1. Introduction
The purpose of this study was to characterize the neurosteroid pregnanolone and its effects on retention using a complex operant task and to compare these effects with flunitrazepam, a prototypic positive GABAA modulator. In order to assess the capacity of pregnanolone to disrupt retention, a repeated-acquisition technique (Thompson, 1986) was used, which assayed retention by requiring subjects to acquire a sequence of responses and then, within that same session, repeat that sequence of responses after some delay. This procedure has been shown to produce both high and low levels of retention depending on the delay and produce drug-induced disruptions of retention in monkeys (Thompson et al., 1986) and rats (Leonard et al., 2009). The procedure is considered to be a repeated-acquistion procedure because a different sequence was acquired each session. Responding was maintained under a second-order fixed-ratio (FR) 2 schedule of food reinforcement, and incorrect responding (errors) produced a 5-sec timeout.
Sarter (1992) has argued that many of the most popular behavioral tasks for assessing drug-induced memory effects in rodents (e.g., Morris water maze and passive-avoidance procedures) fail to fulfill the criterion for predictive validity across species. He points to the results with “memory enhancers” in particular, which enhance memory under many of these existing tasks, but rarely enhance memory in humans (Sarter et al., 1992a; Sarter et al., 1992b). One advantage of the repeated-acquisition technique, in particular, is that it has been used to characterize the effects of a variety of drugs on learning and memory in animals and humans, and these effects have been consistent across species (Auta et al., 1995; Desjardins et al., 1982; Leonard et al., 2009; Savage et al., 1996; Thompson et al., 1986).
In general, both direct agonists (e.g., muscimol) and positive allosteric modulators (e.g., lorazepam, pentobarbital, and ethanol) of the GABAA receptor complex impair memory, likely through the interaction of GABA-ergic neurons with the cholinergic system (Brioni et al., 1991). A greater understanding of the effects of the neurosteroids on learning and memory is needed, however, because there is an increased interest in the potential use of these substances as anxiolytics, sedative hypnotics and anticonvulsants (Belelli and Lambert, 2005; Gasior et al., 1999). Pregnanolone is a naturally-occuring metabolite of progesterone, which was previously undetectable in rat plasma (Kim et al., 2000), but it has recently been shown to be more abundant than either progesterone or allopregnanolone (Ocvirk et al., 2009). Pregnanolone has been shown to potentiate GABAA-mediated neuronal inhibition in a manner similar to allopregnanolone (Wang et al., 2000) as well as directly activate this receptor at supraphysiological concentrations (Callachan et al., 1987). Behaviorally, pregnanolone also has effects very similar to other positive modulators. For example, in rats responding under a differential-reinforcement-of-low-rate (DRL) schedule of reinforcement, pregnanolone produced effects that were comparable to both lorazepam and pentobarbital, but not to the low-efficacy positive modulator flumazenil or the negative modulator beta-carboline-3-carboxylate methyl ester (β-CCM) (Amato et al., 2010). If pregnanolone has amnestic effects similar to other positive allosteric modulators, then it should produce both anterograde and retrograde amnestic effects; however, this study will focus only on its retrograde amnestic effects.
2. Methods
2.1 Subjects
Twenty-four male Long-Evans hooded rats (Harlan Laboratories, Indianapolis, IN) served as subjects. All subjects were housed singly in polypropylene cages with hardwood chip bedding in a colony room maintained at 21±2°C with 50±10% relative humidity on a 14-h light/10-h dark cycle (lights on at 0600 h CST). Each animal earned 45-mg food pellets (TestDiets, a division of LabDiet, Richmond, IN) during the experimental sessions and, when necessary, were provided standard rodent chow (Rodent Diet 5001, PMI Inc., Brentwood, MO) in the home cage after the test sessions in order to maintain them at 90% of their free-feeding weight. Water was available ad libitum in their home cage. This study was carried out in accordance with the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and the guidelines of the Committee on Care and Use of Laboratory Animal Resources, as adopted and promulgated by the U.S. National Institutes of Health.
2.2 Apparatus
Twelve identical modular test chambers (Coulbourn Instruments, Allentown, PA, Model E10-10TC) configured specifically for rats were used. Located on the front wall of each chamber were a houselight, auditory feedback relay, pellet trough (5.5 cm above the floor and centered), and three response keys aligned horizontally (8 cm apart, center to center, and 14.5 cm above the floor). Each response key could be illuminated by a Sylvania 28ESB indicator lamp with a yellow plastic cap. Response keys required a minimum force of 0.25 N for activation and correct responses produced an audible click of the feedback relay. Each chamber was enclosed within a sound-attenuating cubicle equipped with a fan for ventilation and white noise for masking extraneous sounds. All test chambers were connected to a computer programmed in MED-PC for Windows, Version IV (MED Associates, Inc., St. Albans, VT), and to cumulative recorders (Gerbrands, Arlington, MA) located within the same room.
2.3 Behavioral Procedures
In the acquisition and delayed-performance procedure, each session was composed of three phases: acquisition, delay, and retention test. Preliminary training for this task included magazine training, shaping of the response (nose press), and reinforcing responses on individually illuminated keys after shaping. To train acquisition, all three response keys were illuminated with yellow light, but only one of the three response keys was correct for a particular session and each response emitted on that key extinguished all of the key lights, illuminated the stimulus light in the feeder trough for 0.4 s, and delivered a 45-mg food pellet from the pellet dispenser. Responding on either of the other two illuminated keys was considered an incorrect response (error) and resulted in a 5-sec timeout during which the key lights were extinguished and responding had no programmed consequence. For each additional session during this stage of training, the position for the correct response was varied in a mixed order. After rats reliably acquired this single-response task, regardless of the key position, a second response was added to create a sequence of responding such that two correct responses were necessary to obtain reinforcement (e.g., left-right (LR), left-center (LC), RL, RC, CL, and CR). This type of sequential responding is procedurally defined as a “tandem” sequence because the same stimuli (yellow) are correlated with each response in the sequence (Thompson, 1970) even though the position for the correct responses varied both within the two-response sequence and across sessions (i.e., there is still repeated acquisition). Errors emitted during the acquisition of the two-response sequences produced a 5-sec timeout as described above, but did not reset the response sequence (the position of the next correct response was the same before and after a timeout).
When stable responding was obtained under the two-response tandem sequence, a third and fourth response were added to the sequence. These four-response sequences were carefully selected to be equivalent in several ways and there were restrictions on their ordering across sessions (Thompson, 1973). Briefly, 17, four-response sequences were arranged in a specific order and presented to each subject one after another until the list was completed. Once completed, the same list of sequences was presented again. In this list, each of the 17 sequences occurred three to five times and adhered to the other restrictions on their ordering (i.e., adjacent positions within a sequence were different from day to day and no position within a sequence was duplicated across days). This eliminated sequence presentations such as RRCL, and sequence ordering such as RLCR followed by CLRC where the second response in the sequence would be the left (L) key across days. Thus, each subject was exposed to a given sequence three to five times every 61 test sessions.
