Abstract
Objective
To investigate the correlations between aqueous concentrations of interleukin 1β, 6, 8, 10, 12p (IL-1β, IL-6, IL-8, IL-10, IL-12p), and tumor necrosis factor α (TNF-α) and the parameters of macular edema acquired by optical coherence tomography (OCT) in patients with choroidal neovascularization.
Methods
IL-1β, IL-6, IL-8, IL-10, IL-12p, and TNF-α in the aqueous humor samples of 17 patients with exudative age-related macular degeneration (AMD), ten patients with pathological myopia (PM), seven patients with idiopathic choroidal neovascularization (CNV), and 14 patients with cataract and idiopathic epiretinal membrane or macular hole in the control group were measured with cytometric bead array. The maximum macular thickness and macular volume within 1 mm, 3 mm, and 6 mm were measured with OCT.
Results
In the CNV groups, the aqueous levels of IL-6 and IL-8 were significantly associated with macular volume within 6 mm (p=0.011, p=0.008, respectively), while IL-1β, IL-10, IL-12p, and TNF-α showed no significant correlation with either the maximum macular thickness or the macular volume. By further selecting patients with CNV who had accepted their last intravitreal injection of bevacizumab within 3 months, the level of IL-6 still significantly correlated with the maximum macular thickness (p=0.019) and macular volume within 1 mm (p=0.018), 3 mm (p=0.018), and 6 mm (p=0.022). In patients with exudative AMD, the level of IL-6 was significantly associated with the maximum macular thickness (p=0.025) and macular volume within 1 mm (p=0.025), 3 mm (p=0.006), and 6 mm (p=0.002). The aqueous level of all cytokines did not vary significantly between the CNV patients who had accepted their last intravitreal injection of bevacizumab within 3 months and the other patients, nor was a difference found among patients with exudative AMD, PM, and idiopathic CNV, and the control group.
Conclusions
Intraocular concentrations of IL-6 and IL-8 (particularly IL-6) are significantly associated with the volume of macular edema in patients with CNV. However, intravitreal injection of antivascular endothelial growth factor drugs did not change the intraocular level of these inflammation cytokines.
Introduction
Choroidal neovascularization (CNV), either idiopathic CNV [1], exudative age-related macular degeneration (AMD) [2], or secondary to pathological myopia (PM) [3], is one of the main causes of vision impairment throughout the world.
Vascular endothelial growth factor (VEGF), first discovered as a vasopermeability factor [4], has been reported to be associated with CNV. Many clinical trials have showed encouraging outcomes for intravitreal injections of anti-VEGF drugs for managing CNV [5-7]. Some authors even recommend anti-VEGF as first-line treatment for some types of CNV [8-10]. However, in addition to anti-VEGF pharmacotherapy, intravitreal administered anti-inflammatory substances, such as triamcinolone acetonide (TA) [11-13], a widely used anti-inflammatory drug, and infliximab [14], an antibody of tumor necrosis factor α (TNF- α), have also shown positive effects in treating CNV in patients and animal models. Therefore, in the era of anti-VEGF, one may postulate that investigating the role of inflammatory factors in the development of CNV has become more prominent.
In this study, we explored the relationship between levels of inflammatory cytokines in aqueous humor of patients with CNV after and not after recent anti-VEGF therapy and the parameters of the macula acquired with optical coherence tomography (OCT).
Methods
This study included 17 patients (17 eyes) with exudative AMD, ten patients (ten eyes) with pathological myopia (PM), seven patients (seven eyes) with idiopathic choroidal neovascularization (CNV) who underwent intravitreal injection of bevacizumab (Avastin, Genentech Inc., San Francisco, CA) as the study group, and 14 patients (14 eyes) with cataract and idiopathic epiretinal membrane or a macular hole who underwent combined cataract and vitrectomy surgery (CCVS) as the control group. The patients were consecutive in each group. The inclusion criterion in the study group was the presence of active CNV. Exclusion criteria included glaucoma, previous photodynamic therapy, and other retinal diseases, such as diabetic retinopathy and retinal vascular occlusion.
