Abstract
Adverse early experiences are associated with a range of deleterious health outcomes in humans, including higher risk for affective disorders. Studies using a long-standing model of nonhuman primate model of early adversity have demonstrated that nursery-reared monkeys exhibit alterations in multiple aspects of biobehavioral development; however, few studies have evaluated the persistence of socioaffective behavioral changes through adulthood. We evaluated the effects of early rearing experience on adult animals’ response to a well-validated assessment of anxiety-like behavior, the Human Intruder Paradigm (HIP). We tested twenty-two rhesus monkeys who were either nursery-reared (NR) or reared with their mothers (mother-reared; MR). NR monkeys were inhibited in their behavior compared to MR monkeys, with reduced locomotion and exploratory behaviors. NR animals showed a marginal increase in freezing. Together these findings demonstrate that the consequences of differential infant rearing experience on socioaffective behavior persist into adulthood, with evidence of greater inhibition in NR monkeys.
Keywords: Rhesus Monkey, Early Experience, Human Intruder, Affective Behavior, Adult, Lifespan
Introduction
Childhood stress and deprivation contribute to increased risk for a wide-range of deleterious health outcomes that include increased vulnerability for affective disorders and developmental psychopathology (Nelson & Winslow, 2009; Ames, Fraser, & Burnaby, 1997; O’Connor & Rutter, 2000; Rutter, 1980; Rutter, 1998). Among these is increased risk for anxiety disorders (for review Penza, Heim, & Nemeroff, 2003; Heim & Nemeroff, 2001; Kim & Gorman, 2005). In humans the effects of early trauma often persist into adulthood. Understanding how early adverse experiences contribute to individual differences in development across the lifespan is important to developing targeted prevention, intervention, and treatment strategies for affective disorders. Animal models contribute to this effort by providing an avenue for controlled comparison of individuals that vary in early experience.
In nonhuman primates, the effects of early differential environments have been studied by comparing animals reared by their mothers to those maternally separated at birth and reared in a nursery in relative social impoverishment. Over the past five decades, a wide range of studies have demonstrated that various nursery-rearing paradigms produce monkeys that differ from mother-reared monkeys in aspects of behavior (Clarke & Snipes, 1998; Meyer, Novak, Bowman, & Harlow, 1975; Young, Suomi, Harlow, & McKinney, Jr., 1973), physiology (Shannon, Champoux, & Suomi, 1998; Barr et al., 2004; Lewis, Gluck, Petitto, Hensley, & Ozer, 2000), and neurobiology (Ichise et al., 2006; Sanchez, Hearn, Do, Rilling, & Herndon, 1998; Struble & Riesen, 1978). Many of the behavioral and socio-emotional characteristics of nursery-reared monkeys parallel features of the affective disorders for which humans with early adverse experiences are at higher risk (Machado & Bachevalier, 2003; Sanchez, Ladd, & Plotsky, 2001; Gilmer & McKinney, 2003; Teicher, Tomoda, & Andersen, 2006; for discussion and review). For example, compared to their mother-reared counterparts, nursery-reared animals are more reactive to stressful situations (Young et al., 1973; Shannon et al., 1998; Meyer et al., 1975). The great majority of previous findings on behavioral differences between mother- and nursery-reared monkeys, however, are from group comparisons of animals still within the early life maturational period. As a result, there are few data that speak to the persistence of early rearing effects on monkeys’ socioaffective behavior in adulthood. Neither are there longitudinal studies that address the potential for differential developmental changes in anxious behavior in monkeys with different early rearing experiences.
The goal of the study reported here was to evaluate the consequences of differential early rearing on adult rhesus monkeys’ behavioral response to a mild challenge by using the human intruder paradigm (HIP). The HIP is a well-validated and widely-used test of anxiety-related behavior and behavioral inhibition in response to social separation and to ecologically-relevant threats (see Table 1). A series of studies by Kalin and his colleagues (Kalin & Shelton, 2003 for review) have established that young monkeys’ behavior in response to each of these challenges varies in a manner that appears adaptive when the type of threat is considered within an ecological context (Kalin & Shelton, 1989; Kalin & Shelton, 1998a; Kalin, Shelton, & Takahashi, 1991). Animals that exhibit high levels of freezing in response to challenge are categorized as behaviorally inhibited. Neurobiological correlates of behavioral inhibition in these animals are consistent with those in human populations with anxiety disorders, including increased hypothalamic-pituitary-adrenal (HPA) axis activity (Kalin, Shelton, Rickman, & Davidson, 1998b), increased cerebrospinal fluid levels of corticotrophin releasing factor (Kalin, Shelton, & Davidson, 2000), and extreme right frontal brain activity (Kalin, Larson, Shelton, & Davidson, 1998). Furthermore, studies using HIP tests have shown that behavioral responses elicited by the challenge conditions are affected by targeted brain lesions to the limbic system (Izquierdo & Murray, 2004; Izquierdo, Suda, & Murray, 2005; Kalin, Shelton, & Davidson, 2004; Kalin, Shelton, & Davidson, 2007; Machado & Bachevalier, 2008; Mason, Capitanio, Machado, Mendoza, & Amaral, 2006), can be modulated by benzodiazepines (Kalin, Shelton, & Turner, 1991), intensified by anxiogenics (Kalin & Shelton, 1992), altered by changes in diet (Golub et al., 2006; Golub, Hogrefe, Widaman, & Capitanio, 2009; Sullivan et al., 2010), and are associated with immune response (Friedman, Reyes, & Coe, 1996; Willette, Lubach, & Coe, 2007). Although monkeys with early adverse experiences show exacerbated response to a range of challenges (e.g., social separation, response to novelty), only a few studies have examined the behavior of nursery- and mother-reared animals by using a variant of the HIP procedure (Capitanio, Mason, Mendoza, DelRosso, & Roberts, 2006; Karere et al., 2009; Kinnally et al., 2010). Capitanio et al. (2006) found that nursery-reared animals (aged 90-120 days) had lower levels of whole body activity and higher frequencies of vocalizations across four conditions of intruder challenges (profile near, profile far, stare near, and stare far).