Initially, acquisition of these four-response sequences was maintained by reinforcing the completion of each sequence; however, sequence acquisition was eventually transitioned to a second-order fixed-ratio (FR) schedule. Under this schedule, completion of every sequence extinguished the response keys and illuminated the pellet trough, but pellet delivery only occurred after every two sequence completions. As in all of the other stages of acquisition training, errors emitted during the acquisition of the four-response sequences produced a 5-sec timeout, but did not reset the response sequence. When response rates and the percentage of errors did not vary by more than ±20% or ±10% of the mean, respectively, for 10 consecutive sessions, acquisition of the 4-response tandem sequences was considered stable, and the subjects were moved to the final procedure comprised of acquisition, delay, and retention test.
During the acquisition phase, subjects were required to meet an acquisition criterion in which they had to complete 7 sequences errorlessly (i.e., emit 28 consecutive correct responses) within 45 min. Otherwise, the session ended. This 45 min time limit for acquisition in the retention task was included to prevent opportunities for “overlearning”, which can increase retention above control levels and modulate drug effects (cf. Thompson et al., 1986). If the criterion was met before the 45 minutes elapsed, the key lights were turned off and the delay phase (“retention interval”) began. Initially, delays of varying length (5 min, 30 min, 180 min, 6 h, 24 h and 48 h) were presented in a mixed or semi-random order to establish a retention curve; however, only the 30- and 180-minute delays were used for testing with each drug. Following the delay, the response keys and the houselight were illuminated for a 30-min retention test. In this procedure, the houselight served as a discriminative-stimulus for the retention test. Similar to the acquisition phase, responding during the retention test was maintained by food presentation under a second-order FR-2 schedule of reinforcement, and errors produced a 5-sec timeout, but did not reset the sequence. In summary, subjects acquired a sequence in the acquisition phase and after a delay of varying length, demonstrated retention by responding on the same tandem sequence during the retention test.
2.4 Drugs and Drug Administration
The acute effects of pregnanolone (1 – 18 mg/kg), flunitrazepam (0.056 – 1 mg/kg), and flumazenil (5.6 mg/kg) were determined after responding under the acquisition and delayed-performance procedure stabilized. Pregnanolone (5β-pregnan-3α-ol-20-one; Steraloids Inc., Newport, RI) was dissolved in 45% 2-hydroxypropyl γ-cyclodextrin (Sigma-Aldrich Corp., St. Louis, MO) in saline. Flunitrazepam (Sigma-Aldrich Corp., St. Louis, MO) was dissolved in a vehicle consisting of propylene glycol (60%) and sterile water. Flumazenil (Ascent Scientific Ltd., Princeton, NJ) was dissolved in a vehicle containing polyethylene glycol (10%), benzyl alcohol (2%), propylene glycol (44 %), dimethyl sulfoxide (11%), and sterile water (33%). Injections for all three drugs were administered i.p. after subjects met criterion in the acquisition phase and 15 min before the retention test regardless of the delay. The injection volume was always 0.1 ml/100 grams, and all the dosages for a given drug were administered in a mixed order. Responding was always allowed to return to baseline levels before administration of the next dose. However, to avoid the development of acute tolerance or any “carry over” effects, the highest doses of each drug were administered only once per week. A drug-free period of at least 10 days occurred between drugs.
2.5 Statistical Analysis
The data collected were analyzed in terms of: (1) the overall response rate (total responses/min, excluding timeouts), and (2) the overall accuracy, expressed as the percentage of errors [(incorrect responses/total responses) × 100]. However, when the response rate was less than 5 responses per min, data were excluded from the analyses for percent errors because of the small number of responses emitted. Retention was quantified using a percent savings measure (Ebbinghaus, 1964). Percent savings was calculated by subtracting the errors-to-criterion (ETC) during the retention test from the ETC during the acquisition phase, and then expressing this difference as a percentage of the ETC in the acquisition phase. For example, if a subject made 200 errors before the acquisition criterion was met, but made only 50 errors before the same criterion was met in the retention test, the percent savings would be 75 (i.e., (200-50/200) × 100). If there was no retention, then the ETC in the retention test would be equal to, or greater than, the ETC in the acquisition phase; thus, the percent savings would be 0 (any negative values were considered to be zero retention as well).
The mean data for each subject were grouped and analyzed statistically for an effect of drug treatment using a one-way ANOVA for repeated measures (SigmaStat Statistical Software, SYSTAT Software, Inc. Point Richmond, CA, USA). A two-way ANOVA for repeated measures was used to analyze drug combinations, with a one-way ANOVA used to isolate those effects if a significant interaction occurred. Holm-Sidak post-hoc tests were used to compare drug sessions with control (saline or vehicle) sessions. Significance was accepted at α level ≤ 0.05 for all statistical tests. In addition to these measures based on session totals, within-session changes in responding were monitored by a cumulative recorder and computer. ED50 values were determined by linear regression using two or more data points reflecting the slopes of the descending portions of the curve for each drug.
3. Results
3.1 Retention Curve
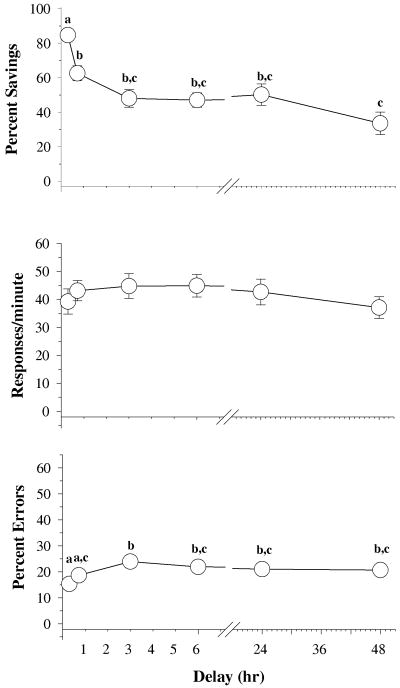
Figure 1 shows the data obtained over several different delays during the retention test. In general, there was a main effect of delay on percent savings [F (5,79)=12.89, p<0.001], response rate [F(5,79)=2.84, p=0.021], and percent errors [F(5,79)=8.21, p < 0.001]. Holm-Sidak post-hoc tests also indicated that percent savings and percent errors significantly differed across delays. For example, percent savings after 5 minutes was significantly different than percent savings after each of the other delays, and percent savings was significantly different after 48 hours than after 30 minutes. No differences in percent savings occurred between the 3-, 6-, 24- and 48-hour delays. In terms of the percentage of errors, fewer errors occurred after the 5- and 30-minute delays than after the rest of the delays, and there were no differences in the percentage of errors after the longer delays.
Fig. 1.
Effects of delay on overall percent savings, response rates and percentage of errors in 23 subjects. Symbols with error bars represent the mean ± SEM, while any points without vertical lines indicate instances in which the SEM is encompassed by the data point. Letters indicate significant differences within delay (α ≤ 0.05) as determined by a one-way ANOVA and Holm-Sidak post-hoc tests.