All patients underwent an ophthalmic examination including best-corrected visual acuity (BCVA) recording using manifest refraction and the logMAR visual acuity chart, non-contact tonometry, slit lamp assisted biomicroscopy of the anterior segment and posterior segment of the eye, fundus fluorescein angiography (FFA), and OCT (Optovue OCT-IV, Optovue Inc., Fremont, CA). All patients in the study group showed leakage on fluorescein angiographies (active CNV), and they were further divided into two subgroups according to the interval between the last intravitreal injection of bevacizumab (IVB) and this time (>3 month group, more than 3 months, or <3 month group, less than 3 months). OCT was analyzed by one investigator, and the maximum macular thickness and macular volume within 1 mm, 3 mm, and 6 mm were measured using built-in software. The OCT measurements were converted from millimeters to proportionally corresponding micrometers.
Aqueous humor was collected during intravitreal injection or CCVS. All procedures conformed to the Declaration of Helsinki for research involving human subjects. Informed consent was obtained from all participants. Undiluted aqueous humor samples (100–200 µl) were obtained through anterior chamber paracentesis. All injections and sample collections were performed using a standard sterilization procedure that included the use of topical povidone-iodine and levofloxacin drops. Samples were stored in a sterilized plastic Corning (2 ml, Corning Inc., NY) at −70 °C until use.
The concentration of aqueous interleukin 1β, 6, 8, 10, 12p (IL-1β, IL-6, IL-8, IL-10, IL-12p) and TNF-α were measured with cytometric bead array (CBA), a method for capturing a soluble analyte or set of analytes with beads of known size and fluorescence, making it possible to detect analytes using flow cytometry. Each capture bead has been conjugated with a specified antibody. The detection reagent is a mixture of phycoerythrin (PE)-conjugated antibodies, which provides a fluorescent signal in proportion to the amount of bound analyte. When capture beads and detector reagent are incubated with an unknown sample containing recognized analytes, sandwich complexes (capture bead+analyte+detection reagent) are formed that can be measured using flow cytometry (BD FACSCalibur, BD Bioscience, San Jose, CA) to identify particles with the fluorescence characteristics of the bead and detector. Six bead population with distinct fluorescence intensities coated with specific capture antibodies for IL-1β, IL-6, IL-8, IL-10, IL-12p, and TNF-α were mixed together to form the bead array. Thus, the concentration of each cytokine in one unknown sample can be calculated from regressive analysis of the standard curve simultaneously. In this study, 50 μl of undiluted aqueous humor sample was used for each test. Quality control procedures were performed according to the CBA kit instructions.
The Pearson correlation test was used to explore the relationship between the aqueous level of a cytokine and the OCT parameters (Statistical Package for Social Sciences [SPSS], ver. 17.0; SPSS, Chicago, IL). Parameters were compared using the Mann–Whitney U test between two groups and the Kruskal–Wallis H test to compare variables among various groups. The chi-square test and the Fisher exact t test were used to compare noncontinuous variables. Values were reported as mean±standard deviation (SD). Statistical significance was set at p<0.05 for two-tail analysis.
Results
A total of 48 aqueous humor samples from 48 patients were collected (Table 1). The age of AMD and control group was significantly older than the other two groups (p<0.001), while gender, BCVA and IOP did not significantly vary among groups (all p>0.05).
Table 1. Composition of the study population (mean±SD).
| Control | Exudative AMD | PM | Idiopathic CNV | p-value | |
|---|---|---|---|---|---|
| Number |
14 |
17 |
10 |
7 |
|
| Age (years) |
62.4±14.5 |
68.6±12 |
49.4±10 |
33.7±10.4 |
<0.001* |
| Female-no (%) |
11 (79%) |
5 (29.4%) |
5(50%) |
4 (57%) |
0.057** |
| BCVA |
0.2±0.13 |
0.24±0.19 |
0.20±0.23 |
0.36±0.36 |
0.787* |
| IOP (mmHg) | 14.3±2.6 | 13.6±3.0 | 12.3±2.6 | 14.86±3.08 | 0.313* |
*Kruskal–Wallis H test, compared among control group, exudative AMD, PM and idiopathic CNV groups. **Fisher’s exact t-test, compared among control group, exudative AMD, PM and idiopathic CNV groups. AMD, age-related macular degeneration; PM, pathological myopia; CNV, choroidal neovascularization; BCVA, best corrected visual acuity; IOP, intraocular pressure.
