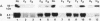Abstract
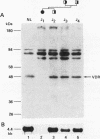
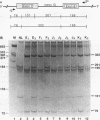
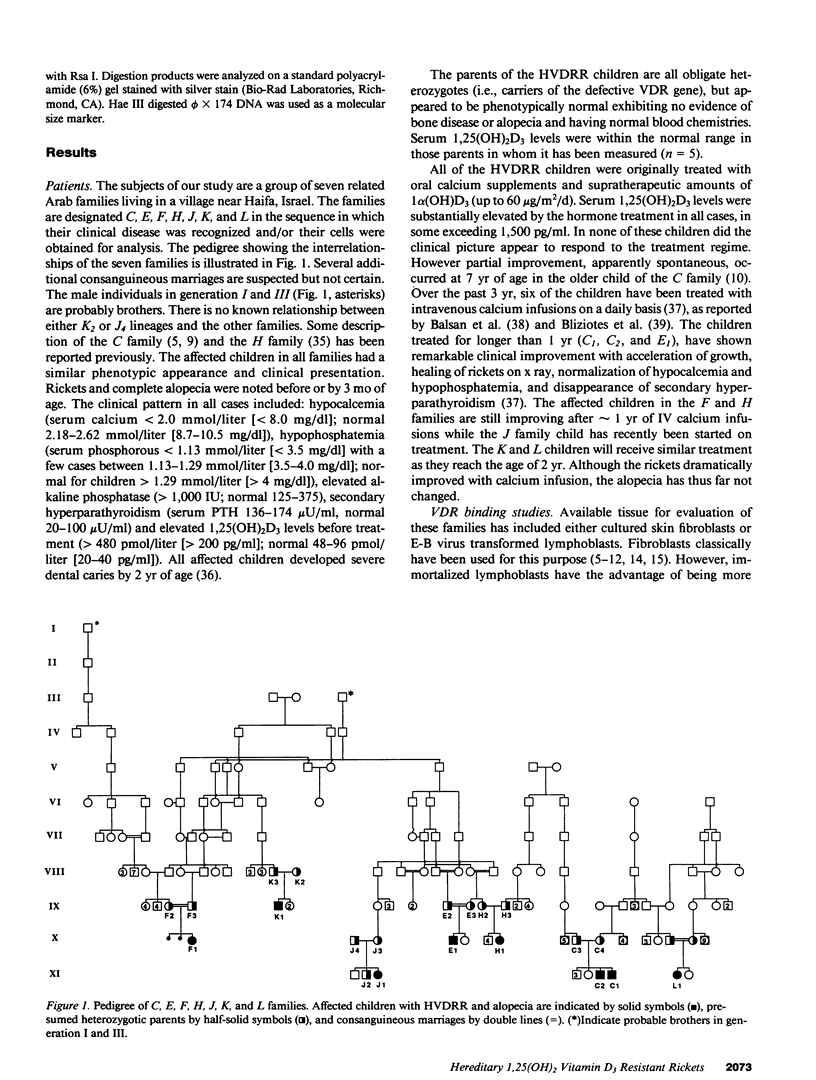
Hereditary 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] resistant rickets (HVDRR) is an autosomal recessive disease caused by target organ resistance to the action of 1,25(OH)2D3, the active form of the hormone. The defect in target cells is heterogenous and commonly appears to be a mutation in the gene encoding the vitamin D receptor (VDR). We have studied cultured skin fibroblasts and Epstein-Barr virus transformed lymphoblasts of seven family branches of an extended kindred having eight children affected with HVDRR. We have previously shown that cells from three affected children in this group contain an "ochre" nonsense mutation coding for a premature stop codon in exon 7 within the steroid-binding domain of the VDR gene. In the current studies, we found that cells from affected children failed to bind [3H]1,25(OH)2D3 and had undetectable levels of VDR as determined by immunoblots using an anti-VDR monoclonal antibody. Measurement of VDR mRNA by hybridization to a human VDR cDNA probe showed undetectable or decreased abundance of steady-state VDR mRNA. Parents, expected to be obligate heterozygotes, showed approximately half the normal levels of [3H]1,25(OH)2D3 binding, VDR protein, and mRNA. The mutation at nucleotide 970 (counting from the mRNA CAP site) results in the conversion of GTAC to GTAA, which eliminates an Rsa I restriction enzyme site and facilitates identification of the mutation. We found that polymerase chain reaction (PCR) amplification of exons 7 and 8 from family members and subsequent Rsa I digestion allows detection of the specific genotype of the individuals. When Rsa I digests of PCR-amplified DNA are subjected to polyacrylamide gel electrophoresis, children with HVDRR exhibit a homozygous banding pattern with loss of an Rsa I site. Parents exhibit a heterozygotic DNA pattern with detection of both normal and mutant alleles. In summary, our data show that the genetic abnormality is a point mutation within the steroid-binding domain of the VDR in all seven related families with HVDRR. Analysis of restriction fragment length polymorphism at the 970 locus of PCR-amplified DNA fragments can be used to diagnose this mutation in both affected children and parents carrying the disease.
Full text
PDF

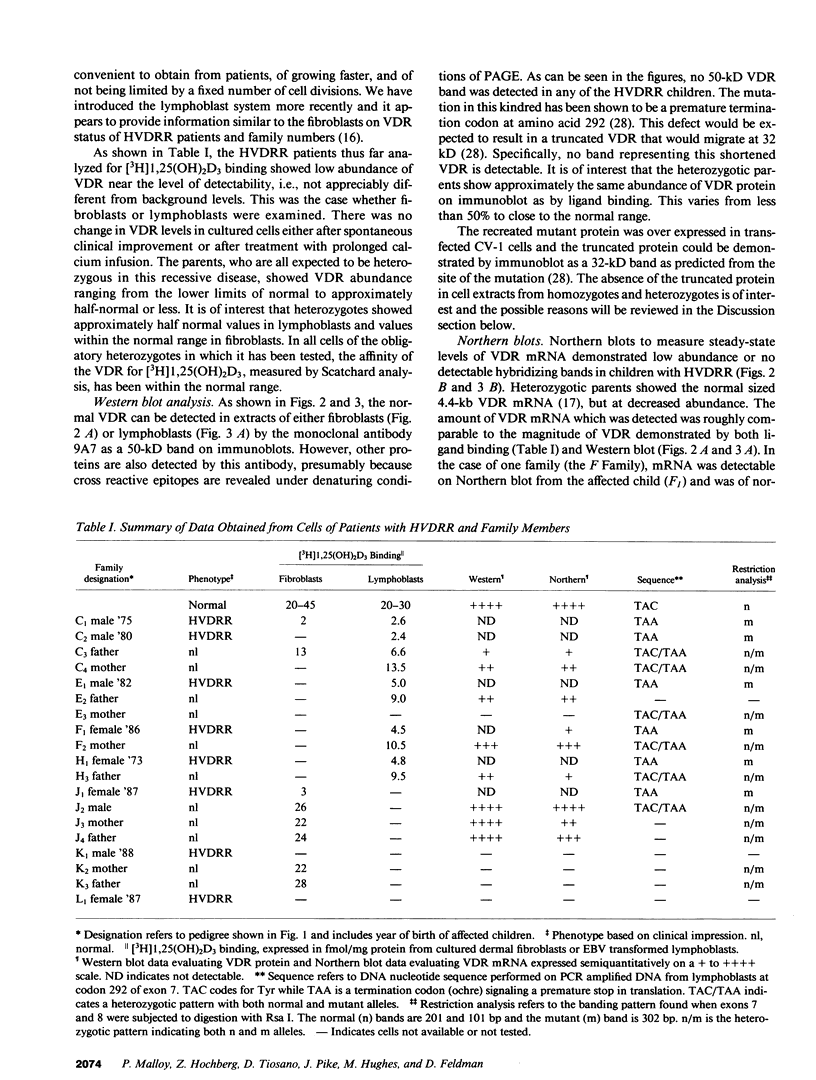