3.2 Effects of pregnanolone and flunitrazepam alone
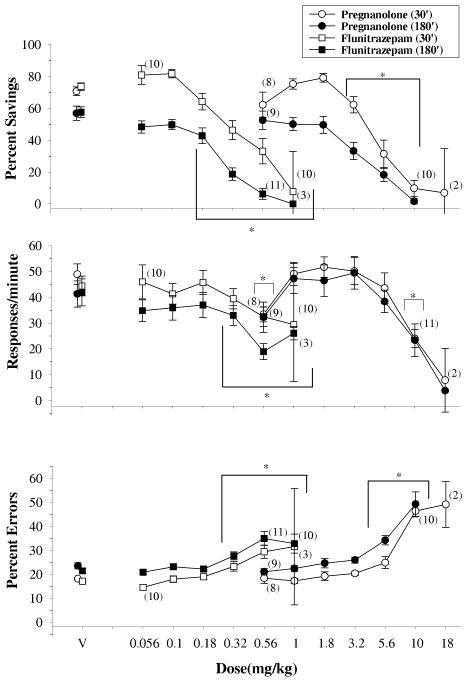
The data plotted in Figure 2 show the effects of increasing doses of both pregnanolone (circles) and flunitrazepam (squares) on percent savings, response rate and percent error after a delay of 30 (unfilled symbols) or 180 minutes (filled symbols). A two-way ANOVA indicated there was a significant main effect for delay [pregnanolone: F(1,123)=19.110,p<0.001; flunitrazepam: F(1,113)=39.962, p<0.001], and dose [pregnanolone: [F(6,123)= 60.044, p<0.001; flunitrazepam: F(6,113)=55.155, p<0.001] on percent savings. However, the effects of delay did not depend on the effects of dose as there was no interaction between delay and dose for either pregnanolone [F(6,123)=2.110, p=0.057] or flunitrazepam [F(6,113)=1.049, p=0.398]. Given the main effect of delay, Holm-Sidak post-hoc tests confirmed the significant difference between the 30- and 180-minute delays on percent savings, whereas post-hoc analyses for the effects of dose found differences between vehicle administration and dosages of each drug. More specifically, when the dose-effect data for percent savings were collapsed across delay and compared to vehicle administration, 3.2 – 10 mg/kg of pregnanolone were significantly different and 0.18 – 1 mg/kg of flunitrazepam were significantly different.
Fig. 2.
Effects of pregnanolone (circles) and flunitrazepam (squares) on overall percent savings, response rates and the percentage of errors after a 30- (open shapes; n=11) and 180-min (closed shapes; n=12) delay. Symbols with error bars represent the mean ± SEM, while any points without error bars indicate instances in which the SEM is encompassed by the data point. Data points and vertical lines above ‘V’ indicate the mean and SEM for control sessions in which vehicle was administered. Asterisks indicate doses that were significantly (α ≤ 0.05) different from control as determined by Holm-Sidak post-hoc tests. The numbers in parentheses adjacent to some data points indicate the total number of subjects represented by that point.
A two-way ANOVA on the data for response rate indicated there was a main effect of dose for both pregnanolone [F(6,123)=22.034, p<0.001] and flunitrazepam [F(6,123)=10.860, p<0.001], but no effect of delay for either drug [pregnanolone: F(1,123)=0.277, p<0.604; flunitrazepam: F(1,123)=2.190, p=0.154]. Furthermore, there was no interaction between delay and dose on response rate for either pregnanolone [F(6,123)=0.557, p=0.764] or flunitrazepam [F(6,113)=1.393, p=0.223]. This limited the statistical analyses to the effect of dose for each drug, and these tests indicated that 0.56 and 10 mg/kg of pregnanolone, as well as 0.32 - 1 mg/kg of flunitrazepam, were significantly different from vehicle administration.
Finally, a two-way ANOVA on the data for percent errors indicated there was a main effect of delay [pregnanolone: F(1,123)=12.632, p=0.002; flunitrazepam: F(1,123)=13.287,p=0.001], and dose for both drugs [pregnanolone: F(6,123)=58.478,p<0.001; flunitrazepam: F(6,123)=15.759,p<0.001]. Similar to the data for percent savings, however, there was no interaction between delay and dose for either pregnanolone [F(6,123)=.688, p=0.660] or flunitrazepam [F(6,113)=205, p=0.974] on percent error. Although the effect of delay was less obvious on percent error than percent savings, Holm-Sidak post-hoc tests detected significant differences between the 30- and 180-minute delay, and significant differences from vehicle administration for pregnanolone (5.6 - 10 mg/kg) and flunitrazepam (0.32 - 1 mg/kg).
3.3 Effects of flumazenil and flunitrazepam in combination
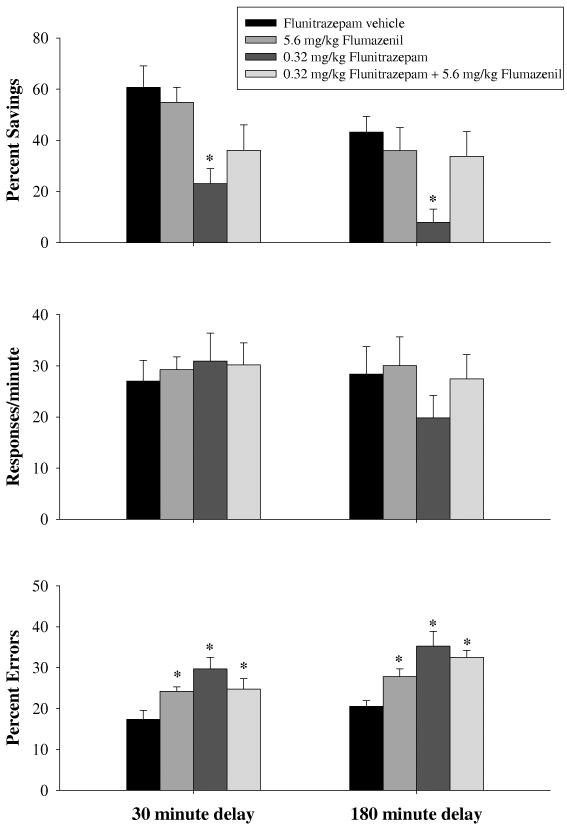
Figure 3 shows the data obtained for percent savings, response rate, and percent errors after subjects received vehicle, 5.6 mg/kg of flumazenil, 0.32 mg/kg of flunitrazepam or a combination of the two drugs after both the 30- and 180-minute delay. A two-way ANOVA detected significant main effects of both delay [F(1,37)=4.956, p=0.036] and drug [F(3,37)=7.748, p<0.001], but no interaction between delay and drug [F(3,37)=0.208, p=0.890] on percent savings, indicating that the drugs produced a similar pattern of effects after both delays. Holm-Sidak post-hoc tests indicated that 0.32 mg/kg of flunitrazepam was significantly different than vehicle when the data were collapsed across delay. Thus, flumazenil had no effect when administered alone and attenuated the disruption produced 0.32 mg/kg of flunitrazepam on percent savings. A two-way ANOVA on response rate detected no significant differences for delay [F(1,38)=0.661, p=0.427] or drug [F(3,38)=1.177, p=0.331], as well as no significant interaction between delay and drug [F(3,37)=1.067, p=0.375]. Lastly, a two-way ANOVA detected main effects of both delay [F(1,37)=10.534, p=0.004] and drug [F(3,37)=11.502, p<0.001] on percent erros, but there was no significant interaction between delay and drug [F(3,37)=1.026, p=0.392] on percent error. For this dependent measure of responding, post-hoc analyses indicated that all three drug treatments were significantly different from vehicle administration.