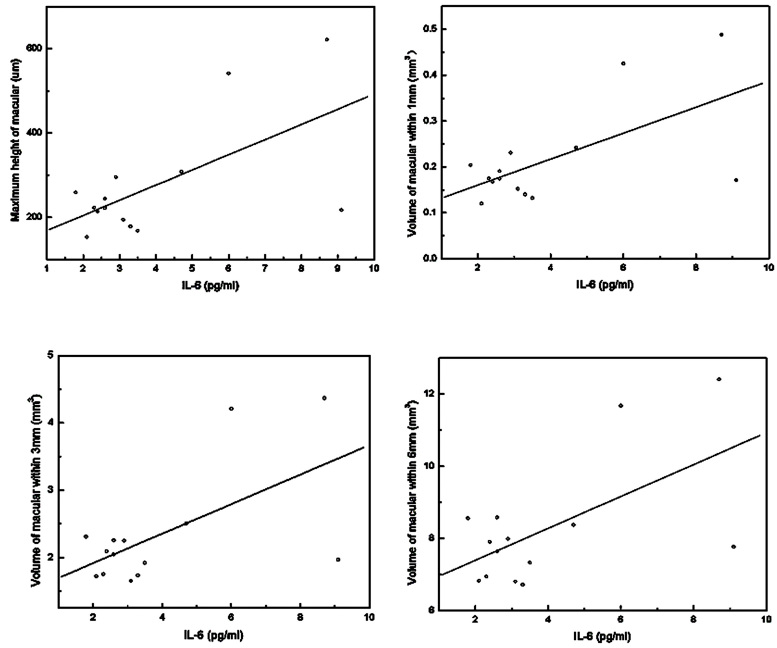
In the CNV group, the aqueous levels of IL-6 and IL-8 were significantly associated with macular volume within 6 mm (PIL-6=0.011, R2=0.176, PIL-8=0.008, R2=0.187; Table 2), while IL-1β, IL-10, IL-12p, and TNF-α showed no significant correlation with the maximum macular thickness or macular volume within 1 mm, 3 mm, or 6 mm (all p>0.05). Moreover, in the CNV group who had accepted their last IVB within the last 3 months (<3 months), the level of IL-6 correlated significantly with the maximum macular thickness and macular volume within 1 mm (p=0.019, R2=0.382), 3 mm (p=0.018, R2=0.382), and 6 mm (p=0.022, R2=0.366; Table 2, Figure 1). However, in the CNV group who had accepted their last IVB more than 3 months before or never (>3 months), no significant correlation was found between the cytokine concentrations and the macular parameters (all p>0.05).
Table 2. P values of each Pearson correlation test between level of cytokines and OCT parameters in CNV groups.
|
CNV |
<3 mon |
>3 mon |
||||||||||
|
Cytokines |
MMT |
MV
(1 mm) |
MV
(3 mm) |
MV
(6 mm) |
MMT |
MV
(1 mm) |
MV
(3 mm) |
MV
(6 mm) |
MMT |
MV
(1 mm) |
MV
(3 mm) |
MV
(6 mm) |
| IL-1b |
0.614 |
0.134 |
0.331 |
0.186 |
0.951 |
0.950 |
0.981 |
0.851 |
0.373 |
0.071 |
0.057 |
0.052 |
| IL-6 |
0.063 |
0.393 |
0.094 |
0.011* |
0.019* |
0.018* |
0.018* |
0.022* |
0.640 |
0.989 |
0.809 |
0.549 |
| IL-8 |
0.074 |
0.212 |
0.081 |
0.008* |
0.517 |
0.515 |
0.950 |
0.901 |
0.195 |
0.870 |
0.219 |
0.066 |
| IL-10 |
0.602 |
0.816 |
0.399 |
0.120 |
0.117 |
0.119 |
0.184 |
0.079 |
0.350 |
0.628 |
0.727 |
0.935 |
| IL-12p |
0.851 |
0.443 |
0.936 |
0.898 |
0.487 |
0.487 |
0.591 |
0.519 |
0.924 |
0.172 |
0.700 |
0.631 |
| TNF-α | 0.051 | 0.185 | 0.309 | 0.480 | 0.225 | 0.225 | 0.434 | 0.560 | 0.150 | 0.437 | 0.503 | 0.644 |
CNV, choroidal neovascularization group; <3mon, patients who receive last IVB within 3 months; >3mon, patients who receive last IVB for more than 3 months and never; MMT, maximum macular thickness; MV, volume of macula; IL, interleukin; TNF, tumor necrosis factor.