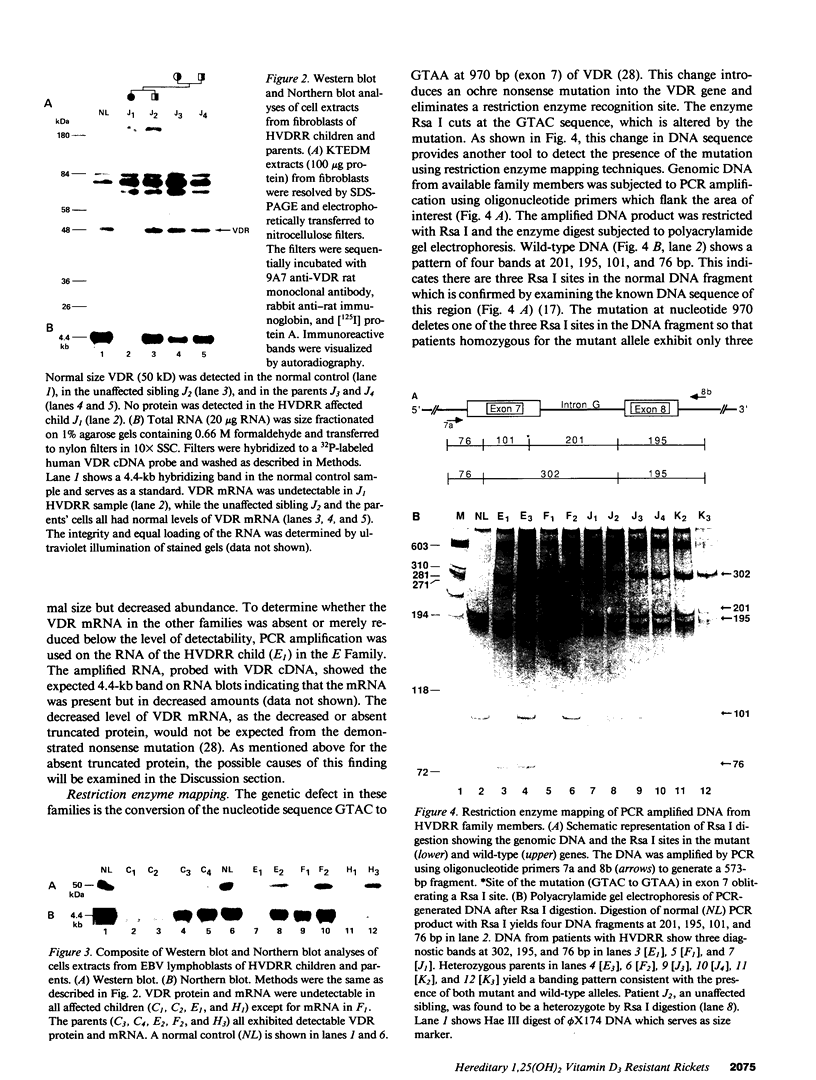




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armanini D., Kuhnle U., Strasser T., Dorr H., Butenandt I., Weber P. C., Stockigt J. R., Pearce P., Funder J. W. Aldosterone-receptor deficiency in pseudohypoaldosteronism. N Engl J Med. 1985 Nov 7;313(19):1178–1181. doi: 10.1056/NEJM198511073131902. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Baker A. R., McDonnell D. P., Hughes M., Crisp T. M., Mangelsdorf D. J., Haussler M. R., Pike J. W., Shine J., O'Malley B. W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988 May;85(10):3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsan S., Garabédian M., Larchet M., Gorski A. M., Cournot G., Tau C., Bourdeau A., Silve C., Ricour C. Long-term nocturnal calcium infusions can cure rickets and promote normal mineralization in hereditary resistance to 1,25-dihydroxyvitamin D. J Clin Invest. 1986 May;77(5):1661–1667. doi: 10.1172/JCI112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer S., Tieder M., Kohelet D., Liberman O. A., Vure E., Bar-Joseph G., Gabizon D., Borochowitz Z. U., Varon M., Modai D. Vitamin D resistant rickets with alopecia: a form of end organ resistance to 1,25 dihydroxy vitamin D. Clin Endocrinol (Oxf) 1981 Apr;14(4):395–402. doi: 10.1111/j.1365-2265.1981.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Berger U., Wilson P., McClelland R. A., Colston K., Haussler M. R., Pike J. W., Coombes R. C. Immunocytochemical detection of 1,25-dihydroxyvitamin D receptors in normal human tissues. J Clin Endocrinol Metab. 1988 Sep;67(3):607–613. doi: 10.1210/jcem-67-3-607. [DOI] [PubMed] [Google Scholar]
- Bliziotes M., Yergey A. L., Nanes M. S., Muenzer J., Begley M. G., Vieira N. E., Kher K. K., Brandi M. L., Marx S. J. Absent intestinal response to calciferols in hereditary resistance to 1,25-dihydroxyvitamin D: documentation and effective therapy with high dose intravenous calcium infusions. J Clin Endocrinol Metab. 1988 Feb;66(2):294–300. doi: 10.1210/jcem-66-2-294. [DOI] [PubMed] [Google Scholar]
- Brooks M. H., Bell N. H., Love L., Stern P. H., Orfei E., Queener S. F., Hamstra A. J., DeLuca H. F. Vitamin-D-dependent rickets type II. Resistance of target organs to 1,25-dihydroxyvitamin D. N Engl J Med. 1978 May 4;298(18):996–999. doi: 10.1056/NEJM197805042981804. [DOI] [PubMed] [Google Scholar]