Fig. 3.
Effects of flunitrazepam, alone and in combination with flumazenil, on overall percent savings, response rates and percentage of errors. Vertical lines associated with each bar represent the SEM. Asterisks reflect doses that were significantly (α ≤ 0.05) different from flunitrazepam vehicle as determined by two-way ANOVA and Holm-Sidak post-hoc tests for both the 30-min (n=8) and the 180-min delay (n=8).
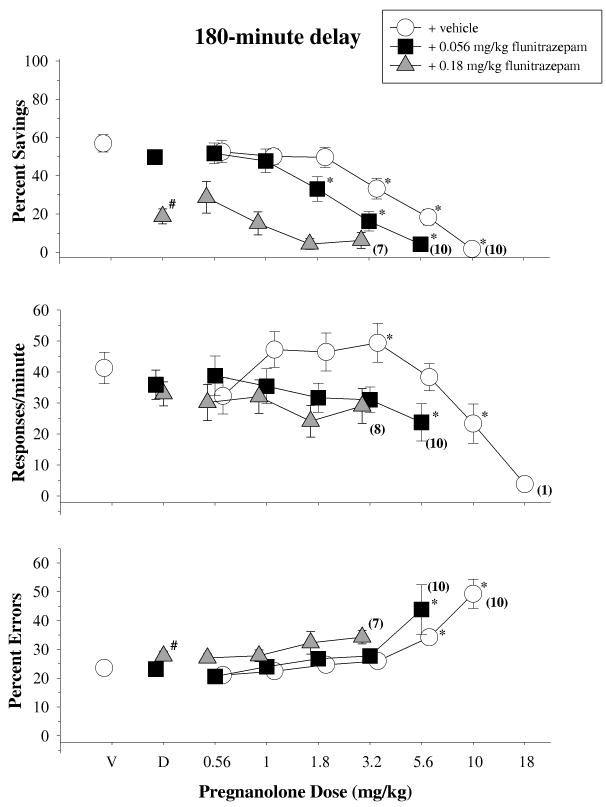
3.4 Effects of flunitrazepam and pregnanolone in combination
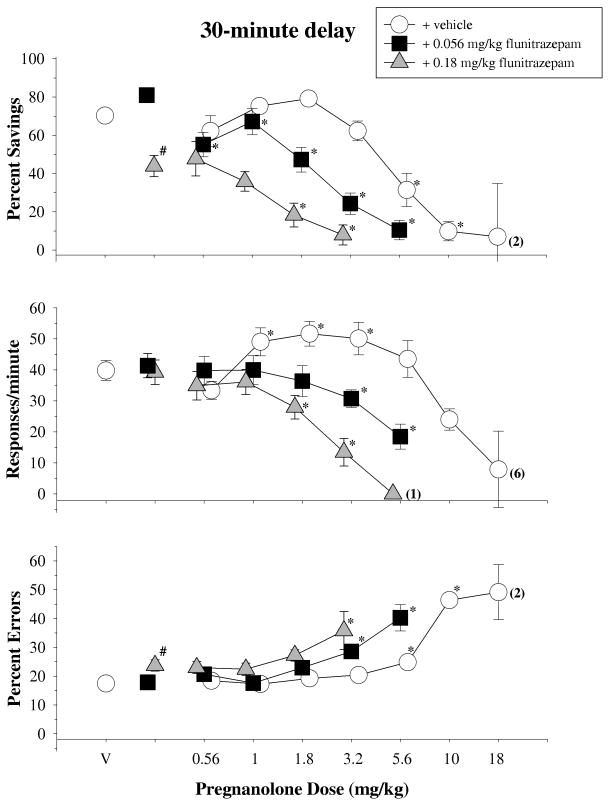
The top panel of Figure 4 shows the data obtained for percent savings after a 30-min retention interval when pregnanolone was given alone and in combination with both an ineffective (0.056 mg/kg) and an effective (0.18 mg/kg) dose of flunitrazepam [F(2,20)=19.586,p<0.001]. Pregnanolone in combination with flunitrazepam vehicle dose-dependently decreased percent savings, and this curve was shifted leftward when pregnanolone was administered with increasing doses of flunitrazepam. These effects were confirmed statistically by one-way repeated-measures ANOVA tests, which indicated there was a significant main effect of dose for every combination (pregnanolone + flunitrazepam vehicle: [F(6,58)=30.725, p<0.001]; pregnanolone + 0.056 mg/kg of flunitrazepam: [F(5,50)=21.021,p<0.001]; pregnanolone + 0.18 mg/kg of flunitrazepam: [F(4,34)=5.490,p=0.002]). Although the leftward shifts could not be compared statistically as a repeated measure, they were evident in the ED50 values for the different combinations. For example, the ED50 value for pregnanolone and vehicle was 5.88 mg/kg, whereas the value was 2.14 and 1.66 mg/kg when pregnanolone was administered with 0.056 and 0.18 mg/kg of flunitrazepam, respectively.
Fig. 4.
Effects of pregnanolone, alone and in combination with 0.056 and 0.18 mg/kg of flunitrazepam, on overall percent savings, response rate and percentage of errors after a 30-min delay in 11 subjects. Symbols with error bars represent the mean ± SEM, while any points without error bars indicate instances in which the SEM is encompassed by the data point. Data points and vertical lines above ‘V’ indicate the mean and SEM for control sessions in which vehicle was administered. Asterisks reflect doses that were significantly (α ≤ 0.05) different from control as determined by a one-way ANOVA of the data for each condition and Holm-Sidak post-hoc tests. The numbers in parentheses adjacent to some data points indicate the total number of subjects represented by that point.
With respect to response rate, neither 0.056 nor 0.18 mg/kg of flunitrazepam alone significantly disrupted response rate on the retention test when compared to control injections [F(2,20)=1.186, p=0.326]; however, flunitrazepam significantly altered both the rate-increasing and rate-decreasing effects of pregnanolone. These effects were confirmed statistically by a significant main effect of dose for every combination (pregnanolone + flunitrazepam vehicle: [F(6,58)=20.515, p<0.001]; pregnanolone + 0.056 mg/kg of flunitrazepam: [F(5,52)=7.342,p<0.001]; pregnanolone + 0.18 mg/kg of flunitrazepam: [F(4,38)=15.057,p<0.001]). Furthermore, the relative shifts in the dose-effect curves were evident in the ED50 values, which tended to be larger than the ED50 values for percent savings and reflected the differential sensitivity of these dependent measures (see Table 1) to the various dosages and dosage combinations. For example, the ED50 for pregnanolone and vehicle was 13.80 mg/kg, whereas it was 5.13 and 2.34 mg/kg when pregnanolone was administered with 0.056 and 0.18 mg/kg of flunitrazepam, respectively.