Figure 1.
Interleukin (IL)-6 associated significantly with maximum macular thickness (p=0.019, R2=0.381), volume of macula within 1 mm (p=0.018, R2=0.382), 3 mm (p=0.018, R2=0.382) and 6 mm (p=0.022, R2=0.366) in choroidal neovascularization (CNV) group who accepted IVB within 3 months.
In exudative AMD (Table 3), the level of IL-6 was also significantly associated with the maximum macular thickness (p=0.025, R2=0.258) and macular volume within 1 mm (p=0.025, R2=0.258), 3 mm (p=0.006, R2=0.386), and 6 mm (p=0.002, R2=0.461).
Table 3. P values of each Pearson correlation test between level of cytokines and OCT parameters in exudative AMD.
| Cytokines | MMT | MV (1 mm) | MV (3 mm) | MV (6 mm) |
|---|---|---|---|---|
| IL-1b |
0.730 |
0.730 |
0.888 |
0.922 |
| IL-6 |
0.025* |
0.025* |
0.006* |
0.002* |
| IL-8 |
0.150 |
0.152 |
0.110 |
0.087 |
| IL-10 |
0.306 |
0.309 |
0.174 |
0.092 |
| IL-12p |
0.535 |
0.534 |
0.588 |
0.408 |
| TNF-α | 0.123 | 0.122 | 0.2414 | 0.128 |
MMT, maximum macular thickness; MV, volume of macula; IL, interleukin; TNF, tumor necrosis factor.
The aqueous level of all cytokines investigated in this study showed no significant difference between the patients with CNV who had accepted their last IVB within the last 3 months (<3 months), patients with CNV who had accepted their last IVB more than 3 months before or never (>3 months), and the control group (all p>0.05, Table 4). Moreover, no difference was found among the patients with exudative AMD, PM, and idiopathic CNV compared to the control group, either (all p>0.05, Table 4).
Table 4. Levels of cytokines in CNV and control group (mean±SD).
| Cytokines | <3 mon | >3 mon | Control | P* | Exudative AMD | PM | Idiopathic CNV | p* |
|---|---|---|---|---|---|---|---|---|
| IL-1b |
1.98±0.28 |
1.99±0.23 |
1.94±0.20 |
0.672 |
2.1±0.3 |
1.9±0.2 |
2.0±0.2 |
0.287 |
| IL-6 |
3.94±2.37 |
4.49±3.09 |
4.47±2.07 |
0.610 |
3.6±1.8 |
4.9±2.0 |
3.1±1.5 |
0.124 |
| IL-8 |
4.18±1.22 |
5.52±2.59 |
5.51±2.90 |
0.118 |
4.5±1.5 |
4.9±0.8 |
4.3±1.0 |
0.145 |
| IL-10 |
1.82±0.23 |
1.85±0.25 |
1.90±0.17 |
0.257 |
1.8±0.3 |
1.9±0.1 |
1.8±0.3 |
0.258 |
| IL-12p |
2.08±0.19 |
2.19±0.31 |
2.19±0.27 |
0.444 |
2.2±0.3 |
2.2±0.2 |
2.1±0.3 |
0.755 |
| TNF-α | 1.90±0.22 | 1.85±0.27 | 1.95±0.28 | 0.507 | 1.9±0.2 | 1.8±0.2 | 2.0±0.4 | 0.621 |
*Kruskal–Wallis H test was used. CNV, choroidal neovascularization group; <3mon, patients who receive last IVB within 3 months; >3mon, patients who receive last IVB for more than 3 months and never; Control, patients with cataract and idiopathic epiretinal membrane or macular hole who underwent combined cataract and vitrectomy surgery; AMD, age-related macular degeneration; PM, pathological myopia; CNV, choroidal neovascularization; IL, interleukin; TNF, tumor necrosis factor.