- Brown T. R., Lubahn D. B., Wilson E. M., Joseph D. R., French F. S., Migeon C. J. Deletion of the steroid-binding domain of the human androgen receptor gene in one family with complete androgen insensitivity syndrome: evidence for further genetic heterogeneity in this syndrome. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8151–8155. doi: 10.1073/pnas.85.21.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester J. K., Wiese R. J., Maeda N., DeLuca H. F. Structure and regulation of the rat 1,25-dihydroxyvitamin D3 receptor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9499–9502. doi: 10.1073/pnas.85.24.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells S., Greig F., Fusi M. A., Finberg L., Yasumura S., Liberman U. A., Eil C., Marx S. J. Severely deficient binding of 1,25-dihydroxyvitamin D to its receptors in a patient responsive to high doses of this hormone. J Clin Endocrinol Metab. 1986 Jul;63(1):252–256. doi: 10.1210/jcem-63-1-252. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Hirst M. A., Cone C. M., Hochberg Z., Tietze H. U., Feldman D. 1,25-dihydroxyvitamin D resistance, rickets, and alopecia: analysis of receptors and bioresponse in cultured fibroblasts from patients and parents. J Clin Endocrinol Metab. 1984 Sep;59(3):383–388. doi: 10.1210/jcem-59-3-383. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Li J. M., Ye T. V., Cone C. M., Feldman D. Hormonal responses to 1,25-dihydroxyvitamin D3 in cultured mouse osteoblast-like cells--modulation by changes in receptor level. J Cell Physiol. 1986 Jan;126(1):21–28. doi: 10.1002/jcp.1041260104. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Vingerhoeds A., Brandon D., Eil C., Pugeat M., DeVroede M., Loriaux D. L., Lipsett M. B. Primary cortisol resistance in man. A glucocorticoid receptor-mediated disease. J Clin Invest. 1982 Jun;69(6):1261–1269. doi: 10.1172/JCI110565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens T. L., Adams J. S., Horiuchi N., Gilchrest B. A., Cho H., Tsuchiya Y., Matsuo N., Suda T., Holick M. F. Interaction of 1,25-dihydroxyvitamin-D3 with keratinocytes and fibroblasts from skin of normal subjects and a subject with vitamin-D-dependent rickets, type II: a model for study of the mode of action of 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1983 Apr;56(4):824–830. doi: 10.1210/jcem-56-4-824. [DOI] [PubMed] [Google Scholar]
- Colston K., Feldman D. 1,25-Dihydroxyvitamin D3 receptors and functions in cultured pig kidney cells (LLC PK1). Regulation of 24,25-dihydroxyvitamin D3 production. J Biol Chem. 1982 Mar 10;257(5):2504–2508. [PubMed] [Google Scholar]
