenetic profile, global gene expression profile, induced pluripotent stem (iPS) cells, regenerative medicine, tissue regeneration
1. Introduction
The discovery of a consistent and reliable technology to reprogram somatic cells into pluripotent stem cells, termed induced pluripotent stem (iPS) cells, opened a new page for medicine.1–3 iPS cells were first established by delivering the four factors c-Myc/Klf4/Oct4/Sox2 or Lin28/Nanog/Oct4/Sox2 into dermal fibroblasts (DFs). Because the introduction of these factors has been via viral vectors, making the reprogrammed cells not clinically useful, significant efforts have been put into removing the vectors from cells after they have been reprogrammed into iPS cells.4–8 To completely circumvent the use of vectors, delivery of recombinant protein-based four factors to generate iPS cells in the mouse and human system has been reported.9,10 However, the protein method is extremely difficult and inefficient. Delivering mRNA of the four factors has also been tested to reprogram human fibroblasts, but the data presented are currently only preliminary and not convincing.11 Another alternative is not to use these genes and their products at all, but to reprogram by chemical stimulation. Small molecule screening by an established mouse cell line carrying a reporter gene (e.g., Oct4-GFP) was able to filter out a list of chemicals that were able to induce pluripotency by substituting some of the four genes.12–15 Although the evidence showing a total replacement of the four factors by chemicals has not been reported, it is anticipated that this may emerge in the future.
The most important contribution of iPS cells to biology and medicine may be summarized as follows: (1) understanding of cell differentiation and dedifferentiation; (2) possibility of establishing individualized iPS cells for clinical applications without the need to harvest allogeneic human (h) embryonic stem (ES) cells from embryos or deal with nuclear transfer; and (3) generation of patient-specific iPS cells to study genetic background and disease mechanisms. Since the isolation of mouse (m) and hES cells,16–18 this cell source that is capable of giving rise to all types of cells has served as a powerful tool to understand stem cell “stemness” and differentiation. The ES cell properties have been considered as a gold standard for pluripotent stem cell-based regenerative medicine. The major drawback of hES cells for clinical use is the lack of identical genetics between the donor cells and the recipients. iPS cells basically resolve this hurdle. More importantly, certain genetic diseases are rare and lack animal study models. Custom iPS cells can be generated from the patients and studied using in vitro or in vivo study models.
With respect to the source of cells for iPS cell generation, most studies used a mouse system in which iPS cells are much easier to derive. Several types of mouse cells have been used to generate iPS cells successfully, including DFs, dermal papilla cells, β cells, liver cells, gut epithelial cells, neural stem cells, mouse adult bone marrow mononuclear cells and B cells.2,19–26 Other types of cells in the mouse system, such as subpopulations of neural stem cells, have been found to be easily reprogrammed with fewer than the four factors.12,13,21 In the human system, DFs, amniotic fluid-derived cells, skin keratinocytes, ES-derived fibroblasts, CD34 blood cells, mesenchymal stem cells (MSCs), adipose stem cells, umbilical cord blood cells, dental stem cells, and oral mucosa fibroblasts have been shown to be reprogrammed into iPS cells.1,27–36 Currently, only one factor—Oct4— is needed to generate hiPS cells if fetal neural stem cells are used.37 Also, human cord blood cells can be reprogrammed into iPS cells with only two factors: Oct4 and Sox2.33 It appears that, in general, it is easier to reprogram more immature cells than adult cells. From the perspective of clinical applications, cells that are not easily accessible, such as neural stem cells, are not a suitable cell source for iPS cell generation. Initially, iPS cells were thought to have reached the ground state as ES cells once they are fully reprogrammed and able to form teratomas, chimeras or adult animals through tetraploid complementation in the mouse system.38–40 However, the possibility of iPS cells retaining “memory” of the original cells has been speculated. Recent investigation in the mouse system has verified this speculation that iPS cells in low passages retain epigenetic memory.41,42 Genome-wide gene expression and epigenetic profile analyses showed that hiPS cells have differential gene expression signatures and methylated regions compared to hES cells.43,44 Therefore, iPS cells are likely not fully reprogrammed to the ground state of ES cells. In view of the impact that iPS cells have on medicine, current and future research on iPS cells will inevitably focus on: (1) mechanisms of iPS cell derivation; (2) characterization of iPS cells; (3) iPS cells for cell-based therapy; and (4) iPS cells for studying genetics and disease mechanism. This article will focus on the review and discussion of these four areas.
2. Mechanisms of iPS Cell Derivation
2.1. The process of iPS cell reprogramming
In mammals, germ cells undergo a different epigenetic modification process from somatic cells throughout the developmental stage. This process continues until after fertilization, at which point the epigenetic modification is completed. This final epigenetic change allows the zygote to enter a state of totipotency such that a new cycle of normal development to form an entire organism can be initiated.45 In the case of nuclear transfer, the egg nucleus carrying the haploid number of chromosomes is replaced by the diploid somatic nucleus. The creation of Dolly the Sheep by somatic cell nuclear transfer implicated that, in mammals, an adult somatic cell nucleus can be reprogrammed, resulting in extensive epigenetic modification of the genome to a state that confers totipotency.46 Cloning of a mammalian organism is made possible because of this phenomenon. However, human nuclear transfer has so far not been successful in giving rise to embryos for the purpose of obtaining ES cells for therapeutic applications, although cloning in many mammalian species can be achieved.47–50 Besides nuclear transfer, one approach to rejuvenating somatic cells into the pluripotent stage is by fusion with ES cells. Although this does lead to transforming the somatic cell into pluripotency resulting from the nuclear effect of the ES cells, this approach causes the cell to become tetraploid.51,52 Additionally, just like using allogeneous hES cells for therapy, it has immune-rejection potential. Another approach is parthenogenesis, a phenomenon that does not occur in mammals but which can be artificially induced. Human parthenogenetic approaches that generate hES cells are potentially useful for clinical applications.53,54 In mouse systems, primordial germ cells or germline stem cells and bone marrow cells may become pluripotent in cultures.55–57 In summary, from a perspective of clinical applications using pluripotent stem cells, those aforementioned methods are either technically difficult or have only been observed in mouse systems.
By studying ES cells, many genes have been known to be associated with the pluripotent state, and these genes are repressed in differentiated cells. The question is whether or not forcing the expression of these repressed genes can dedifferentiate cells and reverse them to the pluripotent state. Yamanaka and his team asked this question and selected a list of 24 genes known to be expressed in ES cells. These genes are mostly transcriptional factors, intracellular signaling factors or involved in epigenetic modifications. By using a Fbx15βgeo/βgeo mouse model, they found that forced expression of all these genes in Fbx15βgeo/βgeo mouse fibroblasts turned them into ES-like cells morphologically. Subsequently, they meticulously narrowed them down to four factors, c-Myc, Klf4, Oct4 and Sox2, that were sufficient to reverse fibroblasts to ES-like cells—the birth of “induced pluripotent stem (iPS) cells”.2 These miPS cells demonstrate the following features: (1) show ES cell morphology in cultures; (2) have similar cell expansion rate to ES cells; (3) express key ES cell genes; (4) their global gene profiles resemble those of ES cells; (5) have similar epigenetic profiles of genes involved in ES cell maintenance and in development; (6) form embryoid bodies in cultures; (7) can differentiate into cells of all germ layers in embryoid bodies in cultures; (8) form teratomas in vivo containing tissues of all germ layers; and (9) form chimeras after iPS cells have been injected into blastocysts.
All the above features are characteristic of ES cells. This finding demonstrates that instead of requiring a myriad of nuclear and cytoplasmic factors to reprogram a somatic cell nucleus into the ground state of pluripotency,58 a delivery of three to four defined factors can lead to a cascade of intracellular events resulting in activation and inactivation of genes that lead to reversing somatic cells to a pluripotent state.
Yamanaka's group further asked if these four factors will do the same for human fibroblasts, although mouse and human systems are very different. They used the same four factors c-Myc, Klf4, Oct4 and Sox2 in humans and introduced them using the same retroviral vector systems. Human DFs from human donors were able to be reprogrammed into iPS cells exhibiting similar features outlined above for miPS cells except for the formation of chimeras which cannot be performed for the human system.1 At the same time, a team led by Thomson independently selected 14 potential genes with enriched expression in ES cells relative to myeloid precursors and known to be involved in the establishment of maintenance of pluripotency to reprogram human DFs. Using a differentiated derivative of an Oct4 knock-in human ES cell line previously generated, the team was able to test the 14 genes and screen which subsets could activate Oct4 promoter in the differentiated cells and reprogram back to ES cell state. They identified a core set of four genes, Oct4, Sox2, Nanog and Lin28, that were able to reprogram human fetal and foreskin fibroblasts into iPS cells with ES cell characteristics.3 These two papers confirmed that hiPS cells can be generated with defined factors, implying that this reprogramming technology may be a powerful tool for clinical applications, especially in generating patient-specific pluripotent stem cells.
