Abstract
Background
Cultivated peanut (Arachis hypogaea L.) is an important crop worldwide, valued for its edible oil and digestible protein. It has a very narrow genetic base that may well derive from a relatively recent single polyploidization event. Accordingly molecular markers have low levels of polymorphism and the number of polymorphic molecular markers available for cultivated peanut is still limiting.
Results
Here, we report a large set of BAC-end sequences (BES), use them for developing SSR (BES-SSR) markers, and apply them in genetic linkage mapping. The majority of BESs had no detectable homology to known genes (49.5%) followed by sequences with similarity to known genes (44.3%), and miscellaneous sequences (6.2%) such as transposable element, retroelement, and organelle sequences. A total of 1,424 SSRs were identified from 36,435 BESs. Among these identified SSRs, dinucleotide (47.4%) and trinucleotide (37.1%) SSRs were predominant. The new set of 1,152 SSRs as well as about 4,000 published or unpublished SSRs were screened against two parents of a mapping population, generating 385 polymorphic loci. A genetic linkage map was constructed, consisting of 318 loci onto 21 linkage groups and covering a total of 1,674.4 cM, with an average distance of 5.3 cM between adjacent loci. Two markers related to resistance gene homologs (RGH) were mapped to two different groups, thus anchoring 1 RGH-BAC contig and 1 singleton.
Conclusions
The SSRs mined from BESs will be of use in further molecular analysis of the peanut genome, providing a novel set of markers, genetically anchoring BAC clones, and incorporating gene sequences into a linkage map. This will aid in the identification of markers linked to genes of interest and map-based cloning.
Background
Cultivated peanut (Arachis hypogaea L.) originated in South America, and is grown in tropical and sub-tropical regions across 100 countries on six continents between 40°N and 40°S [1]. Seed dry matter is an important source of digestible protein (25 to 34%), cooking oil (44 to 56%) and vitamins such as thiamine, riboflavin, and niacin, which are particularly important for human nutrition in many developing countries [2]. As a legume, peanuts improve soil fertility by fixing nitrogen, providing up to 60 kg/ha nitrogen to the soil, thus benefiting crops subsequently planted in the same field [3].
Cultivated peanut is a tetraploid (2n = 4 × = 40), self-pollinating species with a DNA content of about 2813 Mbp/1 C [4]. Very limited genetic variation in peanut has been detected by using molecular markers such as restriction fragment length polymorphisms (RFLPs), isozymes, and random amplified polymorphic DNA (RAPDs) [5-10]. Low genetic diversity among cultivated accessions likely derives from the origin of tetraploid peanut in a single, relatively recent allopolyploid event that is thought to have involved genotypes of the wild diploid species Arachis duranensis and Arachis ipaensis [11-15]. Due to the lack of polymorphism at the DNA level, the crop has not been subject to marker-assisted selection or resistance gene cloning. As a consequence, there is a significant need to pursue genomic strategies in cultivated peanut, with the specific goal of increasing the availability of useful molecular tools.
Importantly, while genetic diversity is low, polymorphisms do exist in numbers that are likely to be sufficient for molecular breeding strategies. The challenges to identifying such polymorphisms involve both scale and data mining: How do we survey a sufficient quantity of the peanut genome to identify large numbers of polymorphisms? One possibility is the use of simple sequence repeat (SSR) markers, which now exist as a set of more than 5,000 assayable loci, many of which are published and show promise as tools for peanut research [16-25].
Genetic linkage maps composed of SSR markers have been constructed for wild diploid genomes (AA [21,24,26] and BB [27]), as well as for a tetraploid synthetic AABB genome derived from a cross between cultivated and wild amphidiploid species [28]. For these genetic maps it was relatively easy to identify polymorphic markers because the corresponding populations incorporated high rates of polymorphism that are typical of wild Arachis species. More recently, several low resolution linkage maps have been produced for purely cultivated tetraploid AABB genome crosses that harbor much lower polymorphism [29-33]. For these latter linkage maps polymorphic markers were limiting, containing less than 200 SSR markers. However the construction of these genetic maps demonstrates the potential to produce a higher resolution genetic linkage map for cultivated peanut.
Although individual SSR markers could be highly valuable for marker-assisted selection in cases where they are linked to traits of interest, in general the number of polymorphic SSR markers in peanut remains insufficient as a tool for routine genetic analysis. Ideally, plant geneticists and breeders should have access to a sufficient number of polymorphic genetic markers to ensure identification of high value markers that are tightly linked to traits of interest, including disease resistance phenotypes. Therefore, there is a need to expand the density and availability of genetic polymorphisms in peanut, and to survey the status of polymorphic alleles across peanut germplasm.
Bacterial artificial chromosome (BAC) libraries have been constructed for many plant species because they are useful tools for physical mapping, map based cloning, gene structure and function analysis. SSR markers mined from BAC clones (BAC-derived SSR markers) have advantages over other markers without targeted sequence [34,35]. BAC-derived SSR markers have been used to facilitate the merging of the genetic and physical maps [36,37], and have streamlined high resolution mapping and map-based cloning of genes and QTLs of interest [35].
Here we report the identification and characterization of SSRs derived from peanut BAC end sequences (BES). The resulting SSR set is likely to be a good representation of SSRs from the peanut genome as a whole because they were derived from a set of randomly selected BAC sequences. These BES-SSR markers were combined with other publicly available peanut SSR markers to construct a new and higher density genetic linkage map in cultivated peanut, and to initiate the process of integrating physical and genetic map resources in peanut.
Results
Sequencing and annotation of BAC clone ends
3,784 resistance gene homolog (RGH)-containing BAC clones of A. hypogaea cv Tifrunner were identified by means of hybridization with a set of peanut disease resistance gene probes, representing ~580 unique nucleotide binding (NB) domains (Rosen, He and Cook unpublished data). These BAC clones were sequenced from their ends to yield 3,905 BAC end sequences (BES) representing 3.5 Mbp of the cultivated Tifrunner genome sequence. Sequencing of an additional 25,000 randomly-selected BAC clones from a library of diploid A. duranensis [38] yielded 32,530 BAC end sequences representing 29.3 Mbp of the A. duranensis genome (Table 1). BAC end sequences were annotated based on a combination of sequence homology using BLAST and comparison to the InterPRO database [39]. BES sequences were divided into five primary categories according to their sequence similarities: (1) putative gene-containing, (2) putative DNA transposable element-containing, (3) putative retroelement-containing, (4) putative organelle- or ribosomal rDNA-containing, and (5) sequences with no detectable similarities, as shown in Table 2. The last of these classes was the largest and represented 48.5% and 58.0% in wild and cultivated BACs, respectively. Similar results were obtained for pigeonpea [37], where 53.6% of BES lacked similiarity to known genes or proteins. BES sequences in the putative gene-containing category represented 44.9% of total BES in A. duranensis and 38.7% in A. hypogaea. We stress, however, that many BES annotated as "gene-containing" may in fact derive from retroements, due the wide diversity in retroelement sequenes and frequent overlap in annotation vocabularies. There were 4-fold more BES sequences explicitly annotated as retroelements than as DNA transposable elements in both BAC libraries.