Discussion
Although CNV is not a classic inflammatory disease like uveitis, much evidence from anatomic studies [15,16] and molecular experiments [17-19] has supported the hypothesis that inflammation plays an important role in disease pathogenesis and progression. Innate immunity and autoimmune components are believed to be heavily involved in CNV development. Macrophage, lymphocytes, and neutrophils have been found to be important in animal models and CNV patients [20-22]. IL-6 is a cytokine derived from activated T lymphocytes with multiple functions, including induction of B-cell growth, induction of B-cell differentiation and antibody production, induction of differentiation and proliferation of T cells, synergistic induction with IL-3 of hematopoietic cell growth, and induction of hepatocyte secretion of acute-phase inflammatory proteins [23,24]. IL-8 is a group of peptides produced by various types of cell, which activate and recruit polymorphonuclear leukocytes in acute and chronic inflammatory process, and are probably involved in initiating labor and delivery in pregnant women [25,26]. Both were found elevated in the synovial fluid of patients who had rheumatoid arthritis. This evidence suggested the role of cytokines in inflammatory diseases [27].
Our results indicated that the aqueous level of IL-6 and IL-8 was significantly associated with the macular volume within 6 mm in patients with CNV, and the aqueous level of IL-6 was significantly associated with the maximum macular thickness and macular volume within 1 mm, 3 mm, and 6 mm in patients who had received IVB within the last 3 months. This implied that IL-6 and IL-8, especially IL-6, not only participate in the process of CNV but also may be another target molecule in treating idiopathic CNV, exudative AMD, and CNV secondary to PM. Furthermore, a macular volume within 6 mm could also be an indicator of the aqueous level of these cytokines in the follow-up process, especially for patients who had accepted IVB within the last 3 months. We also found that the concentration of IL-6 had a linear correlation with the maximum height of macula and macular volume within 1 mm, 3 mm, and 6 mm in exudative AMD. Thus, anti-IL-6 therapy may be further considered in this anti-VEGF era.
Our results also suggested that the aqueous level of cytokines investigated in this study did not significantly vary between patients with CNV who had undergone IVB within the last 3 months and the other patients with CNV. This may explain why some patients were resistant to anti-VEGF therapy. IVB could downregulate only the VEGF concentration, not other cytokines such as IL-6 and IL-8. Anti-inflammatory agents may still be necessary for those patients after failed anti-VEGF therapy.
We could not find any significant correlation between the concentrations of IL-1β, IL-10, IL-12p, and TNF-α and any OCT parameters in any group in this study, which suggested that their role in the progress of CNV may be not as pivotal as that of anti-IL-6 and anti-IL-8.
Our results were in agreement with several previous studies. Higgins [28] found that the response to reactive oxygen species in the photoreceptor outer segments of inflammatory injury, commonly believed to be an initiation of AMD, could lead to increased expression and secretion of IL-8. Goverdhan [29] also suggested that the −251A allele (rs4073) of the IL-8 promoter is more prevalent in AMD cases than controls. Izumi-Nagai et al. [30] found that the IL-6 receptor mediated activation of the signal transducer and activator of transcription-3 promotes CNV generation and the IL-6 receptor blockade could inhibit in vivo and in vitro expression of inflammation-related molecules such as monocyte chemotactic protein, intercellular adhesion molecule-1, VEGF, and macrophage infiltration, which then could be a therapeutic strategy to suppress CNV-associated AMD.
There were limitations in this study. First, the selection of the control group may have caused selection bias, since the aqueous level of all cytokines investigated in this study did not vary significantly between the CNV group and the control group (all p>0.05). Patients with idiopathic epiretinal membrane or a macular hole [31-35] were the preferred control in most previous cytokine research of fundus diseases. However, a slight inflammation process may also potentially be involved in the formation of idiopathic epiretinal membrane or a macular hole; therefore, we have to acknowledge that the aqueous level of all the cytokines investigated in this study of patients with CNV may possibly differ from that of normal cataract patients without epiretinal membrane or a macular hole. Further study will be conducted to evaluate this issue. Second, the number of enrolled patients was not large, which may cover some minor significant association between factors. With a larger number of study participants, the correlations between the aqueous concentrations of the cytokines and the macular thickness or macular volume may become statistically significant. Third, only six cytokines were detected in this study, which fail to represent the main pathological mechanism involved in the development of CNV. Fourth, the aqueous humor samples may be not as valuable as vitreous fluid for detecting cytokine concentrations for fundus diseases. However, obtaining vitreous samples needs to expand the surgical intervention while CNV was not the surgical indication of vitrectomy for most cases.