2.2. A stochastic event of the reprogramming
The activation of the endogenous genes needed to reach the pluripotent state involves at least a change in the epigenetic conditions of those key genes that play a pivotal role in the maintenance of cell pluripotency and differentiation as well as in the development of the organism. Although the defined factor reprogramming method is relatively straightforward compared to other means of obtaining pluripotent cells, this method is slow and highly inefficient. In humans, approximately 30 days are needed to derive iPS cells with a success rate of only ∼0.01% of the transduced fibroblasts, and most cells are partially reprogrammed or undergoing apoptosis. Yamanaka proposed the stochastic model to explain this phenomenon.59 In this model, all cells have the potential to become iPS cells after the four-factor transduction. However, because a successful reprogramming requires the right amount, balance, continuity and silencing of the four-factor transgene expression, only a small number of cells are in the perfect condition to be reprogrammed in the right direction when receiving the transgenes. Because of this subtle balance of transgene expression, the vector system used to deliver the transgenes may be a determining factor of successful reprogramming. Our group initially subcloned the four factor genes (c-Myc, Klf4, Oct4 and Sox2) as well as Nanog, each into the pLenti6.2/C-Lumio/V5-DEST vector system containing a CMV promoter. Produced virus was added to the cultured human dental stem cells (four factors or four factors plus Nanog). Approximately 30–50% of transduced cells underwent cell death in the first few days. The surviving cells proliferated faster than before the transduction and began morphological changes (fibroblastic to epithelial cell-like). The cells were seeded onto feeder cells within 7 days following transduction to allow reprogramming. Within 2–3 weeks, a few cell colonies similar to ES cell colonies emerged (Figure 1). These colonies were passaged to new feeder cells but later all underwent cell death. The same phenomenon was observed after several attempts. Chan et al categorized these ES-like colonies emerging on feeder cells during reprogramming into three types (types I, II and III), with only type III being fully reprogrammed as they expressed Nanog, while types I and II are partially reprogrammed and they do not (type I) or only minimally (type II) express Nanog.60 Subsequently, we used lentiviral vectors pSin-EF2-gene-Pur with PEF1a promoter carrying one of the four factors Lin28, Nanog, Oct4 and Sox2 generated by Thomson's group,3 and were able to obtain iPS cells reprogrammed from dental stem cells.35 We also subcloned human c-Myc, Klf4, Oct4 and Sox2 into the retroviral vector pMXs with 5′ LTR MMLV to drive gene expression. The vector system was also able to successfully reprogram dental stem cells into iPS cells.35 It is possible that certain promoters built in the vector systems provide optimal expression levels and continuity of those four to five factors in the transduced cells allowing successful reprogramming, while some vector systems do not lead to this optimal condition for a full reprogramming.
Figure 1.
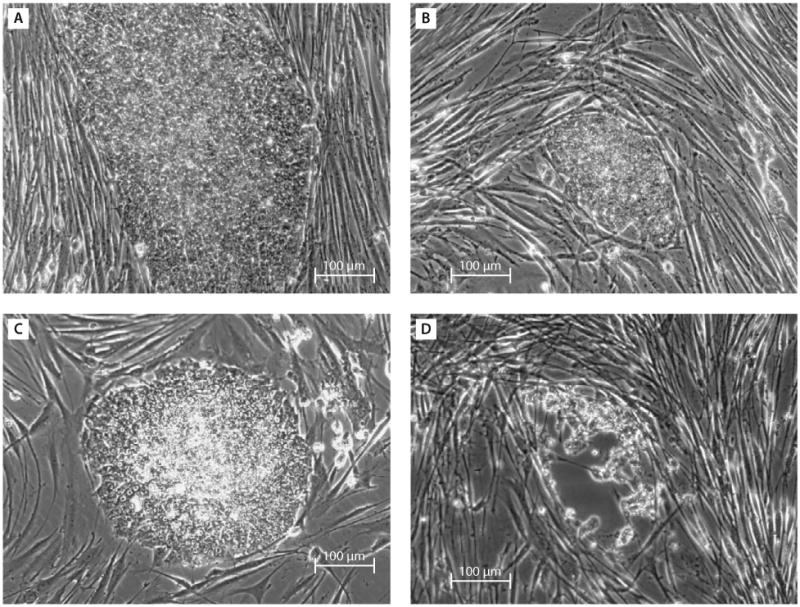
Emergence of embryonic stem (ES) cell-like colonies. Dental pulp stem cells (DPSCs) or stem cells from exfoliated deciduous teeth (SHED) were transduced with the five factors (c-Myc, Klf4, Oct4, Sox2 and Nanog) each carried by the pLenti6.2/C-Lumio/V5-DEST vector system. Trasduced cells were then seeded onto the feeder cell layer of human foreskin fibroblasts (hFFs). (A, B) Twenty-five days after transduction of DPSCs, ES-like colonies began to emerge. However, cells eventually underwent cell death. (C, D) Twenty-one days after transduction of SHED. After being passaged once, shown in (C), cells began dying off 33 days after transduction, (passage 3) shown in (D). Note: pluripotent-associated gene expression was negative in the colonies represented in (C).
Smith et al summarized the successful rate of iPS generation with the highest at 0.1% in a mouse system using embryonic fibroblasts.61 With a single lentiviral vector expressing all four of Yamanaka's factors, Sommer et al were able to demonstrate a reprogramming efficiency of 0.5% using mouse tail-tip fibroblasts.62 In human systems, adipose tissue stem cells can reach a successful reprogramming rate of 0.2%.34 In general, it is difficult to assess the absolute efficiency as different laboratories use various vector systems and viral activities can vary widely as well.
Regarding silencing of the transgenes, iPS cells generated by Thomson's four factors and the vector systems do not become completely silenced after reprogramming but were able to differentiate and form teratomas.3,35 Whether or not these unsilenced exogenous genes would affect specific differentiation processes is unclear. It was mentioned by Yu et al in their first paper on generation of iPS cells (Thomson's four factors) that neural differentiation was common in teratomas from some of their iPS cell clones, while it was largely absent in those from other clones.3 They found that this difference appeared to correlate with a failure to downregulate Nanog and Oct4, and speculated that these differences may be due to the specific integration sites that allowed continued high expression of the lentiviral transgenes partially blocking differentiation.3 Additionally, Yu observed (unpublished) that most of the iPS cells generated with lentiviral vectors (Thomson's four factors) from newborn or adult fibroblasts produced mostly mesodermal and endodermal tissues in teratomas, with less ectodermal tissues compared to those from human ES cells, which was likely due to significant residual transgene expression in these iPS cells (e-mail communication with Yu, April 1 and September 24, 2010).
3. Characterization of iPS Cells
3.1. Global gene and epigenetic profiles
The first set of miPS cells created by Yamanaka and his colleagues was compared to ES cells in terms of global gene profiles, epigenetic characteristics, pluripotency in vitro and teratoma formation in vivo. The global gene expression profiles of the original miPS cells were compared to those of mES cells and their parent cells.2 iPS cell clones clustered well with ES but not their parental cells. Observable differences exist between different miPS cell clones as well as between mES and miPS cells; therefore, miPS cells are not the same as mES cells in their global expression profiles. The promoter regions of Oct4 and Nanog are hypomethylated in mES cells, whereas they are still heavily methylated in miPS cells.2 Subsequently, Yamanaka's team applied a different strategy by introducing Oct4, Sox2 and Klf4 into Nanog-GFP-IRES-Puror mouse embryonic fibroblasts to generate Nanog iPS cells.63 These cells have greater mES-cell-like gene expression compared with Fbx15 iPS cells. Furthermore, the promoter regions of Nanog, Oct4 and Fbx15 are largely unmethylated in Nanog iPS cells, unlike Fbx15 iPS cells. Nanog iPS cells contribute to chimeras and some clones showed germ line transmission in the testis and were able to generate F1 mice cross breeding with C57BL/6 female mice as well as F2 mice by intercrossing the F1.63
In the human system, the global gene expression patterns between iPS and ES cells were also compared and shown to be similar.1,3 Epigenetic profiles of the DNA and histone methylation were analyzed, focusing on the promoter regions of pluripotent-associated and development-associated genes. Oct4, Rex1 and Nanog of the iPS cells were highly unmethylated. The histone modification status in hiPS cells showed H3K4 methylation and H3K27 demethylation in the promoter regions of Oct4, Sox2 and Nanog. There were also bivalent patterns of development-associated genes including Gata6, Msc2, Pax6 and Hand1.1
3.2. iPS cells confer pluripotency of somatic cells and form live adult animals via tetraploid complementation
ES cells have the ability to reprogram the somatic cell genome following cell fusion.51,52,64 The cell hybrids exhibit characteristics of pluripotency. Recent reports demonstrated that at least in the mouse system, iPS cells are also capable of reprogramming somatic cells by cell fusion.65,66 The iPS-somatic hybrids can differentiate into cell types indicative of all three germ layers. This demonstrates that once a somatic cell nucleus is reprogrammed by defined factors, it acquires the capacity and potency to reprogram other somatic cells by cell fusion and shares this functional property with normal ES cells.