Table 1.
SSRs identified from BAC end sequences
| BAC library | A. hypogaea (clones contain RGH) | A. duranensis (random clones) |
|---|---|---|
| Clones sequenced | 3,784 | 25,000 |
| End sequences | 3,905 | 32,530 |
| BES-SSRs | 128 | 1,296 |
| Primers designed | 105 | 1,047 |
Table 2.
Annotation of peanut BAC clone end sequences
| Genes | TEa | REb | Organelle/rRNA | No annotation | Total | |||
|---|---|---|---|---|---|---|---|---|
| EST | R-gene | others | ||||||
| A. duranensis | 5,983 | 92 | 8,536 | 426 | 1,614 | 96 | 15,783 | 32,530 |
| A. hypogaea | 621 | 50 | 842 | 16 | 79 | 32 | 2,265 | 3,905 |
| Total | 6,604 | 142 | 9,378 | 442 | 1,693 | 128 | 18,050 | 36,435 |
| SSRs mined from A. duranensis and A. hypogaea | ||||||||
| SSRs in Ad | 108 | 2 | 302 | 5 | 19 | 3 | 857 | 1,296 |
| SSRs in Ah | 15 | 5 | 26 | 82 | 128 | |||
aTransposable element; bRetroelement
Identification and characterization of BES-SSRs
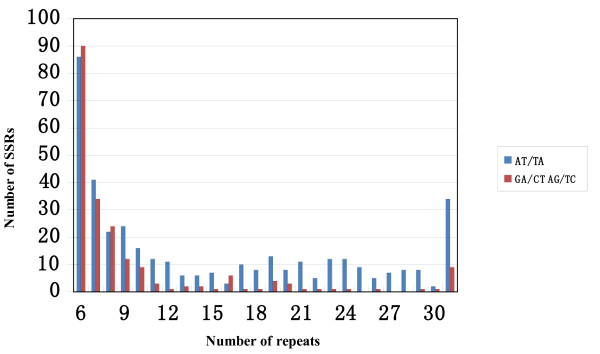
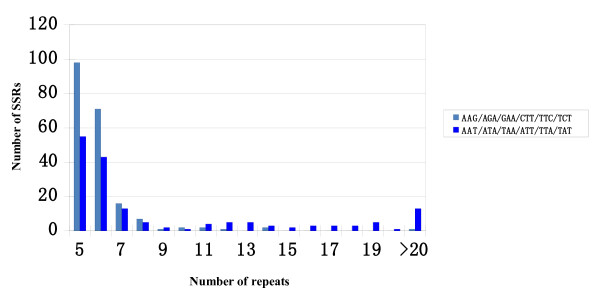
To enlarge the pool of SSRs in peanut, BES sequences were used for mining SSRs. One hundred twenty eight and 1,296 SSRs were identified from BES sequences in the cultivated (tetraploid A. hypogaea) and wild (diploid A. duranensis) BAC clones, respectively (Table 2). For both wild and cultivated species, SSRs were more frequent in the non-annotated fraction of BES. As expected, among these SSRs dinucleotide (47.4%) and trinucleotide (37.1%) SSRs were predominant (Table 3). Survey of EST-SSRs in peanut [40] revealed a similar trend, with 59.5% dinucleotide and 33.7% trinucleotide SSRs. Dinucleotide SSRs composed of AT repeats accounted for 27.4% of all SSRs, followed by SSRs with AG repeats (15.0%). For trinucleotide SSRs, the AAG SSRs occurred at slightly higher frequency (15.2%) than AAT SSRs (12.0%) (Table 4). Moretzsohn et al. [21] also reported an abundance of AAT SSRs in EST sequences, with only AAG SSRs at a higher frequency. Because AT, AG, AAG, and AAT SSRs represented 67% of all SSRs in the BES data set, SSRs with these four motifs were compared for their quantity and frequency of polymorphism. For purposes of this analysis, SSRs were further subdivided into two classes based on length (in the sense of Temnykh et al. [41]), with Class I SSRs ≥ 20 bp and Class II SSRs ≤ 19 bp in length. For dinucleotide SSRs, the number of SSRs with AT motif in Class I was higher than those in Class II, while SSRs with AG motif were less frequent in Class I than in Class II (Figure 1, Table 5). For trinucleotide SSRs, both SSRs with AAT and AAG motifs in Class II were more frequent than in Class I (Figure 2, Table 6).
Table 3.
Percentage of each type of motifs in peanut genome
| Repeat motif | Genomic SSR (%) | EST-SSR* (%) |
|---|---|---|
| Dinucleotide | 47.4 | 59.5 |
| Trinucleotide | 37.1 | 33.7 |
| Tetranucleotide | 4.3 | 1.6 |
| Pentanucleotide | 1.4 | 0.2 |
| Hexanucleotide | 0.5 | 0.3 |
| Compound | 9.3 | 4.5 |
| Total SSRs | 1,424 | 593 |
*cited from Guo et al. 2009
Table 4.
Frequency of individual SSR motifs
| Repeat motif | Number of SSRs | Frequency (%) |
|---|---|---|
| AT/TA | 390 | 27.4 |
| AG/GA/CT/TC | 214 | 15.0 |
| AC/CA/TG/GT | 68 | 4.8 |
| GC/CG | 18 | 1.3 |
| AAG/AGA/GAA/CTT/TTC/TCT | 217 | 15.2 |
| AAT/ATA/TAA/ATT/TTA/TAT | 171 | 12.0 |
| ATG/TGA/GAT/CAT/ATC/TCA | 49 | 3.4 |
| AAC/ACA/CAA/GTT/TTG/TGT | 36 | 2.5 |
| ACC/CCA/CAC/GGT/GTG/TGG | 21 | 1.5 |
| AGG/GGA/GAG/CCT/CTC/TCC | 18 | 1.3 |
| AGT/GTA/TAG/ACT/CTA/TAC | 13 | 0.9 |
| AGC/GCA/CAG/GCT/CTG/TGC | 8 | 0.6 |
| ACG/CGA/GAC/CGT/GTC/TCG | 4 | 0.3 |
| GGC/GCG/CGG/GCC/CCG/CGC | 3 | 0.2 |
Figure 1.
Distribution of repeat number in dinucleotide AT and GA SSRs.
Table 5.
Polymorphism rates of dinucleotide AT and AG SSRs in two classes
| Number of repeat | Non-polymorphic | polymorphic | Total | |
|---|---|---|---|---|
| > 10 (Class I) | AT-SSRs | 175 (81.8%) | 39 (18.2%) | 265 |
| AG-SSRs | 34 (66.7%) | 17 (33.3%) | ||
| 6-9 (Class II) | AT-SSRs | 156 (90.2%) | 17 (9.8%) | 333 |
| AG-SSRs | 154 (96.2%) | 6 (3.8%) | ||
| Total dinucleotide SSRs | 519 (86.8%) | 79 (13.2%) | 598 | |
Figure 2.