Table 1.
Summary of human intruder paradigm studies (HIP) and their primary findings
| Age | Authors | Year | Primary Findings in relation to Human Intruder Paradigm |
|---|---|---|---|
| Infant (0-12 mo) | Kalin & Shelton | 1989 | Increased freezing in NEC. Increased bark in ST. Strong correlation across weeks. |
| Kalin, Shelton, Takahashi | 1991 | Differences with intruder present appear between 9-12 weeks old. | |
| Kalin, Shelton, Turner | 1991 | More vocalizations, lip smack, toothgrind, hostiliity in ST. More locomotion without an intruder, more Freezing in NEC. Alprazolam reduces barks, tooth grinding in ST and across all conditions for lipsmack. | |
| Kalin, Shelton, Turner | 1992 | Anxiogenics increase freezing, environment explore, hostility and barking, decreases coo. | |
| Kalin, Shelton, Rickman, Davidson | 1998 | Infants freeze more in NEC. Cortisol and NEC freeze positively correlated. | |
| Kalin & Shelton | 1998 | No sex differences. Coo decreases with age. Freeze stable across tests. | |
| *Williamson, Coleman, Bacanu, Devlin, Rogers, Ryan, Cameron | 2003 | Movement behaviors during AL, NEC, A2 highly heritable. | |
| *Coleman, Dahl, Ryan, Cameron | 2003 | Animals that froze during NEC had higher GH responsiveness, those that didn’t respond to intruder had a lower GH responsiveness. | |
| *Bethea, Streicher, Coleman, Pau, Moessner, Cameron | 2004 | Monkeys with s/s allele of 5HTTLPR make more threats in ST compared to l/l and l/s. | |
| *Capitanio, Mason, Mendoza, DelRosso, Roberts | 2006 | NR infants less active and more vocals than MR infants. | |
| *Golub, Hogrefe, Germann, Capitanio & Lozoff | 2006 | Prenatally iron-depreived animals less active compared to controls except in ST. | |
| *Golub, Hogrefe, Widaman, Capitanio | 2009 | Males increased threat behavior and distress index compared to females. Iron-deficient males had less threat behavior and distress index compared to controls. | |
| *Karere, Kinnally, Sanchez, Famula, Lyons, Capitanio | 2009 | NR animals showed the greatest amount of fear. | |
| *Sullivan, Grayson, Takahashi, Robertson, Maier, Bethea, Smith, Coleman, Grove | 2010 | 78% of high fat diet animals elicited more aggression/anxiety behaviors compared to 11% control animals in Japanese macaques. | |
| *Kinnally, Karere, Lyons, Mendoza, Mason, Capitanio | 2010 | NR infants increased emotional and behavioral reactivity compared to MR. | |
| *Capitanio, Medozza, Cole | 2011 | High-nervous animals have higher rates of negative emotional behaviors, lower rates of positive emotional behaviors, and lower rates of anxious behavior, but were not significantly different from low-nervous animals for durations of activity behaviors. | |
| Juvenile (1-2.3 yr) | Davidson, Kalin, Shelton | 1993 | EEG frontal asymmetry difference score is positively correlated with freeze. |
| Friedman, Reyes, Coe | 1996 | Increased agitation in ST. Both doses of IL-1 significantly lowered frequencies of yawns. 25mg IL-1 significantly increased yawns in ST. | |
| Kalin, Larson, Shelton, Davidson | 1998 | Cortisol positively correlated with ST bark and hostility. Animals scoring high right activation on EEG froze more in AL and NEC and were more hostile in ST. | |
| Kalin, Shelton, Davidson, Kelley | 2001 | No effect of amygdala lesions on freezing in NEC. | |
| Willette, Lubach, Coe | 2007 | Lippolysaccharide injections decreased locomotion in AL. | |
| Rogers, Shelton, Shelledy, Garcia, Kalin | 2008 | Freeze and orient to intruder heritable. No genetic variation relationships. | |
| *Fox, Shelton, Oakes, Davidson, Kalin | 2008 | NEC produced significant brain activation in left and right amygdala, left hippocampus and left brain stem. A1 produced significant activity in right amygdala and right hippocampus. | |
| Peri-pubertal (2.5-4.6yr) | Rosenblum, Forgerg, Noland, Trost, Coplan | 2001 | Variable foraging demands during infancy animals were less responsive to a clown masked intruder compared to normal foraging demand infants. |