Another important hallmark of ES cells as pluripotent stem cells is the capability to form embryos and live animals via a tetraploid-complementation procedure. This process is commonly performed using a mouse system in which a diploid embryo at two-cell stage is artificially fused into a tetraploid cell and allowed to form a blastocyst. ES cells are then injected into the blastocyst, which is then implanted into a surrogate mother mouse for pregnancy. Tetraploid cells will develop into extraembryonic tissues (e.g., placenta) while the injected ES cells will form the diploid embryo which is born to develop into an adult mouse. Not until 2009, iPS cells had failed to demonstrate their complete pluripotency by developing into adult tetraploid-complementation mice. Three groups at the same time reported the generation of live pups by iPS cells and some of them lived to adulthood.38–40 The success rate of giving rise to tetraploid complementation by iPS cells was similar to that by ES cells; however, different iPS cell lines were not equally successful in producing viable offspring, although they are competent for germline transmission through chimeric mice. Some iPS cell lines showed early termination of fetal development at embryonic day.39
The above evidence suggests that iPS cells are functionally similar if not identical to ES cells, except that variability occurs among different iPS cell clones, which indicates that truly reprogrammed iPS cells cannot be easily verified until tetraploid-complementation assays are conducted. However, hiPS cells cannot be tested via tetraploid complementation. Therefore, further characterization at molecular levels must be carried out to understand the level of reprogramming in hiPS cell clones.
3.3. Telomere and telomerase activity of iPS cells
The premature death of Dolly the Sheep was first considered the result of shorter telomere. Subsequently, other reasons were realized to play an important role in the premature aging.67,68 A large percentage of nuclear transfer-derived mammals either die during/after gestation or exhibit shortened lifespans and other abnormalities. This genetic dysregulation has been considered to be significantly attributed to incomplete or imprecise nucleus reprogramming leading to imprinting errors associated with epigenetic effects and abnormal gene expression.69 Different cell types used for nuclear transfer may yield different outcomes in terms of telomere length. Dolly and another sheep derived from mammary epithelial cells and embryonic cells, respectively, did not attain full genetic reprogramming of the nucleus leading to shortened mean telomere restriction fragment lengths, whereas the third sheep derived from a fibroblast nucleus had normal mean telomere restriction fragment lengths. Therefore, although the nuclear transfer does not always lead to a precise nuclear reprogramming, resetting of telomere-based molecular clocks is attainable.69
Regaining telomere length is an important feature to indicate the completeness of reprogramming of iPS cells. Yamanaka's group showed that high levels of telomerase activity were detected by the telomere repeat amplification protocol method in their first demonstration of the establishment of hiPS cells.1 However, whether or not iPS cells regain the telomere length that is similar to that in ES cells after reprogramming is unclear. In a mouse system, Marion et al demonstrated that during reprogramming, telomere elongation is usually mediated by telomerase and that iPS telomeres acquire the epigenetic marks of ES cells, including a low density of trimethylated histones H3K9 and H4K20, and increased abundance of telomere transcripts.70 They also found that while telomerase activity is not needed to generate iPS cells, reprogramming efficiency of cells derived from increasing generations of telomerase-deficient mice shows a dramatic decrease in iPS cell efficiency, a defect that is restored by telomerase reintroduction.
Shiels and Jardine reasoned that regain of telomere length may not be a prerequisite that determines the lifespan of the cloned animal. However, when using cells derived from cloned animal embryos for therapeutic purposes, cell transplantation-induced stress may trigger premature telomere erosion.69 In the same vein, using iPS cells for transplantation therapies may encounter the same issue if iPS cells do not have sufficient telomere length.
One interesting question regarding the telomere regaining length in iPS cells can be addressed in the disease state dyskeratosis congenita (DC), a disorder of telomere maintenance. Agarwal et al asked whether defects in telomerase function would limit derivation and maintenance of iPS cells from patients with DC. They found that reprogrammed DC cells overcame a critical limitation in telomerase RNA component levels to restore telomere maintenance and self-renewal. Reprogramming restores telomere elongation in DC cells despite genetic lesions affecting telomerase; therefore, strategies to increase expression of telomerase RNA component may be therapeutically beneficial in DC patients.71
3.4. Guided differentiation into various tissue-specific cells
In vitro protocols have been developed to guide ES cell differentiation into various cell types. With the emergence of iPS cells, the protocols for ES cells are being tested for iPS cell differentiation. The question is whether these protocols will work in the same way for iPS cells. This review will focus on the discussion of the following guided differentiation of iPS cells into cells of ectodermal, mesodermal and endodermal origin.
3.4.1. Neurogenesis
Parkinson's disease is a common chronic progressive neurodegenerative disorder characterized primarily by major loss of nigrostriatal dopaminergic neurons. Wernig and colleagues used a mouse model and demonstrated that iPS cells can be guided to differentiate into neural precursor cells efficiently, giving rise to neuronal and glial cell types in cultures. Upon transplantation into the fetal mouse brain, the cells migrated into various brain regions and differentiated into glia and neurons, including glutamatergic, GABAergic, and catecholaminergic sub-types.72 These grafted neurons had mature neuronal activity and were functionally integrated in the host brain. Furthermore, iPS cell-derived dopamine neurons transplanted into a rat model of Parkinson's disease improved behavior. To avoid the risk of tumor formation from the grafted cells, the authors used fluorescence-activated cell sorting to deplete SSEA1-positive cells, and they did not observe the occurrence of teratoma formation in vivo while exhibiting desired functions in their studies.72
Soldner et al further demonstrated that iPS cells reprogrammed from the DFs of five Parkinson's disease patients can be guided to differentiate into dopaminergic neurons (Figure 2).4 The viral vectors carrying the reprogramming factors in these iPS cells were removed using a Cre-recombinase excisable viral vector system. Transgene-free iPS cells maintain a pluripotent state and show a global gene expression profile, more closely related to hES cells than to hiPS cells that carry the transgenes.4 Using a 6-OHDA lesioned rat model (induced Parkinson's disease), Cai et al showed that hiPS cells were able to differentiate into dopaminergic neurons and survive in the 6-OHDA rat brain expressing midbrain dopaminergic neuron precursor cell markers. However, no significant recovery from induced Parkinson's disease symptoms were observed.73
Figure 2. (A) In vitro differentiation of induced pluripotent stem cells.