Distribution of repeat number in trinucleotide AAT and AAG SSRs.
Table 6.
Polymorphism rates of trinucleotide AAT and AAG SSRs in two classes
| Number of repeat | Non-polymorphic | polymorphic | Total | |
|---|---|---|---|---|
| > 7 (Class I) | AAT-SSRs | 46 (67.6%) | 22 (32.4%) | 100 |
| AAG-SSRs | 29 (90.6%) | 3 (9.4%) | ||
| 5-6 (Class II) | AAT-SSRs | 91 (92.9%) | 7 (7.1%) | 267 |
| AAG-SSRs | 163 (96.4%) | 6 (3.6%) | ||
| Total trinucleotide SSRs | 329 (89.6%) | 38 (10.4%) | 367 | |
A total of 1,152 primer pairs were designed from 128 SSRs related to RGH containing BAC clones of A. hypogaea and 1,296 SSRs mined from the randomly selected BAC clones of A. duranensis. One hundred forty eight (12.8%) of these 1152 SSR amplicons were polymorphic when compared among eight cultivated genotypes (Additional file 1). In this analysis we observed similar rates of polymorphism for SSRs identified from A. duranensis and those identified from the A. hypogaea (12.9% and 12.4%, respectively), suggesting that rates of SSR polymorphism do not differ between RGH and non-RGH regions of the genome. These frequencies of polymorphism were consistent with that of publicly available SSRs (10-13%) [16,17,19,23,25,29,30]. The BAC-derived polymorphic SSR markers detected an average of 3.2 alleles per locus, with the polymorphism information content (PIC) values ranging from 0.21 to 0.87 and an average of 0.45 (Additional file 1). The comparison of SSRs in Class I and Class II showed that the frequency of polymorphism was significantly higher in Class I than in Class II for both dinucleotide and trinucleotide SSRs using Fisher's exact test at P < 0.0001 (Table 5 and 6), consistent with previous observations that longer SSRs are more polymorphic [41]. The type of motifs also showed a strong relationship with the frequency of polymorphism. Together, the SSRs with AAT motif generated the greatest frequency (17.5%) of length polymorphism, followed by SSRs with AT motif (14.5%), AG motif (10.9%), and AAG motif (4.5%). Similar results were obtained for soybean BES-derived SSRs, with AT, AG, and AAT motifs yielding higher rates of length polymorphisms than other motifs [42]. Among this new set of peanut BES-SSRs, those with the highest rates of polymorphism were long SSRs (Class I), with rates of 33.3% for AG-SSRs, 32.4% for AAT-SSRs, and 18.2% for AT-SSRs (Table 5 and 6), making these the most suitable targets for new SSR marker development in cultivated accessions.
Comparison of BES-SSRs identified in this study with publicly available peanut SSRs revealed that 48.7% of BES-SSRs were identical to SSRs identified previously by other means (see columns I & J in Additional file 1). Not surprisingly, there was less overlap in the identity of 23% primer sequences for PCR (column K & L in Additional file 1), and thus the current data set provides both novel SSRs and novel primer sets for a portion of previously discovered SSRS.
Mapping of BES-SSRs
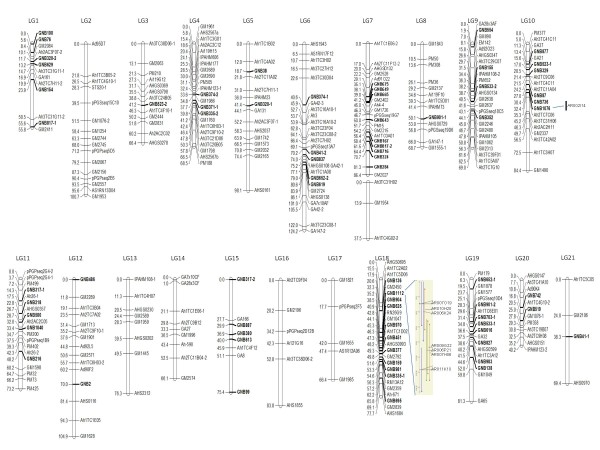
The BES-SSRs developed in this study and ~4,000 SSRs publicly available were evaluated for their genetic polymorphism between two parental genotypes Tifrunner and GT-C20. A total of 347 polymorphic DNA markers, including 68 BES-SSR markers, 7 target region amplification polymorphism (TRAP) markers, and 1 sequence tagged site (STS) marker, were identified and used to detect 385 segregating loci. Detailed information on mapped SSR primer sequences and motifs are given in Additional file 2. Among 347 polymorphic markers, 35 identified 2 independently segregating loci and 1 generated 3 independently segregating loci. These multi-locus SSRs are designated using the suffixes "-1" and "-2" after the locus name, for 2 and 3 independent loci, respectively. Chi-square (χ2) analysis identified 128 (32.3%) loci that deviated from the expected 1:2:1 or 3:1 segregation ratio at P > 0.05 level. Using a LOD score of 4 with the JoinMap4 software, 333 loci were mapped. Twenty-one LGs contained 318 loci that encompassed 1,674.4 cM of total map distance (Figure 3), while an additional 7 linkage groups contained 3 or fewer loci, for a total of 18 markers. The size of the 21 largest linkage groups ranged from 40.2 cM (12 loci) to 124 cM (26 loci), with an average distance of 5.3 cM between adjacent loci. Among the mapped loci, 71 (22.3%) were BAC-derived SSRs distributed among all but 5 of 21 linkage groups, i.e. LG2, LG13, LG14, LG16, and LG17 (Figure 3). Two markers (GNB1076 and GNB1112) from BAC-derived SSRs related to RGH were mapped to two different groups (LG10 and 18), and anchored 1 RGH-BAC contig containing 7 BAC clones and 1 singleton to this genetic linkage map.
Figure 3.
Genetic linkage map of the F2 population of Tifrunner × GT-C20 was constructed using SSR markers. The loci in bold were BES-SSRs. Two loci, GNB1076 and GNB1112, anchored one RGH-BAC singleton and one contig containing 7 RGH-BAC clones to linkage group 10 and 18, respectively.