A previous study investigated the relationship of recurrent CNV and cytokines, but this study may be the first one that states the association between cytokines and CNV volume, suggesting their importance. In conclusion, the aqueous level of IL-6 is positively related to the maximum macular thickness and macular volume within 1 mm, 3 mm, and 6 mm in patients with CNV who had undergone their last IVB within the last 3 months. Intravitreal injection of anti-VEGF drugs did not change the intraocular level of inflammation cytokines, including IL-1β, IL-6, IL-8, IL-10, IL-12p, and TNF-α. Furthermore, IL-6 is positively related to these OCT parameters in exudative AMD. IL-6 and IL-8, especially the former, may be additional target molecules in therapy for CNV.
Acknowledgments
Dr. Yong Tao (drtaoyong@163.com) and Dr. Xiao-xin Li contributed equally to the conduct of this research and are to be considered co-corresponding authors. This study was supported by National Basic Research Program of China (973 Program, No:2011CB510200), National Natural Science Foundation of China (No:30901639), an EFSD/CDS/Lilly grant (No:90561) and Beijing Novel Program (No:2009B04).
References
- 1.Miller DG, Singerman LJ. Vision loss in younger patients: a review of choroidal neovascularization. Optom Vis Sci. 2006;83:316–25. doi: 10.1097/01.opx.0000216019.88256.eb. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 3.Voykov B, Ziemssen F. Myopic CNV. Klin Monbl Augenheilkd. 2011;228:762–70. doi: 10.1055/s-0031-1281583. [DOI] [PubMed] [Google Scholar]
- 4.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–80. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 5.Wolf S, Holz FG, Korobelnik JF, Lanzetta P, Mitchell P, Prünte C, Schmidt-Erfurth U, Weichselberger A, Hashad Y. Outcomes following three-line vision loss during treatment of neovascular age-related macular degeneration: subgroup analyses from MARINA and ANCHOR. Br J Ophthalmol. 2011;95:1713–8. doi: 10.1136/bjophthalmol-2011-300471. [DOI] [PubMed] [Google Scholar]
- 6.Chang TS, Bressler NM, Fine JT. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 2007;125:1460–9. doi: 10.1001/archopht.125.11.1460. [DOI] [PubMed] [Google Scholar]
- 7.Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess. 2008;12:iii–iv. doi: 10.3310/hta12160. [DOI] [PubMed] [Google Scholar]
- 8.Koh A, Lim TH, Au Eong KG. Optimising the management of choroidal neovascularisation in Asian patients: consensus on treatment recommendations for anti-VEGF therapy. Singapore Med J. 2011;52:232–40. [PubMed] [Google Scholar]
- 9.Ouhadj O, Bouarfa A, Akel S. Intravitreal ranibizumab injections for the treatment of choroidal neovascularization complicating high myopia. J Fr Ophtalmol. 2010;33:649–54. doi: 10.1016/j.jfo.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Heimes B, Lommatzsch A, Zeimer M. Anti-VEGF therapy of exudative AMD: Prognostic factors for therapy success. Ophthalmologe. 2011;108:124–31. doi: 10.1007/s00347-010-2210-z. [DOI] [PubMed] [Google Scholar]
- 11.Tao Y, Jonas JB. Intravitreal triamcinolone. Ophthalmologica. 2011;225:1–20. doi: 10.1159/000317909. [DOI] [PubMed] [Google Scholar]
- 12.Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121:57–61. [PubMed] [Google Scholar]
- 13.Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–24. doi: 10.1016/j.ophtha.2003.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Olson JL, Courtney RJ, Mandava N. Intravitreal infliximab and choroidal neovascularization in an animal model. Arch Ophthalmol. 2007;125:1221–4. doi: 10.1001/archopht.125.9.1221. [DOI] [PubMed] [Google Scholar]