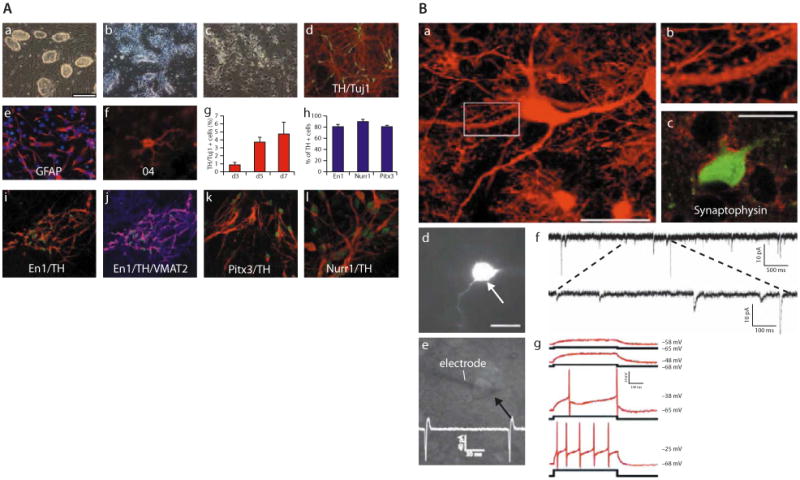
(a) The undifferentiated Oct4-selected iPS cell line O9. (b) FGF2-responsive neural precursor cells. (c) Differentiated neural morphologies 7 days after growth factor withdrawal. (d) A fraction of β-III-tubulin positive neurons (red) are double-labeled with antibodies against TH (green, yellow in merged image), 7 days after growth factor withdrawal. (e, f) At this stage, also, many GFAP-positive astrocytes (red) (e) and rare 04-positive oligodendrocytes (f) are found. (g) The fraction of TH-positive cells over β-III-tubulin positive cells increases along neuronal maturation (mean and standard deviation, three independent experiments). (h) The vast majority of TH-immunoreactive cells coexpress En1, Pitx3, and Nurr1 (mean, standard deviation). (i) Coexpression of En1 (green) and TH (red). (j) VMAT2 and TH [purple in the merged image indicates colocalization of TH (red) and VMAT2 (blue)]. (k, l) Most TH-positive cells (red) are also positive for Pitx3 (green) (k), and Nurr1 (green) (l) after 7 days of neuronal differentiation. [Scale bars: (a, b) 200 μm; (c, d, i, j) 100 μm; (e, k) 50 μm; (f, l) 20 μm.] (B) Synaptic integration of functional iPS cell-derived neurons into the host brain. (a) GFP-immunofluorescence allows the high resolution characterization of dendritic morphologies of transplanted neurons. (b) Higher magnification of the indicated part in a suggests the presence of synaptic spines along this dendrite. (c) Integrated GFP-positive neurons are adjacent to many synaptophysin-positive patches (red), indicating synaptic contacts from host axon terminals. (d) A GFP-expressing neuron (arrow) in the dorsal midbrain was detected in acute brain slices of a P20 mouse brain after in utero transplantation. (e) GFP-positive neurons were identified by infrared differential interference contrast (arrow) and approached by a recording electrode (left). The trace indicates spontaneous generation of action potentials. (f) Voltage-clamp recording at −70 mV in extracellular solution containing 3 mM Mg2+. Traces show spontaneous slow and fast currents that indicate that this transplanted neuron receives synaptic contacts from host cells. All six recorded GFP-positive neurons from two mice (age P20 and P22) exhibited similar spontaneous currents. (g) Current-clamp recordings during current injection. Shown are superimposed membrane potential changes (upper traces, red), which demonstrates the capability of the grafted neurons to fire action potentials in response to a series of current injection (lower traces, black) from a holding potential of −68 mV. All six analyzed GFP-neurons showed these active membrane characteristics. (Scale bars: 20 μm.) (Adapted with permission from reference 72.)
A study by Hu et al investigated neural differentiation comparing hES H9 line with several hiPS cell lines derived from dermal or lung fibroblasts from donors of various ages.74 These hiPS cell lines either carried the transgenes or without the transgenes (reprogrammed by Yamanaka's or Thomson's four factors). Cells were converted to neuroepithelial cells, functional neurons, glia, or neuronal subtypes (motor neurons), and the results showed that all iPS cell lines tested followed developmental principles as hES cells but with variable potency (Figure 3).74 hiPS cells could also be differentiated to form motor neurons with a similar efficiency as hES cells. The differentiation of iPS cells appeared to follow a normal developmental progression associated with motor neuron formation and possessed prototypical electrophysiological properties.75
Figure 3.
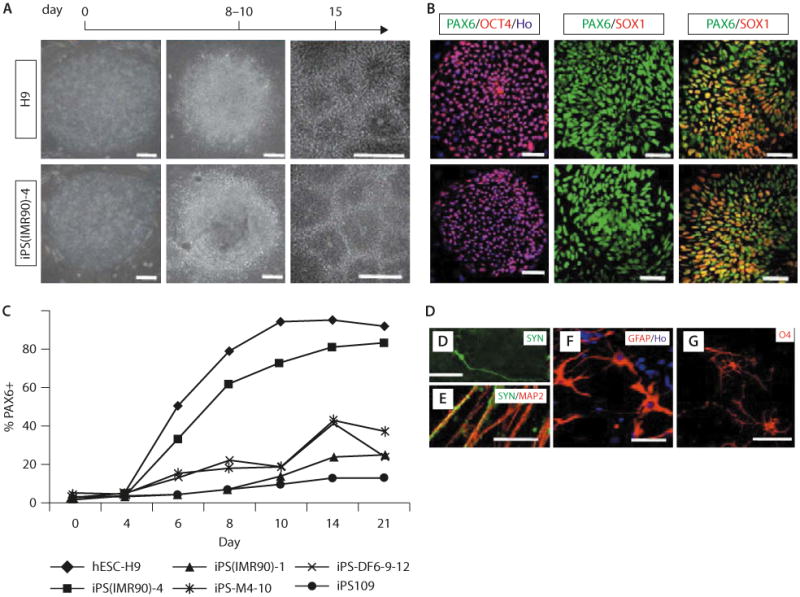
Human iPS and hES cells follow the same temporal course of neural differentiation. (A) Phase contrast images show that hES and iPS cells grew as individual colonies, differentiated to columnar epithelial cells at days 8–10, and formed neural tube-like rosettes at day 15. (B) Both iPS and hES cells were positive for Oct4 at day 0, for Pax6 but not Sox1 at days 8–10, and for both Pax6 and Sox1 at day 15. (C) FACS analyses indicate that differentiating cells from H9 hES, iPS(IMR90)-1 and 4, iPS-M4-10, iPS-DF6-9–12, and iPS109 cells began to generate Pax6-expressing cells at days 6–8, and this reached a plateau at day 14 but with different efficiency. Shown are curves of the average from three replicates for each cell line. (D, E) By 12 weeks in culture, many MAP2+ neurons also expressed synapsin; higher magnification indicated a punctuate staining pattern on the cell bodies and neurites. (F) GFAP+ astrocytes were present in differentiated cultures at 12 weeks. (G) O4 + ramified oligodendrocytes were observed in cultures after 16 weeks. Except when noted elsewhere, images of iPS cells are presented with iPS(IMR90)-4 in this and the following figures. (Scale bar: 50 μm.) (Adapted with permission from reference 74.)
These proof-of-principle studies shed light on the possibility of using a patient's own cells to treat Parkinson's disease and motor neuron diseases.
3.4.2. Hematopoietic and endothelial cell derivation from iPS cells
Protocols that have been used to guide the differentiation of hES cells into hematopoietic and endothelial cell lineages were tested on iPS cells. When an OP9 differentiation system was employed, multiple human iPS cell lines obtained from human fetal, neonatal and adult fibroblasts through reprogramming with Thomson's four factors showed similar differentiation potential, with some variations, compared to five hES cell lines.76 Another protocol using an embryoid body-mediated approach that has been used to guide the differentiation of hES cells into hemangioblastic lineage, a bipotential progenitor of endothelial and hematopoietic cell lineages, was tested on multiple lines of hiPS cells derived by Thomson's as well as by Yamanaka's four factors. While hiPS cells are capable of generating hemangioblasts/blast cells, endothelial cells and hematopoietic cells similar to those derived from hES cells, the efficiency is dramatically lower. Also, there is significantly increased apoptosis, severely limited growth and expansion capability, as well as a substantially decreased hematopoietic colony-forming capability and early senescence of endothelial cells.77 The variation of iPS cell lines on hematopoietic differentiation was also noted by Chang et al in their studies on globin phenotype of erythroid cells.78 In the mouse system, iPS cells derived from embryonic fibroblasts appeared to be more potent to generate hematopoietic cells than those derived from adult cells.79
3.4.3. iPS cell-derived skeletal muscle cells
Mizuno et al demonstrated that miPS cells have the potential to develop in vitro into skeletal muscle stem/progenitor cells, which are almost equivalent to the potential of mES cells.80 Transplanting these cells into mdx mice, a well-known model for Duchenne muscular dystrophy, led to sustained myogenic lineage differentiation in injured muscles, while providing long-lived muscle stem cell support (24 weeks of experimental observation).80 These data suggest that iPS cells have the potential to be used in the clinical treatment of muscular dystrophies, especially as a large number of cells may be needed for this type of therapy. Adult stem cells are unlikely to fulfill this requirement compared to iPS cells.