Discussion
BAC end sequences have been shown to be a powerful tool for developing molecular markers. BAC-derived markers can be used to integrate physical maps with genetic maps [36,43], and also facilitate map-based cloning [44,45]. About 32.8 Mbp of peanut genomic sequences obtained from BAC end sequences was used for mining of SSR markers in this study. We also report a detailed analysis of these BAC derived SSR. Surprisingly, a large proportion of BES possess similarity to gene sequences (44.9% of the wild A. duranensis BES and 38.7% of the cultivated A. hypogaea BES), though we note that a significant fraction of these gene-containing BES may derive from retroelements. SSR frequency was 44.2 and 36.6 SSR/Mbp in wild and cultivated BAC clones, respectively. These differences may reflect differences between the genomes of the cultivated tetraploid genome and the wild diploid genome, but perhaps more likely reflect differences in the nature of the BAC clones (i.e., enriched for disease resistance genes in the case of A. hypogaea and randomly selected clones in the case of A. duranensis) or differences in the construction methods of the two BAC libraries (random-shear and partial HindIII cleavage, respectively).
Allelic diversity estimated for 148 BES derived SSR polymorphic markers was an average of 3.2 alleles per locus, and ranged from 2 to 8 alleles at each locus based on eight genotypes tested. This level of allelic diversity is lower than that reported in previous studies, including allele of 3 to 19 (mean 6.9) for 48 Valencia genotypes, 2 to 27 (mean 8.4) for 60 Brazilian genotypes [20], and 2 to 20 (mean 10.1) among 141 genotypes from the US mini core collection and wild species. However, Cuc et al. (2008) reported allele numbers ranging from 2 to 5 with a mean of 2.44 in 32 genotypes [23]. Although allelic diversity can be used as an indicator of genetic variation, such values are relative and depend on the number of polymorphic loci and the relatedness of genotypes analyzed. In this study, only 8 genotypes were used, all representing cultivated materials, both of which set upper ranges on the number of polymorphic loci that could be identified.
Much publicly available SSR data has been derived from AG repeat motif sequences using enrichment methods involving hybridization to SSR probes [17,19,23]. The use of AT sequences in such procedures is generally avoided because of the potential for the probe to form a hairpin structure, and thus to function inefficiently. Interestingly the current analysis of BES derived SSRs found that SSRs with AT motifs were the most frequent. The randomly selected BAC clones used for developing SSR markers most likely are a good representation of the diploid peanut genome, and thus the distributions and frequencies of the SSRs identified in this study are likely to be a good reflection of their genome-wide frequencies. Comparison of polymorphism rates among AT SSRs and AG SSRs shows that the former has a somewhat higher polymorphism rate than the latter (Table 5). For trinucleotide SSRs, polymorphism of the AAT SSRs is 3.2-fold higher than the polymorphism rate of AAG SSRs (Table 6). This result suggests that AT-rich SSR loci may have relatively high variability in peanut. Several studies have reported that SSRs with larger numbers of repeats have correspondingly higher rates of polymorphism [21,36,46]. Temnykh et al. [41] have suggested that SSRs could be divided into two classes: Class I were long and hypervariable markers, and Class II were short and typically less variable markers. The mutation rate of SSRs increases with repeat number, but long SSRs in eukaryotic genomes have a mutation bias to become shorter SSRs [47]. Our data also showed that both dinucleotide and trinucleotide SSRs in Class I detected more polymorphism than those in Class II. The finding suggested that it is worth developing markers based on long AT-rich SSRs to provide new informative SSR markers in peanut.
In peanut, several efforts have been made to construct genetic linkage maps and to meet the pre-requisites for marker-assisted selection in breeding and map-based cloning of desirable genes. The first genetic linkage map in peanut was constructed by Halward et al. [48] in an F2 population derived from a cross between two diploid wild species A. stenosperma and A. cardenasii using RFLP markers. Another RFLP-based map was developed from a BC1 tetraploid population of a synthetic amphidiploid A. batizocoi x (A. cardenasii x A. diogoi) crossed with cv. Florunner [49]. However, insufficient variability detected by RFLPs or RAPDs within A. hypogaea germplasm has hindered the construction of a genetic linkage map directly in cultivated peanut [48]. As more SSRs have been developed during the past decade, several SSR-based maps have been constructed, including AA genome and BB genome maps in wild x wild species populations [21,24,26,27], and some genetic maps in cultivated x cultivated populations [29-33]. These cultivated maps contain only ~200 SSR loci and thus require additional markers if they are to have utility for peanut breeding. The genetic map constructed by Hong et al. (2010) consisted of 175 SSR loci with a total coverage of 885.4 cM [50], this map was a consensus constructed using three cultivated x cultivated mapping populations. In this study, a large number of BES-SSRs were incorporated into a cultivated genetic map, increasing the number of mapped markers to 318 in a single cultivated mapping population, enlarging the coverage to 1,674 cM, and reducing average distance between two adjacent markers to 5.3 cM. However, there were seven groups containing only 3 or fewer loci and 49 loci could not be mapped, indicating that the linkage map remains incomplete.
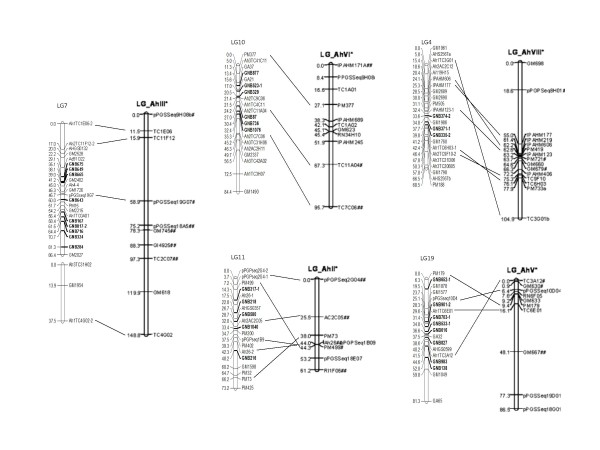
The use of common markers between the present map and previous maps allows a comparison of recombination frequency and marker order among mapping populations. Five linkage groups in the present map were chosen to compare with the first SSR-based peanut map [30], because there were several markers incommon between two maps (Figure 4). Comparison of these two maps reveals both conservation of marker order and rearranged order the between two populations. For instance, LG7 and LG10 in present map had the same marker order as LG_AhIII and LG_AhVI in previous map. Three other linkage groups, however, revealed rearrangements in marker order between two mapping populations (Figure 4).
Figure 4.
Comparison of marker colinear in present and previous linkage maps.
Ten percent of polymorphic SSRs surveyed more than one genetic locus. This situation, which has been noted previously by other authors [30,32], is presumed to derive from the high similarity of the two subgenomes of tetraploid A. hypogaea. In this study, most (82%) of the SSRs that amplified more than one locus were long SSRs (> 30 bp) or compound SSRs. Strachan and Read (1999) reported that SSRs with high repeat numbers are unstable during mitosis and meiosis in humans [51], and as a consequence are highly variable. By analogy, long SSRs are more likely to reveal distinct polymorphisms in the two subgenomes of allotetraploid peanut.