| Kalin, Shelton, Davidson | 2004 | Bilateral central nucleas of the amygdala lesions decrease freezing and increase cooing. | |
| Izquierdo and Murray | 2004 | No group effects of unilateral amygdala and orbital prefrontal cortex on behavior. | |
| Kalin, Shelton, Fox, Oakes, Davidson | 2005 | Activity in the bed nucleus of the stria terminalis and the nucleus accumbens positively correlated with freezing in AL and NEC. | |
| Izquierdo, Suda, Murray | 2005 | Orbital prefrtonal cortex lesioned animals spent more time aggressing in ST. | |
| Kalin, Shelton, Davidson | 2007 | Bilateral orbitofrontal cortex aspiration lesions decrease NEC freezing. | |
| Machado & Bachevalier | 2008 | Ibotonic acid amygala and orbitofrontal asperation lesioned animals froze less following surgery in AL, NEC, and ST conditions. | |
| Kalin, Shelton, Fox, Rogers, Oakes, Davidson | 2008 | Increase in amygdala activation in response to AL. | |
| Young Adult (5.9-6.5yr) | *Capitanio | 1999 | Excitablity personality animals spent more time in front of cage and increased threats in ST. |
| *Mason, Capitanio, Machado, Mendoza, Amaral | 2006 | Bilateral amygdala lesioned animals spend more time at front of cage across all conditions. | |
| Adult (11 yr) | Kalin, Shelton, Rickman, Davidson | 1998 | Mothers following separation freeze more in NEC. Cortisol and NEC freeze positively correlated. |
Study employs a variation of the typical HIP methods described by Kalin and colleagues, but still includes NEC and ST conditions.
Together these findings demonstrate that the HIP is a strong tool for assessing meaningful individual differences in anxiety-like behavior and behavioral inhibition. At the same time nearly all of these studies were conducted with sub-adult animals. To our knowledge only one study of full adult animals has been conducted (Kalin et al., 1998; see Table 1) and that study was limited to females. As a result, there are scarce data from which to evaluate its success in providing a robust measure of individual variation in anxiety-like behavior across the lifespan. In the study reported here, we tested adult male nursery- and mother-reared rhesus monkeys with the HIP. The aim of the study was two-fold: First, to assess adult male monkeys’ behavioral responses to the HIP; and, second, to determine whether early differential rearing (NR vs MR) had the long-term consequence of influencing animals’ anxiety-like behavior and behavioral inhibition in response to the HIP in adulthood.
Methods and Materials
Experimental Subjects
Subjects were 22 adult (M=10.94 years-of-age, SD±3.87, range=6.60-14.61) male rhesus monkeys. All animals were born at the Laboratory of Comparative Ethology at the National Institute of Child Health and Human Development and transferred to Wake Forest University School of Medicine a minimum of three years before this testing occurred. Subjects were either pair- or individually-housed in pens that provided indoor and outdoor access (5.49 × 2.74 × 2.44 m) and fed commercial monkey chow (Purina #5038) twice daily (1000 and 1645 h) or individually-housed in quadrant cages (76 × 60 × 70 cm3) while being fed a diet of Primate Food pellets (Research Diets Inc., New Brunswick, N.J.). All animals had water available ad libitum and were supplemented with fresh fruits and vegetables. This study was conducted in accordance with the Guidelines of the Committee on the Care and Use of Laboratory Animal Resources (NRC, 1996) and approved by the Wake Forest University Animal Care and Use Committee.
Manipulation of Early Rearing Environment
Half of the animals were mother-reared in their infancy. Mother-reared (MR) subjects lived from birth to approximately 7-months of age in social groups housed in indoor-outdoor cages equipped with a variety of climbing and perch substrates. Monkeys were fed chow twice daily and had ad libitum access to water. Over the course of the first 6-months of life the diet of the mother-reared subjects consisted of breast milk and weaning to chow and water. At 7-months old age-matched peer groups were created.