3.4.4. Hepatocytes from iPS cells
Recent evidence has revealed that iPS cells can become fully functional endodermal-derived hepatocytes, demonstrating the true potential of iPS cells for the field of hepatology. iPS cell-derived hepatocytes not only hold promise in therapeutic applications but also facilitate the study of molecular and genetic aspects of human hepatic disease and development. Additionally, they provide a platform for drug toxicity screening and identification of novel pharmaceuticals with potential to treat a wide array of metabolic diseases.81–83
Sullivan et al have shown that iPS cell lines representing both male and female sexes and two ethnic origins can be differentiated to hepatic endoderm at efficiencies of between 70% and 90%.82 The iPS cell-derived hepatic endoderm or hepatocytes exhibit hepatic morphology, express the essential hepatic markers and produce/secrete proteins (e.g., α-fetoprotein), characteristics of functional hepatocytes.82 Similar results were found by Si-Tayeb et al that hepatocyte-like cells derived from either hES cells or hiPS cells express a series of genes encoding phase I and phase II enzymes (characteristic of a fully differentiated hepatocyte). However, the levels of expression of these enzymes were lower in most cases when compared with adult liver samples.83
Taken together, it appears that further studies of large numbers of individual iPS cell lines or clones are needed to understand the extent of variation in differentiation potential that is related to the effects of cell origin or reprogramming methods, especially with regard to any clinical applications. Additionally, iPS cell-derived differentiated cells must be able to fully mature into differentiated cells and have equivalent functions to the naturally formed tissue cells. Fine tuning of the differentiation protocols is needed to achieve these desired goals.
4. iPS Cells for Cell-based Therapy
Although many types of adult stem cells are multipotent and have been rigorously investigated as to their capacity in tissue regeneration, the pluripotency of ES cells that can generate all cell types is unparalleled by adult stem cells. Additionally, the adult stem cell source and lifespan are limited and therefore insufficient in number to regenerate sizable tissues or organs. Since the isolation of hES cells in 1998 by Thomson's group, significant efforts have been made to determine how hES cells can be utilized for clinical applications. However, the clinical trial using hES cells so far is limited to treating spinal cord injury. Although it was approved by the U.S. FDA in 2009, it has been put on hold due to cyst formation in tested animals. After reviewing new data from animal studies, the FDA reapproved the clinical trial in July 2010. Cell-based therapy can be categorized into the clinical needs and applications detailed below.
4.1. Hematopoietic cell therapy to treat blood diseases
A proof-of-principle demonstration by Hanna et al in the use of iPS cells to treat a blood disease is the sickle cell anemia mouse model.84 Sick mice were rescued after transplantation with hematopoietic progenitors obtained in vitro from autologous iPS cells with genetic correction of the sickle hemoglobin allele. Similarly, in a human model, Raya et al showed that, on correction of the genetic defect, somatic cells from Fanconi anemia patients can be reprogrammed into pluripotency to generate patient-specific iPS cells.85 These cell lines appear indistinguishable from hES cells and iPS cells from healthy individuals. Corrected Fanconi anemia-specific iPS cells can give rise to hematopoietic progenitors of the myeloid and erythroid lineages that are phenotypically normal and disease-free. These data indicate that iPS cell technology can be used for the generation of disease-corrected, patient-specific cells with potential value for cell therapy applications.85
4.2. Tissue regeneration to repair lost organs/tissues
While cell-based therapy to repair tissues is more complicated and riskier than non-cell-based therapy, it may be the best option when defected size reaches a point that non-cell-based regeneration cannot repair. Tissue transplant from autologous or allogenic sources has been the most popular mode of treatment to repair lost organs/tissues. However, donor site morbidity is the major shortcoming. The utilization of postnatal or adult stem cells for tissue engineering to regenerate organs/tissues has provided an alternative to tissue transplant. Multipotent stem cells, especially of mesenchymal origin, have drawn much attention recently because of their potential to give rise to different cell lineages, including osteogenic, chondrogenic, adipogenic, neurogenic and myogenic cells. The source of these MSCs has expanded dramatically in the past decade from bone marrow to adipose tissue, umbilical cord blood, and amniotic fluid.86,87 Specific tissues also harbor MSCs. Some, such as dental pulp tissues, may give rise to a large number of cells from a small volume of tissue due to its high capacity of population doubling.88 While MSCs are versatile, their population doublings are still limited. No matter how many sources MSCs can be obtained from, the ultimate problem lies in their limited lifespan. Since ES or iPS cells are potentially immortal, they are an unlimited cell source for tissue regeneration. ES cells have been tested as a cell source to regenerate a variety of tissues under the framework of tissue engineering.89–91 Shifting from the use of ES cells to iPS cells in the same context is obviously the next step.92
5. iPS Cells for Studying Disease Mechanism
One important attribute of iPS cells is their use in the study of disease mechanisms. iPS cells may be generated from patients with specific types of diseases and the disease mechanisms studied in vitro.93
5.1. iPS cells from cells of genetic disease
Park et al proposed to generate iPS cells from patients with a variety of genetic diseases of Mendelian or complex inheritance, including adenosine deaminase deficiency-related severe combined immunodeficiency (ADA-SCID), Shwachman-Bodian-Diamond syndrome (SBDS), Gaucher disease type III, Duchenne muscular dystrophy, Becker muscular dystrophy, Parkinson's disease, Huntington's disease, juvenile-onset type I diabetes mellitus, Down syndrome/trisomy 21, and the carrier state of Lesch-Nyhan syndrome.94 Such disease-specific iPS cells are an excellent tool for the study of both normal and pathologic human tissue formation in vitro, allowing investigations of disease mechanisms and drug development. Park et al obtained patient-derived somatic cells and were able to reprogram them into iPS cells with defined factors (Oct4, Sox2, Klf4 and c-Myc) or three reprogramming factors (minus c-Myc).94
iPS cells generated from patients with single-gene disorders allow experimentation on the diseased cells in vitro. More importantly, the defected gene may be repaired ex vivo and the correct cells transplanted back into the host. Sickle cell leukemia is the best example for this potential clinical application. Diseases caused by point mutations in genes that are inherited in a classical Mendelian manner as autosomal recessive congenital disorders, such as ADA-SCID, SBDS and Gaucher disease type III, may all be thoroughly verified of their genotypic characteristics after generation of respective iPS cells. As for Parkinson's disease and juvenile-onset type I diabetes mellitus, which are conditions that lack a defined genetic basis, genotypic verification is impossible at this time.94
5.2. Cancer-iPS cells
Miyoshi et al reprogrammed cancer cells of endodermal origin, including esophageal, stomach, colorectal, liver, pancreatic and cholangiocellular cancer cells.95 The reprogrammed cancer-iPS cells, termed pluripotent cancer (iPC) cells by the authors, possessed the potential to express morphological patterns of ectoderm, mesoderm and endoderm, but not parental cells. iPC cells showed slow proliferation and were sensitized to differentiation-inducing treatment, and in vivo tumorigenesis was reduced in NOD/SCID mice. Additionally, the tumor-suppressor gene P16(INK4A) was repressed in iPC cells while its expression increased in differentiated iPC cells. This study suggests that the reactivation of tumor suppressor genes by reprogramming may play a role in increased chemosensitivity to fluorouracil and the regression of cell proliferation and invasiveness under differentiation-inducing conditions.95
In the same vein, Carette et al asked the question of whether cancer cell lines can be reprogrammed into iPS cells. They tested KBM7 cells derived from blast crisis stage chronic myeloid leukemia. A near-diploid subclone of KBM7 cells carrying a Philadelphia translocation was used. They infected KBM7 cells with retroviruses expressing the four Yamanaka factors. The cancer-iPS cells formed teratomas containing tissues representing all three germ layers with structures resembling embryonal carcinoma.96 With these KBM7-iPS cells, the authors explored the mechanism of dependency of the cancer cells on BCR-ABL signaling. This dependency forms the basis for successful clinical suppression of disease by imatinib which inhibits the signaling. They found that in contrast to parental KBM7 cells, the two KBM7-iPS cell lines they generated were completely resistant to imatinib. Loss of oncogene addiction was observed in iPS cells but also in neuronal cells or fibroblast-like cells derived from one of the two iPS cell lines. KBM7-iPS cells could differentiate into hematopoietic lineages expressing CD34, CD43 and CD45. However, addition of imatinib reduced the number of cells that were positive for the hematopoietic markers. These experiments indicate that the process of reprogramming can abolish BCR-ABL dependency, which is restored by differentiation into the hematopoietic lineage.96
Taken together, iPS cells created from cancer cells can serve as a useful tool to understand the effectiveness of cancer drugs and to explore the molecular mechanisms in cancer development, especially those that control certain cancer signatures that are lost after reprogramming and reappear after differentiation into the specific cell lineage.
6. Current Issues on iPS for Therapeutic Use
6.1. Use of vectors
Forced expression of exogenous genes is often accomplished by delivering the genes by viral vectors. Stable integration of exogenous genes into the genome gives long-term effects. As in the generation of iPS cells, both vector systems used by Yamanaka's (retroviral vectors) and Thomson's (lentiviral vectors) groups successfully exerted their effects. However, integrating viral vectors with strong promoter driving the exogenous genes in the cells prevents their clinical applications. Interestingly, the exogenous genes carried by the retroviral vectors used by Yamanaka's group were downregulated when iPS cells were fully reprogrammed, whereas the exogenous genes carried by the lentiviral vectors generated by Thomson's group continued to express at various levels after iPS cells complete the reprogramming. The constant high levels of exogenous four factor gene expression do not seem to affect cell differentiation because teratomas were formed by these iPS cells, although preferential formation of certain types of tissues in the teratomas was observed.3 Whether this phenomenon affects other behaviors of these iPS cells requires further investigation.