In parallel to the analysis of BAC-derived SSR markers, we have also used the TRAP marker technique to determine the potential application in peanut. Only seven (~2%) out of 400 TRAP primer pairs were polymorphic and were placed on the linkage map. This indicates that TRAP markers, the generation of which involves the use of arbitrary primers, have only a low chance of detecting polymorphism in the narrow genetic base in peanut.
One advantage of using BAC-derived markers in genetic linkage map construction is that physical contigs can be anchored on genetic map by mapping BAC-derived markers [37]. The integrated map would be useful in marker-assisted selection to introgress genes of interest into elite cultivars when BAC-derived markers reside in these genes, and would facilitate map-based cloning of genes and QTLs. In this study, 105 BAC-derived SSRs related to RGH-based physical contigs and singletons were developed. Only three of these SSRs were polymorphic between the two parents; two of these SSRs were mapped, thus anchoring 1 contig and 1 singleton into the current genetic map. The other marker, related to one RGH contig consisting of three clones was unmapped. Genetic linkage of candidate gene BAC contigs with phenotypes should enhance the opportunity for targeted marker development. In particular, the sequences of the BAC clones can provide the substrate for new marker development. Although we have mapped only a small number of RGH-containing BAC clones in this work, we have identified BAC contigs that contain the vast majority of ~580 peanut NBS-LRR RGH sequences (Rosen, He and Cook, unpublished data). Targeted marker development from these BAC clones holds potential to enhance the current peanut SSR framework with a large number of high value disease resistance candidate genes, and thus define the landscape of R-genes in peanut genome.
Conclusion
The SSRs mined from BAC end sequences of randomly selected clones and RGH containing clones in this study enlarge the pool of informative SSR markers in peanut. These new SSR markers provide useful genetic and genomic tools for molecular analysis of peanut genome, and facilitate the construction of higher density genetic linkage maps. In addition, SSR markers related to RGHs provide a valuable resource for incorporating RGH contigs and singletons into genetic map, which will ultimately increase our understanding of the distribution and organization of disease resistance genes in the peanut genome. Such information will facilitate marker-assisted selection for disease resistance breeding and map-based cloning of resistance genes.
Methods
Sequencing of BAC clone ends
To identify BAC clones which harbored RGHs, we used a BAC library representing an estimated 6x of the genome of peanut cv. Tifrunner together with 589 unique RGH sequences isolated from the genome of Tifrunner (unpublished results). Based on comparison to the sequenced genomes of Medicago truncatula and Glycine max, these 589 RGHs represent the vast majority of resistance gene homologs in peanut genome. Hybridization of the BAC library with a representative set of RGH sequence probes yielded a set of 3,784 BAC clones. These RGH containing BAC clones were assembled using high information content fingerprinting (HICF) [52], and resulted in 344 contigs and 334 singleton loci, for a total of 678 resistance gene regions in the peanut genome. All these 3,784 RGH containing BAC clones were used for end sequencing. In addition, 25,000 clones randomly selected from a BAC library from the wild species A. duranensis [38] were end sequenced. The BESs were subjected to a homology search for annotation by BLAST.
Identification of SSRs from BES and primer designing
After removing short or redundant sequences, BES sequences were used for mining SSRs using the MISA search module [53,54]. SSRs were identified using cutoff values of six repeats for dinucleotide, five repeats for trinucleotide, and four repeats for tetranucleotide SSRs. Based on length of SSRs and their potential as informative DNA markers, the identified SSRs were classified into two groups: Class I consisted of SSRs ≥ 20 bp, and Class II consisted of SSRs ≥ 12 bp < 20 bp [37,41]. Primers were designed to flank the SSR sequence and generate a PCR fragment between 150 and 300 bp. Primers were designed using the Primer3 software [55]. The designed primers were screened for polymorphism against eight cultivated genotypes, Tifrunner, GT-C20, SunOlic 97R, NC94022, D99, H22, Yue you 92, and Xin Hui Xiao Li, the first four of which being US peanut cultivars and the latter four Chinese cultivars or breeding lines. The informative SSRs were measured by PIC value [56].
Construction of a mapping population
The F2 mapping population was constructed at USDA-ARS, Coastal Plain Experimental Station at Tifton, Georgia using cv. Tifrunner and GT-C20 as parents. Tifrunner is a runner market-type cultivar with a high level of resistance to TSWV, and moderate resistance to early (Cercospora arachidicola) and late leaf spot (Cercosporidium personatum), but it is a late maturity cultivar [57]. GT-C20 is a Spanish-type breeding line and highly susceptible to TSWV and leaf spots but resistant to aflatoxin contamination [58]. Young leaves were taken from parents and 94 F2 individual plants for DNA isolation using a modified CTAB method [59].
Mapping of BES-SSRs
The polymorphic SSRs identified from BAC end sequences were used for construction of genetic linkage map. PCR was performed in a total volume of 10 μl of a reaction mixture containing 25 ng genomic DNA, 200 μΜ each dNTP, 0.1 μΜ each of forward primer with M13-tail and reverse primer, 0.1 μM M13 primer labelled by 700- or 800-IR dye (LI-COR Biosciences, NE), 1 × reaction buffer (10 mM KCl,10 mM (NH4)2SO4, 20 mM Tris-HCl, 2 mM MgSO4, 0.1% Triton X-100), and 0.25 U Taq DNA polymerase (BioLabs, Beverly, MA). Amplifications were performed using a DNA Engine Dyad (BioRad, CA) thermal cycler, with the following cycling conditions: an initial 5 min at 95°C; 35 cycles of 30 s at 94°C, 30 s at 55°C, and 30 s at 72°C; and a final 3 min 72°C. Amplicons were analyzed by LI-COR DNA analyzer (LI-COR Biosciences, NE). In addition, we have evaluated the feasibility of TRAP [60] markers using sequence related amplified polymorphism (SRAP) [61] primer paired with RGH primer to detect a polymorphism in peanut. Four hundred such primer pairs were screened. PCR reaction mixture included 25 ng genomic DNA, 200 μM each dNTP, 0.375 μM each primer, 1 × reaction buffer, and 0.2 U Taq DNA polymerase (BioLabs, Beverly, MA) in 10 μl. PCR condition was following the protocol of Hu and Vick [60]. The polymorphic BAC-derived SSR markers and TRAP markers combined with polymorphic SSRs from other public resources were used for mapping. Marker order and map distances were determined by JoinMap4 software (Kyazma, Netherlands) with the Kosambi map function [62].
Authors' contributions
HW, LG, YM, and YZ carried out genotyping and genetic linkage mapping. YM and JG conducted hybridization of BAC library with RGH probes, which were provided by BDR. RVP, ADF, and GH carried out SSR mining, primer designing, sequence annotation, and data analysis for BES-SSRs. BZ, SI, DJB, and RKV provided their developed SSRs. BZ established the mapping population and provided plant materials. DRC conceived the study, designed experiments and coordinated the study. RVP, DJB, RKV, DRC and GH participated in drafting the manuscript. GH finalized the manuscript. All authors read manuscript.