The other group of animals was nursery-reared (NR) in infancy. NR animals were separated from their mothers within 24-hr of birth, moved to a neonatal nursery, and reared under surrogate-peer-reared (n=5; SPR) or peer-reared (n=6; PR) conditions using procedures based on those developed at the University of Wisconsin Harlow Primate Laboratory (Shannon et al., 1998; see Novak & Sackett, 2006 for detailed description and comparison of nursery-rearing conditions). Briefly, infants were housed in an incubator containing an inanimate surrogate equipped with a spring to allow rocking motion, covered with a heating pad, and encased in thick fleece. Infants were fed formula (50:50 Similac:Primilac) and weaned to solid foods and water over the course of 6-months. At 14-days infants were moved to one quadrant of a cage within a room of the nursery and within visual, auditory, and olfactory contact with other monkeys. At approximately 37-days of age, SPR animals were given daily 2hr socialization periods in which four infants were united in a play cage. At approximately 37-days of life, PR animals were housed in peer groups created by uniting four infants within a four-compartment cage. Peer-groups were age-matched as closely as possible. For the purpose of data analysis in this report PR and SPR animals were grouped together as nursery-reared (NR).
Human Intruder Paradigm
The HIP test was conducted following Kalin and his colleagues (1989). To begin, the animals were removed from their home cages, placed in an individual testing cage with a Plexiglas front, and left alone for 10-minutes (A1; Alone). During the next 10-minutes the human intruder entered the room, sat down 2.5 meters away from the front of the cage and presented only his profile, avoiding any eye contact with the animal (NEC; No Eye Contact). Next, the intruder exited the room; following a 3-minute break, he reentered and stared at the animal for 10-minutes while maintaining eye contact and keeping a neutral face (ST; Stare). Finally, the intruder again exited and the animal was left alone for 10-minutes (A2). The human intruder was male and was an individual who was unfamiliar to all animals tested. The monkey’s behavior and vocalizations were scored using a computer equipped with software to record behavior (The Observer v.5.0®, Noldus, Inc., The Netherlands). Frequency and duration of mutually-exclusive categories of behaviors were recorded. Behaviors recorded are listed in Table 2. Observations were coded by observers who met high inter-rater reliability standards, Cohen’s Kappa = 0.91, and were blind to animals’ rearing condition.
Table 2.
Behavioral ethogram
| Behavior | Dur/Freq | Beh. Class1 | Definition |
|---|---|---|---|
| Locomoting | Dur | 1 | Two directed steps in the horizontal and/or vertical plane. |
| Not Locomoting | Dur | 1 | Animal is not taking two directed steps in any direction. |
| Resting | Dur | 1 | Animal is sitting with eyes closed. |
| Lying Down | Dur | 1 | Animal is in a horizontal or flat position along the bottom of the cage. |
| Inactive | Dur | 2 | Animal is sitting or standing with eyes open not performing any other behaviors. |
| Env. Explore | Dur | 2 | Any tactile or oral, active manipulation of the cage, objects attached or in the cage. |
| Self-Direct | Dur | 2 | Any behavior in which the animal manipulates something on its body, such as any biting, picking, scraping, spreading, licking or mouth picking or any sucking of fingers or toes. |
| Tooth Gnash | Dur | 2 | A behavior involves grinding of the bottom teeth on the upper teeth. |
| Lipsmack | Dur | 2 | Pursing the lips together and moving them together to produce a smacking sound, sometimes accompanied by moaning. |
| Freeze | Dur | 2 | A period of at least 3 seconds characterized by tense body posture without vocalizations and movement, other than slow movements of the head. |
| Coo | Freq | 2 | Vocalization made by rounding and pursing the lips with an increase and then a decrease in frequency an intesity. |
| Fear Grimace | Freq | 2 | A grin-like facial expression involving the retraction of the lips exposing clenched teeth. May be accompanied with flattened ears or stiff huddled body posture. |
| Yawn | Freq | 2 | A slow opening of the mouth to an extremely wide position, exposing the teeth. |
| Bark | Freq | 2 | Vocalization made by forcing air through vocal chords from the abdomen, producing a short, rasping, low-frequency sound. |
| Display | Freq | 2 | Any vigorous shaking of the cage. Animal may perch at the top of the mesh and vigorously rock the cage. |
| Head-bobbing | Freq | 2 | Animal is moving entire head side to side and up and down typically seen during hostility. |
| Eyebrow Flicking | Freq | 2 | Animal is moving eye-brow up and down typically seen during hostility. |
| Forward Orient | Dur | 3 | Animal is facing toward the camera with both eyes open and visible. |
| Exp. Hostility | Dur | 3 | Any hostile behaviors directed at the intruder, such as barking, head bobbing, displays and yawns and ear flapping. |
| No Exp. Behavior | Dur | 3 | Either there is no experimenter present or the animal is not eliciting any behavior toward the experimenter. |
All state behaviors within each behavioral class are mutually exclusive and exhaustive.