- Colston K., Hirt M., Feldman D. Organ distribution of the cytoplasmic 1,25-dihydroxycholecalciferol receptor in various mouse tissues. Endocrinology. 1980 Dec;107(6):1916–1922. doi: 10.1210/endo-107-6-1916. [DOI] [PubMed] [Google Scholar]
- Costa E. M., Hirst M. A., Feldman D. Regulation of 1,25-dihydroxyvitamin D3 receptors by vitamin D analogs in cultured mammalian cells. Endocrinology. 1985 Nov;117(5):2203–2210. doi: 10.1210/endo-117-5-2203. [DOI] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Etzioni A., Hochberg Z., Pollak S., Meshulam T., Zakut V., Tzehoval E., Keisari Y., Aviram I., Spirer Z., Benderly A. Defective leukocyte fungicidal activity in end-organ resistance to 1,25-dihydroxyvitamin D. Pediatr Res. 1989 Mar;25(3):276–279. doi: 10.1203/00006450-198903000-00012. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Chen T., Cone C., Hirst M., Shani S., Benderli A., Hochberg Z. Vitamin D resistant rickets with alopecia: cultured skin fibroblasts exhibit defective cytoplasmic receptors and unresponsiveness to 1,25(OH)2D3. J Clin Endocrinol Metab. 1982 Nov;55(5):1020–1022. doi: 10.1210/jcem-55-5-1020. [DOI] [PubMed] [Google Scholar]
- Feldman D., Chen T., Hirst M., Colston K., Karasek M., Cone C. Demonstration of 1,25-dihydroxyvitamin D3 receptors in human skin biopsies. J Clin Endocrinol Metab. 1980 Dec;51(6):1463–1465. doi: 10.1210/jcem-51-6-1463. [DOI] [PubMed] [Google Scholar]
- Gamblin G. T., Liberman U. A., Eil C., Downs R. W., Jr, DeGrange D. A., Marx S. J. Vitamin D-dependent rickets type II. Defective induction of 25-hydroxyvitamin D3-24-hydroxylase by 1,25-dihydroxyvitamin D3 in cultured skin fibroblasts. J Clin Invest. 1985 Mar;75(3):954–960. doi: 10.1172/JCI111796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E., Zerwekh J. E. Impaired stimulation of 25-hydroxyvitamin D-24-hydroxylase in fibroblasts from a patient with vitamin D-dependent rickets, type II. A form of receptor-positive resistance to 1,25-dihydroxyvitamin D3. J Clin Invest. 1983 Oct;72(4):1190–1199. doi: 10.1172/JCI111074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst M. A., Hochman H. I., Feldman D. Vitamin D resistance and alopecia: a kindred with normal 1,25-dihydroxyvitamin D binding, but decreased receptor affinity for deoxyribonucleic acid. J Clin Endocrinol Metab. 1985 Mar;60(3):490–495. doi: 10.1210/jcem-60-3-490. [DOI] [PubMed] [Google Scholar]