Supplementary Material
List of BES-SSRs.
List of mapped SSRs in the F2 mapping population of Tifrunner × GT-C20.
Contributor Information
Hui Wang, Email: wanghuilfx@163.com.
R Varma Penmetsa, Email: rvpenmetsa@ucdavis.edu.
Mei Yuan, Email: yuanbeauty@126.com.
Limin Gong, Email: gonglimin2001@gmail.com.
Yongli Zhao, Email: huobao1314@hotmail.com.
Baozhu Guo, Email: baozhu.guo@ars.usda.gov.
Andrew D Farmer, Email: adf@ncgr.org.
Benjamin D Rosen, Email: bdrosen@ucdavis.edu.
Jinliang Gao, Email: jgao@ucdavis.edu.
Sachiko Isobe, Email: sisobe@kazusa.or.jp.
David J Bertioli, Email: david.bertioli@pq.cnpq.br.
Rajeev K Varshney, Email: r.k.varshney@cgiar.org.
Douglas R Cook, Email: drcook@ucdavis.edu.
Guohao He, Email: hguohao@mytu.tuskegee.edu.
Acknowledgements
Authors are thankful to National Science Foundation (NSF, DBI-0605251) and USDA/CSREES/Capacity Building Program (#2006-38814-17489) for supporting this research.
References
- Naidu RA, Kimmins FM, Deom CM, Subrahmanyam P, Chiyembekeza AJ, van der Merwe PJA. Groundnut rosette: a virus disease affecting groundnut production in sub-Saharan Africa. Plant Dis. 1999;83:700–709. doi: 10.1094/PDIS.1999.83.8.700. [DOI] [PubMed] [Google Scholar]
- Savage GP, Keenan JI. In: The Groundnut Crop: A Scientific Basis for Improvement. Smartt J, editor. London: Chapman & Hall; 1994. The composition and nutritive value of groundnut kernels; pp. 173–213. [Google Scholar]
- Sprent J. In: The Groundnut Crop A Scientific Basis for Improvement. Smartt J, editor. London: Chapman & Hall; 1994. Nitrogen fixation. [Google Scholar]
- Arumuganatham, Earle. Nuclear DNA content of some important plant species. Plant Mol Biol Rep. 1991;9:211–215. [Google Scholar]
- Kochert G, Halward T, Branch WD, Simpson CE. RFLP variability in peanut (Arachis hypogaea L.) cultivars and wild species. Theor Appl Genet. 1991;81:565–570. doi: 10.1007/BF00226719. [DOI] [PubMed] [Google Scholar]
- Halward TM, Stalker HT, Larue EA, Kochert G. Genetic variation detectable with molecular markers among unadapted germplasm resources of cultivated peanut and related wild species. Genome. 1991;34:1013–1020. doi: 10.1139/g91-156. [DOI] [Google Scholar]
- Halward TM, Stalker HT, LaRue E, Kochert G. Use of single-primer DNA amplification in genetic studies of peanut (Arachis hypogaea L.) Plant Mol Biol. 1992;18:315–325. doi: 10.1007/BF00034958. [DOI] [PubMed] [Google Scholar]
- Paik-Ro OG, Smith RL, Knauft DA. Restriction fragment length polymorphism evaluation of six peanut species within the Arachis section. Theor Appl Genet. 1992;84:201–208. doi: 10.1007/BF00224001. [DOI] [PubMed] [Google Scholar]
- Stalker HT, Phillips TD, Murphy JP, Jones TM. Variation of isozyme patterns among Arachis species. Theor Appl Genet. 1994;87:746–755. doi: 10.1007/BF00222901. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Gurtu S, Rao RCN, Nigam SN. Identification of DNA polymorphism in cultivated groundnut using random amplified polymorphic DNA (RAPD) assay. Genome. 2000;43(4):656–660. doi: 10.1139/g00-034. [DOI] [PubMed] [Google Scholar]
- Kochert G, Stalker HT, Gimenes M, Galgaro L, Lopes CR, Moore K. RFLP and cytogenetic evidence on the origin and evolution of allotetraploid domesticated peanut, Arachis hypogaea (Leguminosae) Am J Bot. 1996;83:1282–1291. doi: 10.2307/2446112. [DOI] [Google Scholar]
- Seijo JG, Lavia GL, Fernandez A, Krapovickas A, Ducasse D, Moscone EA. Physical mapping of the 5S and 18S-25S rRNA genes by FISH as evidence that Arachis duranensis and A. ipaensis are the wild diploid progenitors of A. hypogaea (Leguminosae) Am J Bot. 2004;9:1294–1303. doi: 10.3732/ajb.91.9.1294. [DOI] [PubMed] [Google Scholar]
- Seijo GJ, Lavia GI, Fernandez A, Krapovickas A, Ducasse D, Bertioli DJ, Moscone DEA. Genomic relationships between the cultivated peanut (Arachis hypogaea - Leguminosae) and its close relatives revealed by double GISH. Am J Bot. 2007;94:1963–1971. doi: 10.3732/ajb.94.12.1963. [DOI] [PubMed] [Google Scholar]
- Favero AP, Simpson CE, Valls JFM, Vello NA. Study of the evolution of cultivated peanut through crossability studies among Arachis ipaensis, A. duranensis, and A. hypogaea. Crop Sci. 2006;46:1546–1552. doi: 10.2135/cropsci2005.09-0331. [DOI] [Google Scholar]
- Burow MD, Simpson CE, Faries MW, Starr JL, Paterson AH. Molecular biogeographic study of recently described B- and A-genome Arachis species, also providing new insights into the origins of cultivated peanut. Genome. 2009;52:107–119. doi: 10.1139/G08-094. [DOI] [PubMed] [Google Scholar]
- Hopkins MS, Casa AM, Wang T, Mitchell SE, Dean RE, Kochert GD, Kresovich S. Discovery and characterization of polymorphic simple sequence repeats (SSRs) in peanut. Crop Sci. 1999;39:1243–1247. doi: 10.2135/cropsci1999.0011183X003900040047x. [DOI] [Google Scholar]