Tremendous efforts have been made to deliver the defined factors without having viral vector integration. The approaches include transient expression using adenoviral or nonviral vectors,8,23 non-integrating episomal vectors,7 and removing the integrated vectors using piggyBac transposition or loxP/Cre-recombinase excisable viral vector system.4–6,8 Completely circumventing the use of vectors by delivery of recombinant protein-based four factors to generate iPS cells in the mouse and human systems has been reported.9,10 Unfortunately, the protein transduction method is extremely difficult and time-consuming at present. Further improvement of this approach is urgently needed.
Complete replacement of genes or gene products by chemicals is an alternative approach. Small molecule screening by an established mouse cell line carrying a reporter gene (e.g., Oct4-GFP) was able to filter out a list of chemicals that are able to induce pluripotency by substituting some of the four genes.12–14 Currently, only the chemicals that interfere with transforming growth factor-β signaling (RepSox or Alk5 inhibitor) is proven to be capable of replacing Sox2 and c-Myc.15,97,98 Although the evidence showing a total replacement of the four factors by chemicals has not been reported, it is anticipated that this may emerge in the future. It should be noted that chemical stimulation may cause unwanted side effects.
6.2. Difference between iPS and ES cells
The ground state of ES cells is considered as the gold standard for pluripotency. The mechanism underlying this ground state is being rigorously studied. While there are other means to acquire pluripotency, such as parthenogenesis, using defined factors to generate iPS cells is the most feasible means to generate pluripotent cells for clinical applications. Due to its simplicity, in terms of using just a few factors to turn the entire cellular state around, it is questionable as to whether iPS cells really reach a true ground state as ES cells. Generation of live pups from mouse iPS cells through a tetraploid-complementation approach implies that iPS cells are indeed very similar to ES cells. However, recent evidence showed that iPS cells conserve some “memory” of the original cells. Since iPS cell derivation involves epigenetic reprogramming, it is considered that this memory may be determined by the epigenetic status.
It is not clear at present whether iPS cells derived from different types of cells behave in the same manner. More importantly, iPS cells from different cell types may also be different in their abilities to undergo guided differentiation into specialized cells compared to ES cells.99 It has been noted that differences occur between the iPS cells generated from different cell types, e.g., mesenchymal versus endodermal origin, in terms of the kinetics of reprogramming and the outcomes of the generated chimeric mice.100 Doi et al analyzed the DNA methylation patterns on a genome-wide scale and found substantial hypermethylation and hypomethylation of cytosine-phosphate-guanine (CpG) island shores in nine human iPS cell lines compared to their parental fibroblasts.44 The differentially methylated regions (DMRs) in the reprogrammed cells (denoted R-DMRs) were significantly enriched in tissue-specific (T-DMRs) and cancer-specific (C-DMRs) DMRs. Although the iPS cells are derived from fibroblasts, their R-DMRs can distinguish between normal brain, liver and spleen cells and between colon cancer and normal colon cells. Many DMRs are broadly involved in tissue differentiation, epigenetic reprogramming and cancer.44 A recent paper by Kim et al has shown that iPS cells derived by factor-based reprogramming of adult murine tissues harbor residual DNA methylation signatures characteristic of their somatic tissue of origin, which favors their differentiation along lineages related to the donor cell, while restricting alternative cell fates.41 Polo et al confirmed this finding and further noted that the differences among iPS cells derived from different cell types diminished at passage 16 of miPS cells.42
These findings suggest the need for understanding the epigenetic characteristics of hiPS cells as one important aspect of iPS cell biology before they may be used for clinical applications. iPS cells should be generated from various easily accessible human tissues and characterized thoroughly. Because the dental stem cells derive from ectomesenchyme,101,102 it is possible that iPS cells derived from cells of this tissue have the propensity to differentiate into oral, craniofacial and especially neural and odontogenic tissues under the appropriate stimulus. Recent data from our laboratory showed that dental stem cell-derived iPS cells appear to form predominantly ectodermal neural tissues in teratomas (Figure 4).
Figure 4.
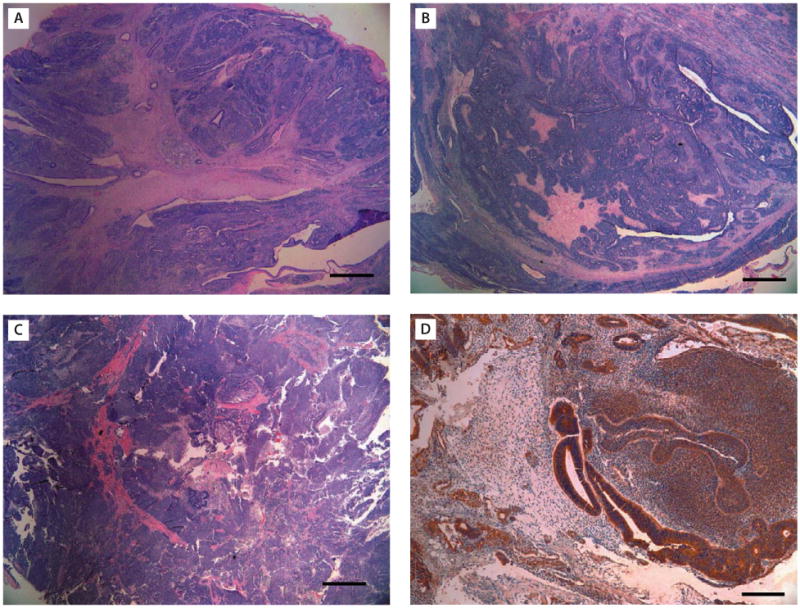
Histological analysis of teratomas derived from: (A, B) SCAP-iPS; and (C, D) SHED-iPS cells. Predominant primitive neural tissues, neural rosettes and retinal epithelium (ectoderm) is shown. (D) β-III-tubulin stain (brown color). [Scale bars: (A–C) 1 mm; (D) 200 μm.] (iPS cells at passages 11–17 were used for teratoma formation in this representative experiment.)
Fine tuning of reprogramming of iPS cells toward the gold standard pluripotent state is another important aspect in this field of research as evidence has shown that the standard three-/four-factor approach is not ideal. Han et al reported that transcription factor Tbx3 significantly improves the quality of iPS cells. iPS cells generated with Oct4/Sox2/Klf4 plus Tbx3 (OSKT) are superior in both germ-cell contribution to the gonads and germ-line transmission frequency. Global gene expression profiling, however, could not distinguish between OSK and OSKT iPS cells. This study suggests that intrinsic qualitative differences exist between iPS cells generated by different methods. Therefore, further studies not limited to in vitro settings to characterize iPS cells generated from different reprogramming approaches are needed.103
6.3. Feasible cell types for iPS cell generation
DFs are considered a feasible cell type to acquire. The procedures involve a 3–6 mm dermal biopsy punch from the arm or thigh and require 2–3 stitches leaving behind a scar. It is not a discarded tissue. A recent report demonstrated that fibroblasts from oral mucosa can be reprogrammed into iPS cells.36 For a similar purpose, oral tissue biopsy of the same size does not leave behind a scar (Figure 5). Skin keratinocytes can also be easily accessed and appear to be a good cell source to produce iPS cells.30 However, culturing keratinocytes is more technically sensitive and procedurally demanding than fibroblasts. Blood cell is another easily obtainable cell type for iPS cell generation. Again, it requires isolation of CD34 subpopulation for successful reprogramming.27 Other types of cells, although shown to be good cell sources for iPS generation, are not feasible to obtain, such as intestinal epithelial cells, hepatocytes, neural stem cells, and bone marrow cells. Therefore, considering the above analysis, oral mucosa fibroblasts appear to be the best cell source for iPS cell derivation in terms of accessibility and absence of scar formation postsurgically.
Figure 5.