Data Analysis
Group means and standard deviations, as well as the number of subjects exhibiting each behavior, are given in Table 3. A number of behaviors were either not exhibited at all or occurred with low frequency (see Results), and thus were not subjected to statistical analysis.
Table 3.
Mean (±SD; n) of behaviors exhibited across HIP conditions for each rearing group
| Behavior | Grp | Dur/Freq | A1 | NEC | ST | A2 |
|---|---|---|---|---|---|---|
| Bark | MR | Freq | 0 (±0; 0) | 0 (±0; 0) | 0 (±0; 0) | 0 (±0; 0) |
| NR | 0 (±0; 0) | 0 (±0; 0) | 0 (±0; 0) | 0 (±0; 0) | ||
| Coo | MR | Freq | 0 (±0; 0) | 0 (±0; 0) | 0 (±0; 0) | 0.09 (±0.30; 1) |
| NR | 0 (±0; 0) | 0 (±0; 0) | 0 (±0; 0) | 0.09 (±0.30; 1) | ||
| Env. Explore | MR | Dur | 91.5 (±90.5; 10) | 86.3 (±78.9; 9) | 139.1 (±146.8; 11) | 76.4 (±178.8; 11) |
| NR | 34.1 (±41.8; 10) | 38.9 (±57.1; 6) | 60.4 (±61.4; 10) | 38.7 (±36.1; 11) | ||
| Exp. Hostility | MR | Dur | - | 1.47 (±4.8; 1) | 0.33 (±1.1; 1) | - |
| NR | - | 0(±0; 0) | 0.19 (±0.6; 1) | - | ||
| Forward Orient | MR | Dur | 123.5 (±53.4; 11) | 93.5 (±36.4; 11) | 46.1 (±30.7; 11) | 163.6 (±89.2; 11) |
| NR | 183.9 (±67.7; 11) | 100.1 (±52.1; 11) | 49.7 (±35.6; 11) | 191.1 (±66.4; 11) | ||
| Freeze | MR | Dur | 1.6 (±5.2; 1) | 33.0 (±59.9; 7) | 11.5 (±17.9; 4) | 9.8 (±27.1; 2) |
| NR | 9.2 (±11.2; 5) | 65.75 (±89.6; 8) | 25.8 (±35.7; 8) | 16.2 (±32.5; 6) | ||
| Lipsmack | MR | Dur | 0.2 (±0.5; 1) | 0 (±0; 0) | 7.53 (±11.3; 6) | 0 (±0; 0) |
| NR | 0.7 (±1.8; 2) | 0.2 (±0.6; 1) | 10.6 (±28.4; 6) | 0.3 (±0.6; 2) | ||
| Locomotion | MR | Dur | 87.8 (±49.8; 11) | 56.2 (±46.0; 11) | 62.9 (±47.5; 11) | 106.1 (±74.7; 11) |
| NR | 52.7 (±82.9; 11) | 28.5 (±46.2; 9) | 70.6 (±128.3; 9) | 83.55 (±136.6; 11) | ||
| Lying Down | MR | Dur | 17.6 (±24.5; 8) | 9.5 (±12.5; 5) | 0.2 (±0.8; 1) | 7.0 (±16.1; 6) |
| NR | 14.8 (±14.9; 8) | 5.99 (±10.5; 5) | 3.35 (±6.3; 4) | 8.2 (±15.7; 6) | ||
| Self Direct | MR | Dur | 1.5 (±4.4; 3) | 0.5 (±1.0; 3) | 1.59 (±5.0; 2) | 3.7 (±8.9; 3) |
| NR | 0 (±0; 0) | 3.21 (±7.0; 4) | 3.9 (±9.8; 2) | 3.0 (±6.4; 3) |
Mean, standard deviation, and number of subjects exhibiting each behavior. Some behaviors occurred infrequently (by one subject): Coo, Fear Grimace, Yawn, Display, Eyebrow Flick, and Self-orient. Other behaviors did not occur at all: Resting, Bark, Head-bob, and Tooth-Gnash. Both infrequently occuring and non-occuring behaviors were excluded from data analysis.
Durations for variables found to be of interest were collapsed across conditions for further between-subjects analysis, including: explore, forward orient, freezing, lipsmack, and locomotion. Main effect between-subject ANOVAs were run across all subjects to evaluate early rearing group effects.
Between-groups and repeated-measures (ANOVA) were used to evaluate rearing group differences between the four testing conditions. All significance levels were set at p<0.05. Fisher’s PLSD tests served for post hoc comparisons. Non-normally distributed duration data was transformed using a LOG(x+1) transformation and homogeneity of variance was confirmed with Levene Test for Equality of Variances.
Results
Adult male monkeys did not exhibit a number of the behavioral responses to the HIP that have previously been reported as typical for much younger animals. Behaviors indicative of heightened anxiety in macaques that were not observed in any monkeys during any of the four conditions included: bark, head-bob, and tooth-gnash. Other behaviors were exhibited infrequently, including: coo, fear grimace, yawn, display, eyebrow flick, self-orient, and hostility directed at the intruder.