- Hochberg Z., Benderli A., Levy J., Vardi P., Weisman Y., Chen T., Feldman D. 1,25-Dihydroxyvitamin D resistance, rickets, and alopecia. Am J Med. 1984 Nov;77(5):805–811. doi: 10.1016/0002-9343(84)90516-3. [DOI] [PubMed] [Google Scholar]
- Hochberg Z., Borochowitz Z., Benderli A., Vardi P., Oren S., Spirer Z., Heyman I., Weisman Y. Does 1,25-dihydroxyvitamin D participate in the regulation of hormone release from endocrine glands? J Clin Endocrinol Metab. 1985 Jan;60(1):57–61. doi: 10.1210/jcem-60-1-57. [DOI] [PubMed] [Google Scholar]
- Hochberg Z., Gilhar A., Haim S., Friedman-Birnbaum R., Levy J., Benderly A. Calcitriol-resistant rickets with alopecia. Arch Dermatol. 1985 May;121(5):646–647. [PubMed] [Google Scholar]
- Hughes M. R., Malloy P. J., Kieback D. G., Kesterson R. A., Pike J. W., Feldman D., O'Malley B. W. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988 Dec 23;242(4886):1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- Kerner S. A., Scott R. A., Pike J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren R., Ravid A., Liberman U. A., Hochberg Z., Weisman Y., Novogrodsky A. Defective binding and function of 1,25-dihydroxyvitamin D3 receptors in peripheral mononuclear cells of patients with end-organ resistance to 1,25-dihydroxyvitamin D. J Clin Invest. 1985 Nov;76(5):2012–2015. doi: 10.1172/JCI112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee B., Vissing H., Ramirez F., Rogers D., Rimoin D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science. 1989 May 26;244(4907):978–980. doi: 10.1126/science.2543071. [DOI] [PubMed] [Google Scholar]
- Liberman U. A., Eil C., Marx S. J. Resistance to 1,25-dihydroxyvitamin D. Association with heterogeneous defects in cultured skin fibroblasts. J Clin Invest. 1983 Feb;71(2):192–200. doi: 10.1172/JCI110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn D. B., Brown T. R., Simental J. A., Higgs H. N., Migeon C. J., Wilson E. M., French F. S. Sequence of the intron/exon junctions of the coding region of the human androgen receptor gene and identification of a point mutation in a family with complete androgen insensitivity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9534–9538. doi: 10.1073/pnas.86.23.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy P. J., Hochberg Z., Pike J. W., Feldman D. Abnormal binding of vitamin D receptors to deoxyribonucleic acid in a kindred with vitamin D-dependent rickets, type II. J Clin Endocrinol Metab. 