Table 1.
ED50s for percent savings, response rate, and percent error for each treatment group.
| Treatment | Percent Savings | Response Rate | Percent Error |
|---|---|---|---|
| 30 minute delay | 5.88 | 13.80 | 5.01 |
| Pregnanolone + Flunitrazepam Vehicle | |||
| Pregnanolone + 0.056 mg/kg Flunitrazepam | 2.14 | 5.13 | 2.24 |
| Pregnanolone + 0.18 mg/kg Flunitrazepam | 1.66 | 2.34 | 2.88 |
| 180 minute delay | 4.79 | 10.96 | 5.89 |
| Pregnanolone + Flunitrazepam Vehicle | |||
| Pregnanolone + 0.056 mg/kg Flunitrazepam | 2.46 | -- | 4.07 |
| Pregnanolone + 0.18 mg/kg Flunitrazepam | 1.23 | -- | -- |
The bottom panel of Figure 4 shows the data obtained for percent errors after the 30-min retention interval. On this dependent measure of responding, 0.056 mg/kg of flunitrazepam alone had no effect, whereas 0.18 mg/kg produced a small, but significant, increase in percent errors [F(2,20)=4.827, p=0.019]. Pregnanolone also dose-dependently increased percent errors, and this curve was shifted leftward when it was combined with 0.056 mg/kg of flunitrazepam. The significance of these effects was confirmed by a main effect of dose for every combination (pregnanolone + flunitrazepam vehicle: [F(6,58)=65.111, p<0.001]; pregnanolone + 0.056 mg/kg of flunitrazepam: [F(5,50)=11.741,p<0.001]; pregnanolone + 0.18 mg/kg of flunitrazepam: [F(4,34)=4.313, p=0.006]). There were also orderly decreases in the ED50 values for each combination as shown in Table 1.
The top panel of Figure 5 shows the data obtained for percent savings after a 180-min retention interval when pregnanolone was given in combination with both an ineffective (0.056 mg/kg) and an effective (0.18 mg/kg) dose of flunitrazepam [F(2,20)=54.890,p<0.001]. As shown, pregnanolone in combination with flunitrazepam vehicle dose-dependently decreased percent savings, and this curve was shifted to the left and downward when pregnanolone was administered with increasing doses of flunitrazepam. These effects were confirmed statistically by one-way repeated-measures ANOVA tests, which indicated there was a significant main effect of dose for every combination (pregnanolone + flunitrazepam vehicle: [F(6,60)=45.591, p<0.001]; pregnanolone + 0.056 mg/kg of flunitrazepam: [F(5,46)=22.404,p<0.001]; pregnanolone + 0.18 mg/kg of flunitrazepam: [F(4,34)=4.342,p=0.006]). Post-hoc tests also indicated that 3 doses of pregnanolone were significantly different from vehicle as were 3 doses of pregnanolone in combination with 0.056 mg/kg of flunitrazepam. However, none of the doses of pregnanolone in combination with the 0.18 mg/kg of flunitrazepam were significantly different from vehicle due to the effect of flunitrazepam alone (i.e., the significant effect of dose was due to the differences among the different dosage combinations). The changes in the slopes of the curves were reflected in the ED50 values for each curve. For example, the ED50 for pregnanolone and vehicle was 4.79 mg/kg, whereas it was 2.45 and 1.23 mg/kg for the combination of pregnanolone and 0.056 and 0.18 mg/kg of flunitrazepam, respectively.
Fig. 5.
Effects of pregnanolone, alone and in combination with 0.056 and 0.18 mg/kg of flunitrazepam, on overall percent savings, response rates and percentage of errors after a 180-min delay in 11 subjects. For additional details, see legend for Figure 4.
In contrast to percent savings, neither 0.056 nor 0.18 mg/kg of flunitrazepam alone significantly disrupted response rate during the 180-min retention test when compared to control injections [F(2,22)=1.289, p=0.295]. Pregnanolone and vehicle significantly increased (3.2 mg/kg) and decreased (10 mg/kg) response rate [F(6,60)=7.834, p<0.001]. When pregnanolone was administered with 0.056 mg/kg of flunitrazepam, only one dose combination significantly disrupted response rate [F(6,58)=20.515, p<0.001]. None of the doses of pregnanolone tested in combination with 0.18 mg/kg of flunitrazepam significantly affected response rate ([F(4,35)= 2.233,p=0.076]). Given the relative lack of effects for the combination of pregnanolone and flunitrazepam on response rate, the only ED50 value that could be calculated was for the combination of pregnanolone and vehicle. In this case, the ED50 was 10.96 mg/kg.
The bottom panel of Figure 5 shows the data obtained for percent errors after a 180-min retention test. On this dependent measure of responding, the low dose of flunitrazepam alone had no effect, while the high dose produced a small, but significant, increase in errors [F(2,22)=4.449,p=0.024]. Likewise, the combination of pregnanolone with either vehicle or 0.056 mg/kg of flunitrazepam significantly increased percent errors. These dose-effects were confirmed statistically by a main effect of dose for both combinations (pregnanolone + flunitrazepam vehicle: [F(6,60)=20.399, p<0.001]; pregnanolone + 0.056 mg/kg of flunitrazepam: [F(5,50)=5.440,p<0.001]) and decreases in the ED50 values for each combination in which an increase in errors occurred (Table 1). Doses of pregnanolone in combination with 0.18 mg/kg of flunitrazepam did not produce a significant increase in errors when compared to control data [F(4,35)=1.695, p=0.172].
4. Discussion
Similar to previous studies conducted in this laboratory (Leonard et al., 2009; Thompson et al., 1986), the present study used a repeated acquisition and delayed-performance baseline to assess the effects of two drugs (pregnanolone and flunitrazepam) on the retention of recently acquired information over both a short (30-minute) and long (180-minute) delay. In general, the observed decrease in retention over time indicated that the baseline was sensitive for measuring time-dependent disruptions in retention, while the observed decreases in retention after drug administration indicated that both drugs have retrograde amnestic effects. More specifically, pregnanolone produced disruptions in retention similar to the positive GABAA modulator flunitrazepam, and both an effective and ineffective doses of flunitrazepam potentiated the disruptions in retention, response rate, and percentage of errors produced by pregnanolone, implicating the GABAA receptor in the behavioral effects of this drug.