These adult animals did, however, provide robust evidence of differentiation between the test components in several elements of behavioral response (see Figure 1). The duration of locomotion, defined as at least two directed steps in the horizontal and/or vertical plane, differed significantly across conditions, F(3,63) = 5.35, p < 0.01, see Figure 1A. Fisher’s PLSD tests revealed that monkeys locomoted more while alone than in the NEC condition (p < 0.01) or while in the ST condition (p < 0.05). There was no difference between the A1 and A2 conditions, p = 0.98, nor between the NEC and ST conditions (p = 0.37) for duration of locomotion. Duration of freezing behaviors, defined as a period of at least three seconds characterized by tense body posture without vocalizations and movement, other than slow movements of the head (Kalin et al., 2004), differed across conditions, F(3,63) = 6.66, p < 0.001, see Figure 1B. Fisher’s PLSD tests revealed that, as hypothesized, monkeys’ duration of freezing was greater in the NEC condition compared to A1 (p = 0.0001) and A2 (p < 0.01, and marginally greater than ST (p < 0.10). Lip-smacking also differed by condition, F(3,63) = 13.89, p < 0.0001. Post hoc tests revealed the duration of lip-smacking was significantly higher in the ST condition when compared to any other condition (p < 0.0001), see Figure 1C. Exploratory behaviors, comprising active tactile or oral manipulation of the cage, differed across conditions of the HIP, F(3,63) = 2.97, p < 0.05. Monkeys’ exploratory behavior was significantly inhibited during the NEC compared to ST condition, p < 0.01, see Figure 1D. Forward orienting, defined as head directed toward the location where the intruder would present himself with both eyes open and visible, also differed significantly across conditions, F(3,63) = 43.04, p < 0.0001, see Figure 1E. Animals oriented forward significantly less in the NEC and ST conditions than in the A1 and A2 conditions (p < 0.001), and less in the ST than in the NEC condition, p < 0.0001.
Figure 1.
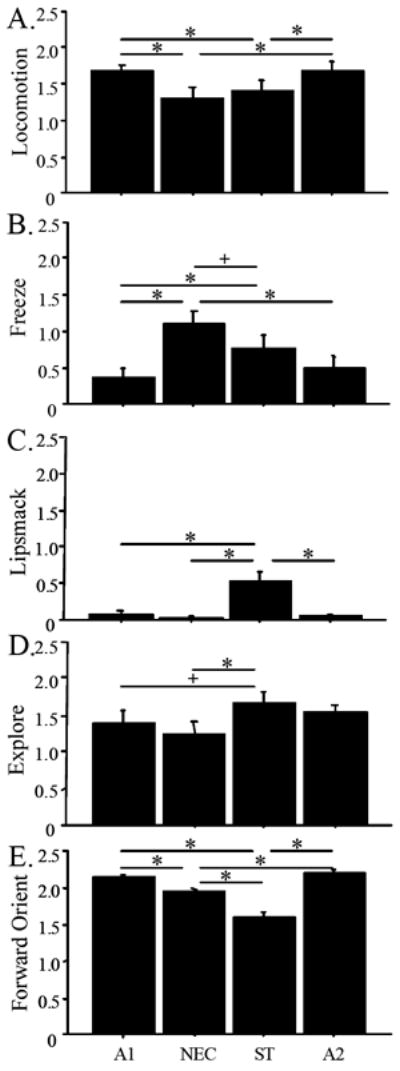
Mean LOG (x+1) transformed duration (+ SEM) of locomotion (A), freezing (B), lipsmack (C) explore (D) and forward orient (E) across HIP conditions. Symbols refer to differences between condition (* < 0.05; + < 0.10).
Nursery- and mother-reared monkeys differed significantly in multiple aspects of their behavioral response in the HIP (see Figure 2). First, NR animals locomoted less than MR animals, resulting in a significant main effect of rearing group on the duration of locomotion, F(1,20) = 6.17, p < 0.05, see Figure 2A. NR animals also engaged in less exploratory behavior than MR animals, F(1,20) = 4.98, p < 0.05, see Figure 2B. There was a marginal main effect of rearing history on the duration of freezing such that NR animals froze more than MR animals, F(1,20) = 3.62, p = 0.07, see Figure 2C.
Figure 2.