- He GH, Meng RH, Newman M, Gao GQ, Pittman RN, Prakash CS. Microsatellites as DNA markers in cultivated peanut (Arachis hypogaea L.) BMC Plant Biol. 2003;3:3. doi: 10.1186/1471-2229-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He GH, Meng RH, Gao H, Guo B, Gao G, Newman M, Pittman RN, Prakash CS. Simple sequence repeat markers for botanical varieties of cultivated peanut (Arachis hypogaea L.) Euphytica. 2005;142:131–136. doi: 10.1007/s10681-005-1043-3. [DOI] [Google Scholar]
- Ferguson ME, Burow MD, Schulze SR, Bramel PJ, Paterson AH, Kresovich S, Mitchell S. Microsatellite identification and characterization in peanut (A. hypogaea L.) Theor Appl Genet. 2004;108:1064–1070. doi: 10.1007/s00122-003-1535-2. [DOI] [PubMed] [Google Scholar]
- Moretzsohn MC, Hopkins MS, Mitchell SE, Kresovich S, Valls JFM, Ferreira ME. Genetic diversity of peanut (Arachis hypogaea L.) and its wild relatives based on the analysis of hypervariable regions of the genome. BMC Plant Biol. 2004;4:11. doi: 10.1186/1471-2229-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretzsohn MC, Leoi L, Proite K, Guimaraes PM, Leal-Bertioli SCM, Gimenes MA, Martins WS, Valls JFM, Grattapaglia D, Bertioli DJ. A microsatellite-based, gene-rich linkage map for the AA genome of Arachis (Fabaceae) Theor Appl Genet. 2005;111(6):1060–1071. doi: 10.1007/s00122-005-0028-x. [DOI] [PubMed] [Google Scholar]
- Proite K, Leal-Bertioli SCM, Bertioli DJ, Moretzsohn MC, da Silva FR, Martins NF, Guimaraes PM. ESTs from a wild Arachis species for gene discovery and marker development. BMC Plant Biol. 2007;7:7. doi: 10.1186/1471-2229-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuc LM, Mace ES, Crouch JH, Quang VD, Long TD, Varshney RK. Isolation and characterization of novel microsatellite markers and their application for diversity assessment in cultivated groundnut (Arachis hypogaea L.) BMC Plant Biol. 2008;8:55. doi: 10.1186/1471-2229-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Bertioli SCM, Jose Ana CVF, Freitas Dione MTA, Moretzsohn MC, Guimaraes PM, Nielen S, Vidigal BS, Pereira RW, Pike J, Favero AP, Parniske M, Varshney RK, Bertioli DJ. Identification of candidate genome regions controlling disease resistance in Arachis. BMC Plant Biol. 2009;9:112. doi: 10.1186/1471-2229-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautami B, Ravi K, Lakshmi NM, Hoisington DA, Varshney RK. Novel set of groundnut SSRs for genetic diversity and interspecific transferability. Int J Integr Biol. 2009;7:100–106. [Google Scholar]
- Bertioli D, Moretzsohn M, Madsen LH, Sandal N, Leal-Bertioli S, Guimarães P, Hougaard BK, Fredslund J, Schauser L, Nielsen AM, Sato S, Tabata S, Cannon S, Stougaard J. An analysis of synteny of Arachis with Lotus and Medicago sheds new light on the structure, stability and evolution of legume genomes. BMC Genomics. 2009;10:45. doi: 10.1186/1471-2164-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretzsohn MC, Barbosa AVG, Alves-freitas DMT, Teizeira C, Leal-Bertioli SCM, Guimaraes PM, Pereira RW, Lopes CR, Cavallari MM, Valls JFM, Bertioli DJ, Gimenes MA. A linkage map for the B-genome of Arachis (Fabaceae) and its synteny to the A-genome. BMC Plant Biol. 2009;9:40. doi: 10.1186/1471-2229-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonceka D, Hodo-Abalo T, Rivallan R, Faye I, Ndoye M, Ndoye O, Favero AP, Bertioli DJ, Glaszmann JC, Courtois B, Rami JF. Genetic mapping of wild introgressions into cultivated peanut: a way toward enlarging the genetic basis of a recent allotetraploid. BMC Plant Biol. 2009;9:103. doi: 10.1186/1471-2229-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YB, Liang XQ, Chen XP, Liu HY, Zhou GY, Li SX, Wen SJ. Construction of genetic linkage map based on SSR markers in peanut (Arachis hypogaea L.) Agric Sci in China. 2008;7(8):915–921. doi: 10.1016/S1671-2927(08)60130-3. [DOI] [Google Scholar]
- Varshney RK, Bertioli DJ, Moretzsohn MC, Vadez V, Krishramurthy L, Aruma R, Nigam SN, Moss BJ, Seetha K, Ravi K, He GH, Knapp SJ, Hoisington DA. The first SSR-based genetic linkage map for cultivated groundnut (Arachis hypogaea L.) Theor Appl Genet. 2009;118(4):729–39. doi: 10.1007/s00122-008-0933-x. [DOI] [PubMed] [Google Scholar]
- Khedikar YP, Gowda MVC, Sarvamangala C, Patgar KV, Upadhyaya HD, Varshney RK. A QTL study on late leaf spot and rust revealed one major QTL for molecular breeding for rust resistance in groundnut (Arachis hypogaea L.) Theor Appl Genet. 2010;121:971–984. doi: 10.1007/s00122-010-1366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi K, Vadez V, Isobe S, Mir RR, Guo Y, Nigam SN, Gowda MVC, Radhakrishnan T, Bertioli DJ, Knapp SJ, Varshney RK. Identification of several small main-effect QTLs and a large number of epistatic QTLs for drought tolerance related traits in groundnut (Arachis hypogaea L.) Theor Appl Genet. 2011;122:1119–1132. doi: 10.1007/s00122-010-1517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvamangala C, Gowda MVC, Varshney RK. Identification of quantitative trait loci for protein content, oil content and oil quality for groundnut (Arachis hypogaea L.) Field Crops Res. 2011. doi:10.1016/j.fcr.2011.02.010.