Initially, each subject was tested over several different delays in order to assess the efficacy of this procedure for measuring the decay of retention over time (i.e., “forgetting”). Similar to forgetting curves generated by Ebbinghaus and others (cf. Woodworth and Schlosberg, 1961), there was a steep initial decline in retention from 0 – 30 minutes and a comparatively slow decline in retention from 30 minutes to 48 hours. After establishing this retention curve, delays of 30 and 180 minutes were chosen to assess the effects of both drugs, alone and in combination on retention. When administered alone, the dose-dependent decreases in retention obtained with pregnanolone were similar to the decreases seen with flunitrazepam, a benzodiazepine that gained notoriety in the 1990's for its use in drug-facilitated rape (Ohshima, 2006; Saum and Inciardi, 1997). More specifically, pregnanolone produced decreases in retention at doses that did not affect response rate or the percentage of errors, similar to flunitrazepam (Leonard et al., 2009; Pompeia et al., 1996) and other benzodiazepines (Auta et al., 1995; Block and Berchou, 1984; Woodworth and Schlosberg, 1961) that have been shown to disrupt retention/retrieval. In the study by Leonard et al. (2009), the disruptions in retention also occurred at doses lower than those that disrupted the acquisition and performance of response sequences under a multiple schedule. The potent effects of the benzodiazepines on the retention of an acquired were not surprising, however, as Thompson et al. (1986) previously showed that “within-session” performance was more sensitive to the amnestic effects of phencyclidine than “between-session” performance in patas monkeys. Thus, the doses required to disrupt the weaker stimulus control established under within-session performance conditions tend to be lower than those required disrupt the strong stimulus control established under between-session performance conditions. The same potency relationship seems to hold for pregnanolone when pregnanolone's effects on retention in this study are compared with its effects on repeated acquisition and performance under a multiple schedule in two previous studies (Gerak et al., 2004; Quinton et al., 2005).
The benzodiazepines are a very well characterized class of GABAA modulator, particularly with respect to their anterograde amnestic effects (i.e., disruptions of memory acquisition and formation), but not necessarily with regard to their retrograde amnestic effects (Gentil et al., 1989; Jensen et al., 1979; Keith et al., 2003; Lister, 1985; Misaki et al., 1998; Nogueira et al., 2006; Pompeia et al., 2000). Nevertheless, retrograde amnestic effects have been reported in both humans (e.g., Koht and Moss, 1997) and animals (Jensen et al., 1979; Platel and Porsolt, 1982), and in animal studies involving repeated-acquisition procedures (Auta et al., 1995; Pakarinen et al., 1996). Due to the limited amount of data indicating that the benzodiazepines produce retrograde amnesia, there is also the concern that the observed deficits only reflect disruptions of state-dependent learning rather than disruptions of memory processes. As indicated by Lister (1985) and others (Petersen and Ghoneim, 1980), however, the data supporting a “state-dependent retrieval” hypothesis for the deficits produced by the benzodiazepines are quite limited. In fact, as Lister states in his review on benzodiazepines and amnesia, “Although state-dependent learning may be observed with benzodiazepine treatment it is a small effect and cannot account for most of the observed impairments (p. 87).” Moreover, the capacity of this delayed-performance baseline to detect the decay of retention over time even in the absence of different states for acquisition and performance would also seem to suggest that the memory processes assayed were not strictly state dependent, as would the graded, dose-dependent nature of the deficits produced by the benzodiazepines.
The involvement of a benzodiazepine binding site on the GABAA receptor complex was demonstrated by administration of the benzodiazepine site ligand flumazenil, which antagonized the disruption of retention produced by 0.32 mg/kg of flunitrazepam. The antagonistic effect of flumazenil is consistent with the literature, though some investigators have reported difficulty antagonizing the amnestic effects of the benzodiazepines compared to their sedative and motor-impairing effects (for a review, see Woods et al., 1992). One unusual finding in the present study was that flumazenil alone produced small increases in the percentage of errors after both the 30- and 180-minute delay. Nevertheless, these increases suggest either that tonic activity at the benzodiazepine binding site is required for retention or that flumazenil exerts its low-efficacy agonist effects at these doses. In a drug-discrimination study by McMahon and France (2006), flumazenil was able to enhance the midazolam-like effects of the neurosteroid alfaxolone similar to L-838,417 and bretazenil, suggesting that flumazenil may share low-efficacy agonist effects with these drugs. This conclusion was also supported by a study in which high concentrations of flumazenil were able to increase GABA-mediated chloride influx (Mehta and Ticku, 1989). In a recent study by Bai and Gerak (2010), a dose of 5.6 mg/kg of flumazenil (the same dose used in the current study) produced a 10-fold shift in the midazolam and flunitrazepam dose-response curves in rats discriminating pregnanolone and midazolam. Thus, there may have been sufficient receptor occupancy in this study for its low-efficacy agonist effects.
When flunitrazepam was given in combination with different doses of pregnanolone prior to the end of a 30-minute delay, the effects of these combinations were greater than those for either drug alone. For example, when 0.056 mg/kg of flunitrazepam was given alone, there was no effect on percent savings after this delay. However, when this dose of flunitrazepam was given with an ineffective dose of pregnanolone (3.2 mg/kg), significant disruptions in retention were produced (> 50% reduction from control data), along with disruptions in both response rate and percent error. Combinations of pregnanolone and flunitrazepam also produced decreases in retention that did not affect response rate or percent error, which is indicative of a selective effect on retention because these doses did not produce motor impairments or disrupt working memory. These findings were also reminiscent of the results in a study by Gerak et al. (2004) in which doses of pregnanolone potentiated the disruptive effects of flunitrazepam and pentobarbital (two positive GABAA modulators), but not of the NMDA receptor antagonist ketamine, on a repeated-acquisition task in rats. There are also several studies from this laboratory implicating GABAergic mechanisms in the behavioral effects of the neuroactive steroids, and pregnanolone in particular (Amato et al., 2010; Gerak et al., 2004; Gerak et al., 2008; Quinton et al., 2005). For instance, Quinton et al. (2005) found that pregnanolone and lorazepam in combination produced greater disruptions in learning than either drug alone when administered to rats responding under a repeated-acquisition procedure. Taken together, these results emphasize the importance of GABAA modulation in the effects produced by pregnanolone on memory.
When flunitrazepam was given in combination with different doses of pregnanolone at the end of a 180-minute delay, the effects of these combinations were similar to those obtained after a 30-minute delay. For example, 1.8 and 3.2 mg/kg of pregnanolone and 0.056 mg/kg of flunitrazepam in combination produced significant disruptions in retention, but did not potentiate disruptions in response rate or percent errors. Such effects show the potency with which these combinations can selectively disrupt retention. However, the disruptions produced by the combination of pregnanolone and flunitrazepam were not as evident as the disruptions observed after the 30-minute delay, because retention was generally poorer after the 180-minute delay.