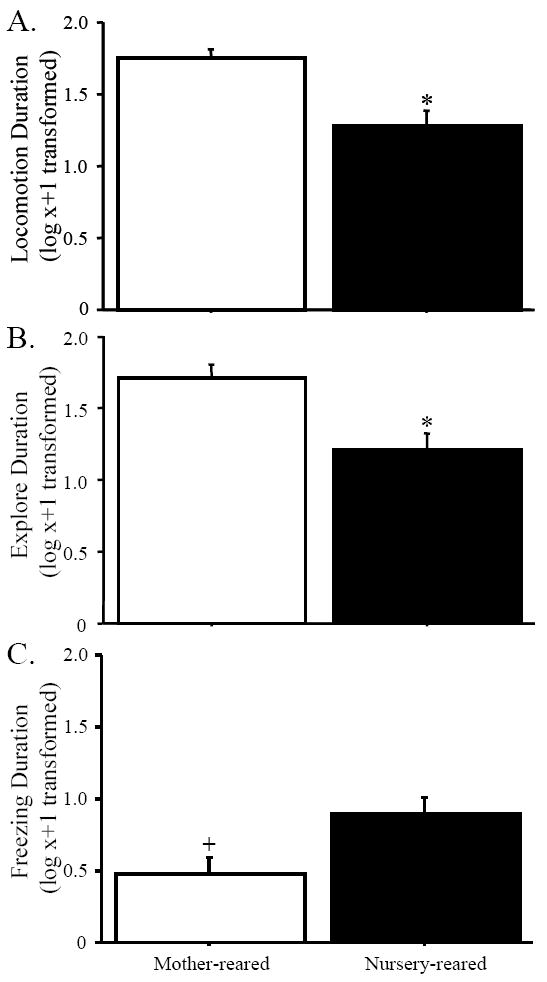
Nursery-reared animals spent less time (in seconds) than their mother-reared counterparts in locomotion (A) and exploration (B) across all HIP conditions.
Discussion
The findings reported here demonstrate that early differential rearing has long-lasting consequences on adult rhesus monkeys’ socioaffective behavior. In adulthood, monkeys nursery-reared in their infancy showed evidence of greater behavioral inhibition in response to the HIP challenge when compared to their mother-reared counterparts. Overall, NR monkeys exhibited reduced locomotion and exploration compared to their mother-reared counterparts and this reduction was maintained across the entire test period regardless of condition. This pattern of findings is congruent with previous observations in infant NR monkeys who exhibited reduced locomotion following a social separation challenge when tested at 6-months of age (Meyer et al., 1975).
Evidence of greater inhibition in nursery-reared animals, seen in differences in locomotion, exploratory, and freezing behaviors, is not only consistent with the findings of other studies that have examined response to social challenges in young mother- and nursery-reared animals, but also extends these findings to adult animals. Kalin and his colleagues previously showed that the HIP NEC condition elicited the highest amount of freezing behavior in young monkeys (Kalin et al., 2003). Freezing is interpreted as an initial adaptive response aimed at reducing the likelihood of detection by a predator. Using a similar behavioral paradigm, Capitanio et al., (2006) found that NR infants were less active in terms of whole body movement when compared to MR monkeys. Although they did not code freezing, increased freezing and decreased activity share the functional consequence of reducing the likelihood of detection by potential predators. Our data, taken together with the findings of Capitanio et al. (2006), provide convergent evidence that NR monkeys are more behaviorally inhibited compared to MR monkeys, both in infancy and in full adulthood. Thus, taken together these findings demonstrate that the consequences of nursery-rearing not only include increased response to challenges that provoke anxiety-like behavior (e.g., HIP and social separation), but also that this differential sensitivity persists into adulthood.
The pattern of results and predominant behaviors exhibited by the animals in this study differ in kind from those previously reported by Kalin, his colleagues, and others for younger monkeys (see Table 1). The difference is not unexpected. The behavioral repertoire of adult monkeys differs from that of infants and younger animals. Of significant interest, however, is that adult monkeys’ exploratory, ambulatory, orient, and anxiety-like behaviors provide evidence of differentiation between the HIP challenge conditions. These differential behavioral responses are parallel in function to those shown by younger animals, and thus provide strong evidence that the test is sensitive enough to detect group and individual differences in anxiety-like behavior in adult monkeys.
The no eye contact and stare conditions of the HIP represent two distinct types of challenge and elicit different behavioral responses in young animals (see Table 1). Our findings provide clear evidence that adult male monkeys also differentiated between the test conditions. The stare condition is typically met with hostile or defensive behavior that may represent attempts to minimize attack (Kalin et al., 1989; Kalin et al., 1998a; Kalin, Shelton, & Takahashi, 1991). In young monkeys, increased frequency of barks and hostility directed at the human intruder are observed in ST (Kalin & Shelton, 1989). Males classified as having a personality of excitable had increased threats in ST (Capitanio, 1999). None of these behaviors were reported for adult females (Kalin et al., 1998b). The animals tested here did not bark in any test condition. Hostility directed at the human intruder occurred infrequently and was exhibited by only few animals (see Table 3); however, the animals did orient toward the human intruder and spent more time doing so during the NEC condition than during the ST. Furthermore, comparison of lip-smacking in the different test conditions provided additional evidence of differentiation between conditions, with animals engaging in more lip-smacking during the ST than in the NEC condition. Although it is not clear why infant and juvenile animals, but not adults, would respond to a human intruder with hostility, one possibility is that the level of threat perceived by the adults was lower than that perceived by younger animals.