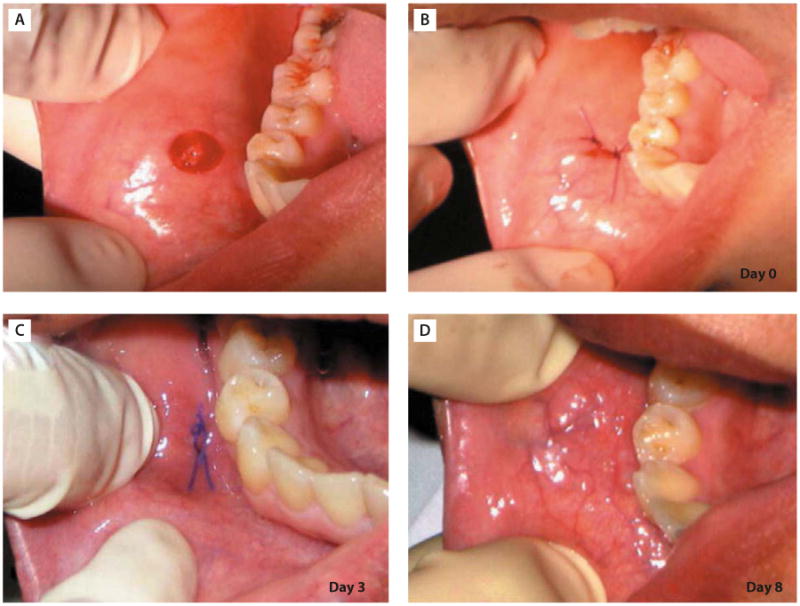
Excision of oral mucosal tissue by punch biopsy. (A) Buccal surface immediately after dissection of oral mucosal tissue from the cheek of the volunteer by punch biopsy. (B) Wound closure. The wound was closed by two stitches of suture (day 0). (C) Buccal surface 3 days after surgery. The wound healed almost completely without a scar. (D) Buccal surface 8 days after surgery. The wound healed completely and became indistinguishable from the surrounding tissue. (Adapted with permission from reference 36.)
There are also a number of discarded tissues/fluids regarded as biomedical waste that can serve as a source for iPS cell reprogramming, such as amniotic fluid, umbilical cord, foreskin, adipose tissues and shed or extracted teeth. Dental tissues derived from ectomesenchyme harbor mesenchymal-like stem/progenitor cells, including dental pulp stem cells (DPSCs), stem cells from exfoliated deciduous teeth (SHED), stem cells from apical papilla (SCAP), periodontal ligament stem cells and dental follicle precursor cells (DFPCs).88 Our laboratory has shown that DPSCs, SHED and SCAP can be reprogrammed into iPS cells at a higher rate than fibroblasts.35 Dental stem cells from teeth are mesenchymal-like stem cells and are different from other MSCs in many aspects.88 The frequency of colony-forming cells derived from dental pulp tissue (22–70 colonies/104 cells plated) is higher than that of MSCs from bone marrow (2.4–3.1 colonies/104 cells plated).104 They proliferate rapidly in culture with an average population doubling time of ∼20 hours105 and can reach a population doubling of up to 100 or more before senescence.106 In addition, from our unpublished work along with the reported, DPSCs and SHED already express a number of ES cell-associated genes such as c-Myc, Oct4, Nanog, SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81 at low levels,107,108 which may facilitate the reprogramming. SHED are from children shedding teeth around the ages of 6–12 years. SCAP and DPSCs are from third molars from young individuals aged 16–22 years. These age groups contain more immature cells suitable for reprogramming purposes.
7. Conclusion and Prospects
Without a doubt, the invention of iPS cells opened a new page for medicine. It is also a giant leap on a multitude of perspectives for cell and molecular biology. Using manageable defined factors instead of the abysmal number of nuclear and cytoplasmic factors in the oocyte needed to reprogram cells into pluripotency (nuclear transfer), it provides a simple starting point to ask the question as to how a somatic cell can be reversed to a pluripotent state. At present, little is known regarding the mechanisms involved during the reprogramming process.109 There is a large void in our knowledge base when attempting to investigate the underlying mechanisms. It is inevitable that the intracellular mechanisms of the reprogramming process needs to be studied. In the meantime, further characterization of iPS cells for the purpose of clinical applications is what is expected by the public and is the ultimate driving force for this field of research. To this end, the following goals must be reached: (1) generating fully reprogrammed iPS cells using effective non-vector approaches; (2) elucidating molecular mechanisms involved in the reprogramming process by defined factors; (3) genome-wide characterization of iPS cells derived from different cell types in comparison with ES cells genetically and epigenetically; (4) establishing reliable protocols to guide iPS cells toward differentiated cells for specific tissue regeneration and understanding the cause of variability among iPS cell clones during differentiation; and (5) determining the genomic stability of iPS cells in long-term cultures in order to address the safety issues in clinical applications.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health R01 DE019156-01 to George T.-J. Huang. The author wishes to thank Dr. Junying Yu (University of Wisconsin, Madison, WI, USA) for providing personal experience on the observation of iPS cells and our postdoctoral fellow Dr. Xing Yan for the work on dental iPS cells.
Footnotes
Disclosure Statement: There are no commercial associations that might create a conflict of interest in connection with this work.
References
- 1.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–77. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–70. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–5. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez F, Barragan Monasterio M, Tiscornia G, Montserrat Pulido N, Vassena R, Batlle Morera L, Rodriguez Piza I, et al. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc Natl Acad Sci USA. 2009;106:8918–22. doi: 10.1073/pnas.0901471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–4. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–6. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Comm. 2010;394:189–93. doi: 10.1016/j.bbrc.2010.02.150. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Desponts C, Do JT, Hahm HS, Scholer HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–74. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Do JT, Desponts C, Hahm HS, Scholer HR, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–8. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Lyssiotis CA, Foreman RK, Staerk J, Garcia M, Mathur D, Markoulaki S, Hanna J, et al. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci USA. 2009;106:8912–7. doi: 10.1073/pnas.0903860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, et al. A small-molecule inhibitor of Tgf-[beta] signaling replaces Sox2 in reprogramming by inducing Nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 18.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 19.Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol. 2008;18:890–4. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton Menno P, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–64. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, Ko K, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–9. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Arauzo-Bravo MJ, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–50. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 23.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–9. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 25.Kunisato A, Wakatsuki M, Kodama Y, Shinba H, Ishida I, Nagao K. Generation of induced pluripotent stem (iPS) cells by efficient reprogramming of adult bone marrow cells. Stem Cells Dev. 2010;19:229–38. doi: 10.1089/scd.2009.0149. [DOI] [PubMed] [Google Scholar]
- 26.Tsai SY, Clavel C, Kim S, Ang YS, Grisanti L, Lee DF, Kelley K, et al. Oct4 and Klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cells. 2010;28:221–8. doi: 10.1002/stem.281. [DOI] [PubMed] [Google Scholar]
- 27.Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC, Kim K, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–9. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotech. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 30.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotech. 2008;26:1276–84. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Zhou J, Shi G, Ma Y, Yang Y, Gu J, Yu H, et al. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum Mol Genet. 2009;18:4340–9. doi: 10.1093/hmg/ddp386. [DOI] [PubMed] [Google Scholar]
- 32.Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodríguez-Pizà I, Vassena R, Raya A, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. 2009;5:353–7. doi: 10.1016/j.stem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giorgetti A, Montserrat N, Rodriguez-Piza I, Azqueta C, Veiga A, Belmonte JCI. Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and Sox2. Nat Protocols. 2010;5:811–20. doi: 10.1038/nprot.2010.16. [DOI] [PubMed] [Google Scholar]
- 34.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci USA. 2009;106:15720–5. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GTJ. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010;19:469–80. doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyoshi K, Tsuji D, Kudoh K, Satomura K, Muto T, Itoh K, Noma T. Generation of human induced pluripotent stem cells from oral mucosa. J Biosci Bioeng. 2010;110:345–50. doi: 10.1016/j.jbiosc.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Kim JB, Greber B, Arauzo-Bravo MJ, Meyer J, Park KI, Zaehres H, Scholer HR. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–3. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 38.Kang L, Wang J, Zhang Y, Kou Z, Gao S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell. 2009;5:135–8. doi: 10.1016/j.stem.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, Hao J, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461:86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- 40.Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Gifford W, Martin G, Kupriyanov S, et al. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–4. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- 41.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–90. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–55. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–23. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, Herb B, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009;41:1350–3. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surani MA. Reprogramming of genome function through epigenetic inheritance. Nature. 2001;414:122–8. doi: 10.1038/35102186. [DOI] [PubMed] [Google Scholar]
- 46.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–3. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 47.Thuan NV, Kishigami S, Wakayama T. How to improve the success rate of mouse cloning technology. J Reprod Dev. 2010;56:20–30. doi: 10.1262/jrd.09-221a. [DOI] [PubMed] [Google Scholar]