In summary, the effects of the neurosteroid pregnanolone on retention closely resemble those of the positive GABAA modulator flunitrazepam. Because neurosteroids have been shown to modulate other receptors including the NMDA and sigma receptor (Elfverson et al., 2008; Maurice et al., 1999), these results cannot unequivocally implicate GABAA receptor modulation in the amnestic effects produced by pregnanolone. However, in a radioligand binding study by Prince and Simmonds (1993), pregnanolone potentiated 1 nM [3H]flunitrazepam binding at the GABAA receptor, with increases in binding ranging from 140-150% of control. These data suggest that pregnanolone can produce behavioral disruptions by potentiation of the effects of other GABAA receptor modulators (Prince and Simmonds, 1993) in addition to its capacity to directly modulate GABAA receptors in the presence of GABA (Callachan et al., 1987; Xu et al., 1997). Recent behavioral studies involving the neurosteroids have been conducted with the idea that these substances could serve as viable alternatives to the benzodiazepines for a number of clinical conditions such as anxiety (Gerak et al., 2004). The putative advantage of the neurosteroids is that they may be able to produce many of the same anxiolytic, sedative and anticonvulsant effects as the benzodiazepines, without producing the same adverse effects (Bai and Gerak, 2010). For example, some of the neurosteroids examined do not seem to produce tolerance to the same extent as the benzodiazepines (Kokate et al., 1998; Reddy and Rogawski, 2000). Another therapeutic advantage of the neurosteroids may be their abuse liability. In a self-administration study by Rowlett et al. (1999), the investigators found that pregnanolone functioned as a reinforcer in rhesus monkeys, whereas the neurosteroid CO 8-7071 did not, suggesting an abuse potential for certain neurosteroids over others. However, as evident from the present study, the neurosteroid pregnanolone and the benzodiazepine flunitrazepam disrupt memory processes similarly, and even ineffective doses of flunitrazepam can potentiate the capacity of pregnanolone to disrupt retention.
Highlights.
Pregnanolone disrupted retention on a repeated acquisition and performance task.
The effects of pregnanolone on retention were similar to those of flunitrazepam.
Flumazenil attenuated the disruptions produced by flunitrazepam on retention.
Flunitrazepam potentiated the disruptions produced by pregnanolone.
Disruptions in retention produced by pregnanolone may be GABAA mediated.
Acknowledgments
This work was supported by USPHS AA09803 from the National Institute on Alcohol and Alcoholism, and DA019625 from the National Institute on Drug Abuse. Special thanks to Dr. Stuart Leonard, Ms. Jessie Sutton, and Ms. Mary Worrel for their expert assistance in completing this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amato RJ, Lewis PB, He H, Winsauer PJ. Effects of positive and negative modulators of the gamma-aminobutyric acid A receptor complex on responding under a differential-reinforcement-of-low-rate schedule of reinforcement in rats. Behav Pharmacol. 2010 doi: 10.1097/FBP.0b013e32833fa7c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auta J, Faust WB, Lambert P, Guidotti A, Costa E, Moerschbaecher JM. Comparison of the effects of full and partial allosteric modulators of GABA(A) receptors on complex behavioral processes in monkeys. Behav Pharmacol. 1995;6:323–332. [PubMed] [Google Scholar]
- 3.Bai X, Gerak LR. Comparing the discriminative stimuli produced by either the neuroactive steroid pregnanolone or the benzodiazepine midazolam in rats. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-2047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 5.Block RI, Berchou R. Alprazolam and lorazepam effects on memory acquisition and retrieval processes. Pharmacol Biochem Behav. 1984;20:233–241. doi: 10.1016/0091-3057(84)90248-x. [DOI] [PubMed] [Google Scholar]
- 6.Brioni JD, Arolfo MP, Jerusalinsky D, Medina JH, Izquierdo I. The effect of flumazenil on acquisition, retention, and retrieval of spatial information. Behav Neural Biol. 1991;56:329–335. doi: 10.1016/0163-1047(91)90514-q. [DOI] [PubMed] [Google Scholar]
- 7.Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- 8.Desjardins PJ, Moerschbaecher JM, Thompson DM, Thomas JR. Intravenous diazepam in humans: effects on acquisition and performance of response chains. Pharmacol Biochem Behav. 1982;17:1055–1059. doi: 10.1016/0091-3057(82)90493-2. [DOI] [PubMed] [Google Scholar]
- 9.Ebbinghaus H. In: Memory: A Contribution to Experimental Psychology. Ruger HA, Bussenius CE, translators. New York: Dover; 1964. originally published in 1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elfverson M, Linde AM, Le Greves P, Zhou Q, Nyberg F, Johansson T. Neurosteroids allosterically modulate the ion pore of the NMDA receptor consisting of NR1/NR2B but not NR1/NR2A. Biochemical and Biophysical Research Communications. 2008;372:305–308. doi: 10.1016/j.bbrc.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 11.Gasior M, Carter RB, Witkin JM. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol Sci. 1999;20:107–112. doi: 10.1016/s0165-6147(99)01318-8. [DOI] [PubMed] [Google Scholar]
- 12.Gentil V, Gorenstein C, Camargo CH, Singer JM. Effects of flunitrazepam on memory and their reversal by two antagonists. J Clin Psychopharmacol. 1989;9:191–197. [PubMed] [Google Scholar]
- 13.Gerak LR, Moerschbaecher JM, Winsauer PJ. Overlapping, but not identical, discriminative stimulus effects of the neuroactive steroid pregnanolone and ethanol. Pharmacol Biochem Behav. 2008;89:473–479. doi: 10.1016/j.pbb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerak LR, Stevenson MW, Winsauer PJ, Moerschbaecher JM. Effects of pregnanolone alone and in combination with other positive GABAA modulators on complex behavior in rats. Psychopharmacology (Berl) 2004;173:195–202. doi: 10.1007/s00213-003-1717-2. [DOI] [PubMed] [Google Scholar]
- 15.Jensen RA, Martinez JL, Jr, Vasquez BJ, McGaugh JL. Benzodiazepines alter acquisition and retention of an inhibitory avoidance response in mice. Psychopharmacology (Berl) 1979;64:125–126. doi: 10.1007/BF00427358. [DOI] [PubMed] [Google Scholar]
- 16.Keith JR, Pitts RC, Pezzuti T, Galizio M. Effects of positive GABA(A) modulators on a multiple-component, repeated-acquisition test of spatial learning. Behav Pharmacol. 2003;14:67–75. doi: 10.1097/00008877-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Kim YS, Zhang H, Kim HY. Profiling neurosteroids in cerebrospinal fluids and plasma by gas chromatography/electron capture negative chemical ionization mass spectrometry. Anal Biochem. 2000;277:187–195. doi: 10.1006/abio.1999.4384. [DOI] [PubMed] [Google Scholar]