- 15.Hageman GS, Luthert PJ, Chong VNH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune mediated processes at the RPE–Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 16.Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in drusen formation and age-related macular degeneration. Exp Eye Res. 2001;73:887–96. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 17.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 18.Dastgheib K, Green W. Granulomatous reaction to Bruch’s membrane in age-related macular degeneration. Arch Ophthalmol. 1994;112:813–8. doi: 10.1001/archopht.1994.01090180111045. [DOI] [PubMed] [Google Scholar]
- 19.Penfold PL, Killingsworth M, Sarks S. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 20.Killingsworth MC, Sarks J, Sarks S. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye (Lond) 1990;4:613–21. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 21.Jones LL, Vignali DA. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol Res. 2011;51:5–14. doi: 10.1007/s12026-011-8209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka T, Narazaki M, Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y, Wang D, Wang X. Role of CCR2 and IL-8 in acute lung injury: a new mechanism and therapeutic target. Expert Rev Respir Med. 2011;5:107–14. doi: 10.1586/ers.10.80. [DOI] [PubMed] [Google Scholar]
- 24.Szarka A, Rigo J, Jr, Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause ML, Davis JM, 3rd, Knutson KL. Assessing immune function by profiling cytokine release from stimulated blood leukocytes and the risk of infection in rheumatoid arthritis. Clin Immunol. 2011;141:67–72. doi: 10.1016/j.clim.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green WR, Key S. Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc. 1977;75:180–254. [PMC free article] [PubMed] [Google Scholar]
- 27.Grindle CFJ, Marshall J. Ageing changes in Bruch’s membrane and their functionalimplications. Trans Ophthalmol Soc U K. 1978;98:172–5. [PubMed] [Google Scholar]
- 28.Higgins GT, Wang JH, Dockery P. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Invest Ophthalmol Vis Sci. 2003;44:1775–82. doi: 10.1167/iovs.02-0742. [DOI] [PubMed] [Google Scholar]
- 29.Goverdhan SV, Ennis S, Hannan SR. Interleukin 8 promoter polymorphism −251A/T is a risk factor for age related macular degeneration. Br J Ophthalmol. 2008;92:537–40. doi: 10.1136/bjo.2007.123190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izumi-Nagai K, Nagai N, Ozawa Y. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol. 2007;170:2149–58. doi: 10.2353/ajpath.2007.061018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehlken C, Rennel ES, Michels D, Grundel B, Pielen A, Junker B, Stahl A, Hansen LL, Feltgen N, Agostini HT, Martin G. Levels of VEGF but not VEGF(165b) are increased in the vitreous of patients with retinal vein occlusion. Am J Ophthalmol. 2011;152:298–303. doi: 10.1016/j.ajo.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 32.Yan H, Cui J, Yu JG. The expression of vascular endothelial growth factor of vitreous in patients with proliferative diabetic retinopathy. Zhonghua Yan Ke Za Zhi. 2009;45:206–9. [PubMed] [Google Scholar]
- 33.Asato R, Kita T, Kawahara S. Vitreous levels of soluble vascular endothelial growth factor receptor (VEGFR)-1 in eyes with vitreoretinal diseases. Br J Ophthalmol. 2011;95:1745–8. doi: 10.1136/bjophthalmol-2011-300139. [DOI] [PubMed] [Google Scholar]
- 34.Kawashima M, Shoji J, Nakajima M. Soluble IL-6 receptor in vitreous fluid of patients with proliferative diabetic retinopathy. Jpn J Ophthalmol. 2007;51:100–4. doi: 10.1007/s10384-006-0411-4. [DOI] [PubMed] [Google Scholar]
- 35.Roh MI, Kim HS, Song JH. Concentration of cytokines in the aqueous humor of patients with naive, recurrent and regressed CNV associated with AMD after bevacizumab treatment. Retina. 2009;29:523–9. doi: 10.1097/IAE.0b013e318195cb15. [DOI] [PubMed] [Google Scholar]