1989 Feb;68(2):263–269. doi: 10.1210/jcem-68-2-263. [DOI] [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Wilson C. M., Wilson J. D., Griffin J. E., McPhaul M. J. A single nucleotide substitution introduces a premature termination codon into the androgen receptor gene of a patient with receptor-negative androgen resistance. J Clin Invest. 1990 May;85(5):1522–1528. doi: 10.1172/JCI114599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx S. J., Bliziotes M. M., Nanes M. Analysis of the relation between alopecia and resistance to 1,25-dihydroxyvitamin D. Clin Endocrinol (Oxf) 1986 Oct;25(4):373–381. doi: 10.1111/j.1365-2265.1986.tb01703.x. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Mangelsdorf D. J., Pike J. W., Haussler M. R., O'Malley B. W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987 Mar 6;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Scott R. A., Kerner S. A., O'Malley B. W., Pike J. W. Functional domains of the human vitamin D3 receptor regulate osteocalcin gene expression. Mol Endocrinol. 1989 Apr;3(4):635–644. doi: 10.1210/mend-3-4-635. [DOI] [PubMed] [Google Scholar]
- Nagler A., Merchav S., Fabian I., Tatarsky I., Weisman Y., Hochberg Z. Myeloid progenitors from the bone marrow of patients with vitamin D resistant rickets (type II) fail to respond to 1,25(OH)2D3. Br J Haematol. 1987 Nov;67(3):267–271. doi: 10.1111/j.1365-2141.1987.tb02346.x. [DOI] [PubMed] [Google Scholar]
- Neufeld E. F. Natural history and inherited disorders of a lysosomal enzyme, beta-hexosaminidase. J Biol Chem. 1989 Jul 5;264(19):10927–10930. [PubMed] [Google Scholar]
- Norman A. W., Roth J., Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev. 1982 Fall;3(4):331–366. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- Ohno K., Suzuki K. A splicing defect due to an exon-intron junctional mutation results in abnormal beta-hexosaminidase alpha chain mRNAs in Ashkenazi Jewish patients with Tay-Sachs disease. Biochem Biophys Res Commun. 1988 May 31;153(1):463–469. doi: 10.1016/s0006-291x(88)81247-6. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- Pillai S., Bikle D. D., Elias P. M. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. J Biol Chem. 1988 Apr 15;263(11):5390–5395. [PubMed] [Google Scholar]