- 18.Koht A, Moss JI. Does midazolam cause retrograde amnesia, and can flumazenil reverse that amnesia? Anesth Analg. 1997;85:211–212. doi: 10.1097/00000539-199707000-00037. [DOI] [PubMed] [Google Scholar]
- 19.Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1998;287:553–558. [PubMed] [Google Scholar]
- 20.Leonard ST, Gerak LR, Delatte MS, Moerschbaecher JM, Winsauer PJ. Relative potency and effectiveness of flunitrazepam, ethanol, and beta-CCE for disrupting the acquisition and retention of response sequences in rats. Behav Pharmacol. 2009;20:33–44. doi: 10.1097/FBP.0b013e3283242f2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lister RG. The amnesic action of benzodiazepines in man. Neurosci Biobehav Rev. 1985;9:87–94. doi: 10.1016/0149-7634(85)90034-x. [DOI] [PubMed] [Google Scholar]
- 22.Maurice T, Phan VL, Urani A, Kamei H, Noda Y, Nabeshima T. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81:125–155. doi: 10.1254/jjp.81.125. [DOI] [PubMed] [Google Scholar]
- 23.McMahon LR, France CP. Differential behavioral effects of low efficacy positive GABAA modulators in combination with benzodiazepines and a neuroactive steroid in rhesus monkeys. Br J Pharmacol. 2006;147:260–268. doi: 10.1038/sj.bjp.0706550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta AK, Ticku MK. Benzodiazepine and beta-carboline interactions with GABAA receptor-gated chloride channels in mammalian cultured spinal cord neurons. J Pharmacol Exp Ther. 1989;249:418–423. [PubMed] [Google Scholar]
- 25.Misaki K, Nakagawa H, Koshino Y, Kishi H, Ota T, Okuda K, Kanda I, Isaki K, Ito T. Effect of flunitrazepam on sleep and memory. Psychiatry Clin Neurosci. 1998;52:327–332. doi: 10.1046/j.1440-1819.1998.00398.x. [DOI] [PubMed] [Google Scholar]
- 26.Nogueira AM, Pompeia S, Galduroz JC, Bueno OF. Effects of a benzodiazepine on free recall of semantically related words. Hum Psychopharmacol. 2006;21:327–336. doi: 10.1002/hup.775. [DOI] [PubMed] [Google Scholar]
- 27.Ocvirk R, Franklin KB, Pearson Murphy BE. Measurement of ring A-reduced progesterone metabolites by enzyme-linked immunoassay with colorimetric detection: baseline levels of six metabolites, including pregnanolone, in male rat plasma. Anal Chem. 2009;81:1191–1197. doi: 10.1021/ac801538c. [DOI] [PubMed] [Google Scholar]
- 28.Ohshima T. A case of drug-facilitated sexual assault by the use of flunitrazepam. J Clin Forensic Med. 2006;13:44–45. doi: 10.1016/j.jcfm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Pakarinen ED, Faust WB, Moerschbaecher JM. Effects of convulsant and anticonvulsant agents on memory in squirrel monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:883–898. doi: 10.1016/0278-5846(96)00066-8. [DOI] [PubMed] [Google Scholar]
- 30.Petersen RC, Ghoneim MM. Diazepam and human memory: influence on acquisition, retrieval, and state-dependent learning. Prog Neuropsychopharmacol. 1980;4:81–89. doi: 10.1016/0364-7722(80)90064-8. [DOI] [PubMed] [Google Scholar]
- 31.Platel A, Porsolt RD. Habituation of exploratory activity in mice: a screening test for memory enhancing drugs. Psychopharmacology (Berl) 1982;78:346–352. doi: 10.1007/BF00433739. [DOI] [PubMed] [Google Scholar]
- 32.Pompeia S, Bueno OF, Lucchesi LM, Manzano GM, Galduroz JC, Tufik S. A double-dissociation of behavioural and event-related potential effects of two benzodiazepines with similar potencies. J Psychopharmacol. 2000;14:288–298. doi: 10.1177/026988110001400318. [DOI] [PubMed] [Google Scholar]
- 33.Pompeia S, Gorenstein C, Curran HV. Benzodiazepine effects on memory tests: dependence on retrieval cues? Int Clin Psychopharmacol. 1996;11:229–236. doi: 10.1097/00004850-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Prince RJ, Simmonds MA. Differential antagonism by epipregnanolone of alphaxalone and pregnanolone potentiation of [3H]flunitrazepam binding suggests more than one class of binding site for steroids at GABAA receptors. Neuropharmacology. 1993;32:59–63. doi: 10.1016/0028-3908(93)90130-u. [DOI] [PubMed] [Google Scholar]
- 35.Quinton MS, Gerak LR, Moerschbaecher JM, Winsauer PJ. Interaction of cocaine with positive GABAA modulators on the repeated acquisition and performance of response sequences in rats. Psychopharmacology (Berl) 2005;181:217–226. doi: 10.1007/s00213-005-2241-3. [DOI] [PubMed] [Google Scholar]
- 36.Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000;295:1241–1248. [PubMed] [Google Scholar]
- 37.Rowlett JK, Winger G, Carter RB, Wood PL, Woods JH, Woolverton WL. Reinforcing and discriminative stimulus effects of the neuroactive steroids pregnanolone and Co 8-7071 in rhesus monkeys. Psychopharmacology (Berl) 1999;145:205–212. doi: 10.1007/s002130051050. [DOI] [PubMed] [Google Scholar]
- 38.Sarter M, Hagan J, Dudchenko P. Behavioral screening for cognition enhancers: from indiscriminate to valid testing: Part I. Psychopharmacology (Berl) 1992a;107:144–159. doi: 10.1007/BF02245132. [DOI] [PubMed] [Google Scholar]
- 39.Sarter M, Hagan J, Dudchenko P. Behavioral screening for cognition enhancers: from indiscriminate to valid testing: Part II. Psychopharmacology (Berl) 1992b;107:461–473. doi: 10.1007/BF02245257. [DOI] [PubMed] [Google Scholar]
- 40.Saum CA, Inciardi JA. Rohypnol misuse in the United States. Subst Use Misuse. 1997;32:723–731. doi: 10.3109/10826089709039372. [DOI] [PubMed] [Google Scholar]
- 41.Savage UC, Faust WB, Lambert P, Moerschbaecher JM. Effects of scopolamine on learning and memory in monkeys. Psychopharmacology (Berl) 1996;123:9–14. doi: 10.1007/BF02246275. [DOI] [PubMed] [Google Scholar]
- 42.Thompson DM. Repeated acquisition as a behavioral baseline. Psychonomic Science. 1970;21:156–157. [Google Scholar]
- 43.Thompson DM. Repeated acquisition as a behavioral base line for studying drug effects. J Pharmacol Exp Ther. 1973;184:506–514. [PubMed] [Google Scholar]
- 44.Thompson DM, Mastropaolo J, Winsauer PJ, Moerschbaecher JM. Repeated acquisition and delayed performance as a baseline to assess drug effects on retention in monkeys. Pharmacol Biochem Behav. 1986;25:201–207. doi: 10.1016/0091-3057(86)90253-4. [DOI] [PubMed] [Google Scholar]
- 45.Wang MD, Backstrom T, Landgren S. The inhibitory effects of allopregnanolone and pregnanolone on the population spike, evoked in the rat hippocampal CA1 stratum pyramidale in vitro, can be blocked selectively by epiallopregnanolone. Acta Physiol Scand. 2000;169:333–341. doi: 10.1046/j.1365-201x.2000.00744.x. [DOI] [PubMed] [Google Scholar]
- 46.Woodworth RS, Schlosberg H. Experimental Pyschology. New York: Holt, Rinehart & Winston; 1961. [Google Scholar]
- 47.Xu TL, Imanishi T, Akaike N. Modulation of the GABAA response in rat ventromedial hypothalamic neurons by pregnanolone. Comp Biochem Physiol A Physiol. 1997;117:219–226. doi: 10.1016/s0300-9629(96)00288-5. [DOI] [PubMed] [Google Scholar]