- 48.Oh HJ, Hong SG, Park JE, Kang JT, Kim MJ, Kim MK, Kang SK, et al. Improved efficiency of canine nucleus transfer using roscovitine-treated canine fibroblasts. Theriogenology. 2009;72:461–70. doi: 10.1016/j.theriogenology.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Gómez MC, Pope CE, Dresser BL. Nuclear transfer in cats and its application. Theriogenology. 2006;66:72–81. doi: 10.1016/j.theriogenology.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Miyashita N, Shiga K, Yonai M, Kaneyama K, Kobayashi S, Kojima T, Goto Y, et al. Remarkable differences in telomere lengths among cloned cattle derived from different cell types. Biol Reprod. 2002;66:1649–55. doi: 10.1095/biolreprod66.6.1649. [DOI] [PubMed] [Google Scholar]
- 51.Yu J, Vodyanik MA, He P, Slukvin II, Thomson JA. Human embryonic stem cells reprogram myeloid precursors following cell-cell fusion. Stem Cells. 2006;24:168–76. doi: 10.1634/stemcells.2005-0292. [DOI] [PubMed] [Google Scholar]
- 52.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–73. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 53.Revazova ES, Turovets NA, Kochetkova OD, Kindarova LB, Kuzmichev LN, Janus JD, Pryzhkova MV. Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;9:432–49. doi: 10.1089/clo.2007.0033. [DOI] [PubMed] [Google Scholar]
- 54.Revazova ES, Turovets NA, Kochetkova OD, Agapova LS, Sebastian JL, Pryzhkova MV, Smolnikova VI, et al. HLA homozygous stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2008;10:11–24. doi: 10.1089/clo.2007.0063. [DOI] [PubMed] [Google Scholar]
- 55.Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–7. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 56.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 57.Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–12. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Do JT, Schöler HR. Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells. 2004;22:941–9. doi: 10.1634/stemcells.22-6-941. [DOI] [PubMed] [Google Scholar]
- 59.Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460:49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- 60.Chan EM, Ratanasirintrawoot S, Park IH, Manos PD, Loh YH, Huo H, Miller JD, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–7. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- 61.Smith KP, Luong MX, Stein GS. Pluripotency: toward a gold standard for human ES and iPS cells. J Cell Physiol. 2009;220:21–9. doi: 10.1002/jcp.21681. [DOI] [PubMed] [Google Scholar]
- 62.Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–9. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 64.Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11:1553–8. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 65.Sumer H, Jones KL, Liu J, Heffernan C, Tat PA, Upton KR, Verma PJ. Reprogramming of somatic cells after fusion with induced pluripotent stem cells and nuclear transfer embryonic stem cells. Stem Cells Dev. 2010;19:239–46. doi: 10.1089/scd.2009.0142. [DOI] [PubMed] [Google Scholar]
- 66.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 67.Fulka J, Miyashita N, Nagai T, Ogura A. Do cloned mammals skip a reprogramming step? Nat Biotech. 2004;22:25–6. doi: 10.1038/nbt0104-25. [DOI] [PubMed] [Google Scholar]
- 68.Shiels PG, Kind AJ, Campbell KHS, Wilmut I, Waddington D, Colman A, Schnieke AE. Analysis of telomere length in Dolly, a sheep derived by nuclear transfer. Cloning. 1999;1:119–25. doi: 10.1089/15204559950020003. [DOI] [PubMed] [Google Scholar]
- 69.Shiels PG, Jardine AG. Dolly, no longer the exception: telomeres and implications for transplantation. Cloning Stem Cells. 2003;5:157–60. doi: 10.1089/153623003322234768. [DOI] [PubMed] [Google Scholar]
- 70.Marion RM, Strati K, Li H, Tejera A, Schoeftner S, Ortega S, Serrano M, et al. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009;4:141–54. doi: 10.1016/j.stem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Agarwal S, Loh YH, McLoughlin EM, Huang J, Park IH, Miller JD, Huo H, et al. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature. 2010;464:292–6. doi: 10.1038/nature08792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci. 2008;105:5856–61. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai J, Yang M, Poremsky E, Kidd S, Schneider JS, Iacovitti L. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem Cells Dev. 2010;19:1017–23. doi: 10.1089/scd.2009.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335–40. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–11. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–67. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng Q, Lu SJ, Klimanskaya I, Gomes I, Kim D, Chung Y, Honig GR, et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28:704–12. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- 78.Chang KH, Huang A, Hirata RK, Wang PR, Russell DW, Papayannopoulou T. Globin phenotype of erythroid cells derived from human induced pluripotent stem cells. Blood. 2010;115:2553–4. doi: 10.1182/blood-2009-11-252650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kulkeaw K, Horio Y, Mizuochi C, Ogawa M, Sugiyama D. Variation in hematopoietic potential of induced pluripotent stem cell lines. Stem Cell Rev. 2010;6:381–9. doi: 10.1007/s12015-010-9150-5. [DOI] [PubMed] [Google Scholar]
- 80.Mizuno Y, Chang H, Umeda K, Niwa A, Iwasa T, Awaya T, Fukada S, et al. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J. 2010;24:2245–53. doi: 10.1096/fj.09-137174. [DOI] [PubMed] [Google Scholar]
- 81.Gallicano GI, Mishra L. Hepatocytes from induced pluripotent stem cells: a giant leap forward for hepatology. Hepatology. 2010;51:20–2. doi: 10.1002/hep.23474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, Dalgetty D, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329–35. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–3. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 85.Raya A, Rodriguez-Piza I, Guenechea G, Vassena R, Navarro S, Barrero MJ, Consiglio A, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460:53–9. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolf C, Cho E, Tuan R. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim J, Lee Y, Kim H, Hwang KJ, Kwon HC, Kim SK, Cho DJ, et al. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Peppo GM, Sjovall P, Lenneras M, Strehl R, Hyllner J, Thomsen P, Karlsson C. Osteogenic potential of human mesenchymal stem cells and human embryonic stem cell-derived mesodermal progenitors: a tissue engineering perspective. Tissue Eng Part A. 2010 Aug 2; doi: 10.1089/ten.TEA.2010.0052. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 90.Cohen S, Leshansky L, Zussman E, Burman M, Srouji S, Livne E, Abramov N, et al. Repair of full-thickness tendon injury using connective tissue progenitors efficiently derived from human embryonic stem cells and fetal tissues. Tissue Eng Part A. 2010 Aug 28; doi: 10.1089/ten.TEA.2009.0716. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 91.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–6. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lengner CJ. iPS cell technology in regenerative medicine. Ann N Y Acad Sci. 2010;1192:38–44. doi: 10.1111/j.1749-6632.2009.05213.x. [DOI] [PubMed] [Google Scholar]
- 93.Nishikawa SI, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–9. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 94.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miyoshi N, Ishii H, Nagai K, Hoshino H, Mimori K, Tanaka F, Nagano H, et al. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc Natl Acad Sci USA. 2010;107:40–5. doi: 10.1073/pnas.0912407107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carette JE, Pruszak J, Varadarajan M, Blomen VA, Gokhale S, Camargo FD, Wernig M, et al. Generation of iPSCs from cultured human malignant cells. Blood. 2010;115:4039–42. doi: 10.1182/blood-2009-07-231845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solanki A, Lee KB. A step closer to complete chemical reprogramming for generating iPS cells. Chembiochem. 2010;11:755–7. doi: 10.1002/cbic.201000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maherali N, Hochedlinger K. Tgf[beta] signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr Biol. 2009;19:1718–23. doi: 10.1016/j.cub.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–7. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 100.Sridharan R, Plath K. Illuminating the black box of reprogramming. Cell Stem Cell. 2008;2:295–7. doi: 10.1016/j.stem.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 101.Cobourne MT, Sharpe PT. Tooth and jaw: molecular mechanisms of patterning in the first branchial arch. Arch Oral Biol. 2003;48:1–14. doi: 10.1016/s0003-9969(02)00208-x. [DOI] [PubMed] [Google Scholar]
- 102.Chai Y, Jiang X, Ito Y, Bringas P, Han J, Rowitch DH, Soriano P, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–9. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 103.Han J, Yuan P, Yang H, Zhang J, Soh BS, Li P, Lim SL, et al. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 2010;463:1096–100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang GT, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–73. doi: 10.1016/j.joen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 106.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang AH, Chen YK, Lin LM, Shieh TY, Chan AW. Isolation and characterization of dental pulp stem cells from a supernumerary tooth. J Oral Pathol Med. 2008;37:571–4. doi: 10.1111/j.1600-0714.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 108.Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–16. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 109.Scheper W, Copray S. The molecular mechanism of induced pluripotency: a two-stage switch. Stem Cell Rev. 2009;5:204–23. doi: 10.1007/s12015-009-9077-x. [DOI] [PubMed] [Google Scholar]