- Ritchie H. H., Hughes M. R., Thompson E. T., Malloy P. J., Hochberg Z., Feldman D., Pike J. W., O'Malley B. W. An ochre mutation in the vitamin D receptor gene causes hereditary 1,25-dihydroxyvitamin D3-resistant rickets in three families. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9783–9787. doi: 10.1073/pnas.86.24.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. F., Fleischman A. R., Finberg L., Hamstra A., DeLuca H. F. Rickets with alopecia: an inborn error of vitamin D metabolism. J Pediatr. 1979 May;94(5):729–735. doi: 10.1016/s0022-3476(79)80139-0. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Takeda K., Ain K., Ceccarelli P., Nakai A., Seino S., Bell G. I., Refetoff S., DeGroot L. J. Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor beta. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8977–8981. doi: 10.1073/pnas.86.22.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989 Jun 30;244(4912):1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Simpson R. U., DeLuca H. F. Characterization of a receptor-like protein for 1,25-dihydroxyvitamin D3 in rat skin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5822–5826. doi: 10.1073/pnas.77.10.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. L., Walworth N. C., Holick M. F. Effect of 1 alpha,25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol. 1986 Jun;86(6):709–714. doi: 10.1111/1523-1747.ep12276343. [DOI] [PubMed] [Google Scholar]
- Sone T., Scott R. A., Hughes M. R., Malloy P. J., Feldman D., O'Malley B. W., Pike J. W. Mutant vitamin D receptors which confer hereditary resistance to 1,25-dihydroxyvitamin D3 in humans are transcriptionally inactive in vitro. J Biol Chem. 1989 Dec 5;264(34):20230–20234. [PubMed] [Google Scholar]
- Underwood J. L., DeLuca H. F. Vitamin D is not directly necessary for bone growth and mineralization. Am J Physiol. 1984 Jun;246(6 Pt 1):E493–E498. doi: 10.1152/ajpendo.1984.246.6.E493. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Tennyson G. E., Bale A. E., Lash R. W., Gesundheit N., Wondisford F. E., Accili D., Hauser P., Weintraub B. D. A base mutation of the C-erbA beta thyroid hormone receptor in a kindred with generalized thyroid hormone resistance. Molecular heterogeneity in two other kindreds. J Clin Invest. 1990 Jan;85(1):93–100. doi: 10.1172/JCI114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman Y., Bab I., Gazit D., Spirer Z., Jaffe M., Hochberg Z. Long-term intracaval calcium infusion therapy in end-organ resistance to 1,25-dihydroxyvitamin D. Am J Med. 1987 Nov;83(5):984–990. doi: 10.1016/0002-9343(87)90666-8. [DOI] [PubMed] [Google Scholar]