- Yu JZ, Kohel RJ, Dong J. Proceeding of Beltwide Cotton Improvement Conference. Atlanta: CD-Rom Published by National Cotton Council of America; 2002. Development of integrative SSR markers from TM-1 BACs. January 8-12. [Google Scholar]
- Guo Y, Sukumar S, Yu JZ, Jenkins JN, Kohel RJ, Scheffler BE, Stelly DM. BAC-derived SSR markers chromosome locations in cotton. Euphytica. 2008;161:361–370. doi: 10.1007/s10681-007-9585-1. [DOI] [Google Scholar]
- Mun JH, Kim DJ, Choi HK, Gish J, Debelle F, Mudge J, Denny R, Endre G, Saurat O, Dudez AM, Kiss GB, Roe B, Young ND, Cook DR. Distribution of microsatellites in the genome of Medicago truncatula: a resource of genetic markers that integrate genetic and physical maps. Genetics. 2006;172:2541–2555. doi: 10.1534/genetics.105.054791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohra A, Dubey A, Saxena RK, Penmetsa RV, Poornima KN, Kumar N, Framer AD, Srivani G, Upadhyaya HD, Gothalwal R, Ramesh S, Singh D, Saxena K, Kavikishor PB, Singh NK, Town CD, May GD, Cook DR, Varshney RK. Analysis of BAC-end sequences (BESs) and development of BES-SSR markers for genetic mapping and hybrid purity assessment in pigeonpea (Cajanus spp.) BMC Plant Biol. 2011;11:56. doi: 10.1186/1471-2229-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães PM, Garsmeur O, Proite K, Leal-Bertioli SCM, Seijo G, Chaine C, Bertioli DJ, D'Hont A. BAC libraries construction from the ancestral diploid genomes of the allotetraploid cultivated peanut. BMC Plant Biol. 2008;8:14. doi: 10.1186/1471-2229-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S, Apweiler R, Attwood TK, Bairoch A, Bateman A, Binns D, Bork P, Das U, Daugherty L, Duquenne L, Finn RD, Gough J, Haft D, Hulo N, Kahn D, Kelly E, Laugraud A, Letunic I, Lonsdale D, Lopez R, Madera M, Maslen J, McAnulla C, McDowall J, Mistry J, Mitchell A, Mulder N, Natale D, Orengo C, Quinn AF, Delengut JD, Sigrist CJA, Thimma M, Thomas PD, Valentin F, Wilson D, Wu CH, Yeats C. InterPro: the integrative protein signature database. Nucl Acids Res. 2009;37(Suppl 1):D211–D215. doi: 10.1093/nar/gkn785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BZ, Chen XP, Hong YB, Liang XQ, Dang P, Brenneman T, Holbrook C, Culbreath A. Analysis of gene expression profiles in leaf tissues of cultivated peanuts and development of EST-SSR markers and gene discovery. Intl J Plant Genomics. 2009. doi:10.1155/2009/715605. [DOI] [PMC free article] [PubMed]
- Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhour S, McCouch S. Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon association, and genetic marker potential. Genome Res. 2001;11:1441–1452. doi: 10.1101/gr.184001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker RC, Grant D, Olson T, Warren WC, Wing R. et al. Microsatellite discovery from BAC end sequences and genetic mapping to anchor the soybean physical and genetic maps. Genome. 2008;51:294–302. doi: 10.1139/G08-010. [DOI] [PubMed] [Google Scholar]
- Ohyama A, Asamizu E, Negoro S, Miyatake K, Yamaguchi H, Tabata S, Fukuoka H. Characterization of tomato SSR markers developed using BAC-end and cDNA sequences from genome databases. Mol Breeding. 2009;23:685–691. doi: 10.1007/s11032-009-9265-z. [DOI] [Google Scholar]
- McNeil MD, Kota R, Paux E, Dunn D, McLean R, Feuillet C, Li D, Kong X, Lagudah E, Zhang JC, Jia JZ, Spielmeyer W, Bellgard M, Appels R. BAC-derived markers for assaying the stem rust resistance gene, Sr2, in wheat breeding programs. Mol Breeding. 2008;22:15–24. doi: 10.1007/s11032-007-9152-4. [DOI] [Google Scholar]
- David P, Sevignac M, Thareau V, Catillon Y, Kami J, Gepts P, Langin T, Geffroy V. BAC end sequences corresponding to the B4 resistance gene cluster in common bean: a resource for markers and synteny analyses. Mol Genet Genomics. 2008;280:521–533. doi: 10.1007/s00438-008-0384-8. [DOI] [PubMed] [Google Scholar]
- Burstin J, Deniot G, Potier J, Weinachter C, Aubert G, Baranger A. Microsatellite polymorphism in Pisum sativum. Plant Breeding. 2001;120:311–317. doi: 10.1046/j.1439-0523.2001.00608.x. [DOI] [Google Scholar]
- Hong CP, Piao ZY, Kang TW, Batley J, Yang TJ, Hur YK, Bhak J, Park BS, Edwards D, Lim YP. Genomic distribution of simple sequence repeats in Brassica rapa. Mol Cells. 2007;23(3):349–356. [PubMed] [Google Scholar]
- Halward TM, Stalker HT, Kochert G. Development of an RFLP linkage map in diploid peanut species. Theor Appl Genet. 1993;87:379–384. doi: 10.1007/BF01184927. [DOI] [PubMed] [Google Scholar]
- Burow MD, Simpson CE, Starr JL, Paterson AH. Transmission genetics of chromatin from a synthetic amphidiploid to cultivated peanut (Arachis hypogaea L.) broadening the gene pool of a monophyletic polyploidy species. Genetics. 2001;159:823–837. doi: 10.1093/genetics/159.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YB, Chen XP, Liang XQ, Liu HY, Zhou GY, Li SX, Wen SJ, Holbrook CC, Guo BZ. A SSR-based composite genetic linkage map for the cultivated peanut (Arachis hypogaea L.) genome. BMC Plant Biol. 2010;10:17. doi: 10.1186/1471-2229-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan T, Read AP. Human Molecular Genetics II. 2. Oxford: BIOS Scientific Publishers Ltd; 1999. [Google Scholar]
- Luo MC, Thomas C, You FM, Hsiao J, Ouyang S, Buell CR, Malandro M, McGuire PE, Anderson OD, Dvorak J. High-throughput fingerprinting of bacterial artificial chromosomes using the SNaPshot labeling kit and sizing of restriction fragments by capillary electrophoresis. Genomics. 2003;82:378–389. doi: 10.1016/S0888-7543(03)00128-9. [DOI] [PubMed] [Google Scholar]
- Varshney RK, Thiel T, Stein N, Langridge P, Graner A. In silico analysis on frequency and distribution of microsatellites in ESTs of some cereal species. Cell Mol Biol Lett. 2002;7:537–546. [PubMed] [Google Scholar]
- Thiel T, Michalek W, Varshney RK, Graner A. Exploiting EST databases for the development and characterization of gene derived SSR-markers in barley (Hordeum vulgare L.) Theor Appl Genet. 2003;106:411–422. doi: 10.1007/s00122-002-1031-0. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. In: Bioinformatics Methods And Protocols: Methods in Molecular Biology. Krawetz S, Misener S, editor. Totowa: Humana Press; 2000. Primer3 on the WWW for general users and for biologist progrmmers; pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- Holbrook CC, Culbreath AK. Registration of 'Tifrunner' peanut. J Plant Reg. 2007;1:124. doi: 10.3198/jpr2006.09.0575crc. [DOI] [Google Scholar]
- Liang X, Holbrook CC, Lynch RE, Guo BZ. Beta-1,3-glucanase activity in peanut seed (Arachis hypogaea) is induced by inoculation with Aspergillus flavus and copurifies with a conglutin-like protein. Phytopathology. 2005;95:506–511. doi: 10.1094/PHYTO-95-0506. [DOI] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Jinguo, Vick BA. Target region amplification polymorphism: A novel marker technique for plant genotyping. Plant Mol Biol Reporter. 2003;21:289–294. doi: 10.1007/BF02772804. [DOI] [Google Scholar]
- Li G, Quiros CF. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theor Appl Genet. 2001;103:455–461. doi: 10.1007/s001220100570. [DOI] [Google Scholar]
- Kosambi DD. The estimation of map distances from recombination values. Ann Eugen. 1944;12:172–175. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of BES-SSRs.
List of mapped SSRs in the F2 mapping population of Tifrunner × GT-C20.