Overall, the results of this study show that the HIP test can be used successfully to measure individual differences in socioaffective behavior in adult animals. Identifying a test that can be used in animals of different ages and across laboratories is important for many reasons, among them to facilitate longitudinal studies, studies aimed at detecting the long-term consequences of early life events, and large-scale behavioral genetic and comparative studies where uniform measurement of meaningful phenotypes is crucial. These findings also demonstrate that the HIP can be used successfully with adult monkeys to detect group differences in behavioral response to a challenge and, specifically, to identify the long-lasting effects of differential early rearing experiences on aspects of socioaffective behavior. In turn, the findings underscore the parallel between this animal model and observations in human populations where the effects of early childhood adversity persist and increase lifelong risk of affective disorders, alcoholism, and other deleterious health outcomes.
Acknowledgments
We are grateful to Maria Blevins, Tara Chavanne, Jessica Christenson, and Ken Szeliga for technical assistance and for the efforts of the Animal Resources Program at the Wake Forest University School of Medicine. We are also indebted to Keith F. Groach for invaluable assistance in preparation of this manuscript. This research was partially supported by the Translational Center for Neurobehavioral Alcohol Research (AA017056), as well as NIH grants MH084980 (AJB, PJP), AA013995 (AJB), and AA014106 (DPF).
Reference List
- Ames EW, Fraser S, Burnaby BC. The development of Romanian orphanage children adopted to Canada. British Columbia: Simon Fraser University; 1997. [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing Condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR) Behavior Genetics. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. American Journal of Primatology. 1999;47:299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, DelRosso L, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal G, Elias K, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. New York: Springer; 2006. pp. 191–214. [Google Scholar]
- Capitanio JP, Mendoza S, Cole SW. Nervous temperament in infant monkeys is associated with reduced sensitivity of leukocytes to cortisol’s influence on trafficking. Brain, Behavior, and Immunity. 2011;25:151–159. doi: 10.1016/j.bbi.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AS, Snipes M. Early behavioral development and temperamental traits in mother- vs peer-reared rhesus monkeys. Primates. 1998;39:433–448. [Google Scholar]
- Coleman K, Dahl RE, Ryan ND, Cameron JL. Growth hormone response to growth hormone-releasing hormone and clonidine in young monkeys: correlation with behavioral characteristics. J Child Adolesc Psychopharmacol. 2003;13:227–241. doi: 10.1089/104454603322572561. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kalin NH, Shelton SE. Lateralized response to diazepam predicts temperamental style in rhesus monkeys. Behavioral Neuroscience. 1993;107:1106–1110. doi: 10.1037//0735-7044.107.6.1106. [DOI] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Reyes TM, Coe CL. Context-dependent behavioral effects of interleukin-1 in the rhesus monkey (Macaca mulatta) Psychoneuroendocrinology. 1996;21:455–468. doi: 10.1016/0306-4530(96)00010-8. [DOI] [PubMed] [Google Scholar]
- Gilmer WS, McKinney WT. Early experience and depressive disorders: Human and non-human primate studies. Journal of affective disorders. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Tarantal AF, Germann SL, Beard JL, Georgieff MK, et al. Diet-induced iron deficiency anemia and pregnancy outcome in rhesus monkeys. Am J Clin Nutr. 2006;83:647–656. doi: 10.1093/ajcn.83.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51:47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, et al. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. Journal of Neuroscience. 2006;26:4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Larson C, Shelton S, Davidson RJ. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behav Neurosci. 1998;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: Environmental cues and neurochemical regulation. Science. 1989;243 doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Ontogeny and stability of separation and threat-induced defensive behaviors in rhesus monkeys during the first year of life. American Journal of Primatology. 1998a;44:125–35. doi: 10.1002/(SICI)1098-2345(1998)44:2<125::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Cerebrospinal fluid corticotropin-releasing hormone levels are elevated in monkeys with patterns of brain activity associated with fearful temperament. Society of Biological Psychiatry. 2000 doi: 10.1016/s0006-3223(99)00256-5. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–74. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Molecular Psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Rickman M, Davidson RJ. Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behav Neurosci. 1998b;112:251–254. doi: 10.1037//0735-7044.112.1.251. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Turner JG. Effects of alprazolam on fear-related behavioral, hormonal, and catecholamine responses in infant rhesus monkeys. Life Sci. 1991;49:2031–2044. doi: 10.1016/0024-3205(91)90646-s. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Turner JG. Effects of B-carboline on fear-related behavioral and neurohormonal responses in infant rhesus monkeys. Biological Psychiatry. 1992;31:1008–1019. doi: 10.1016/0006-3223(92)90094-g. [DOI] [PubMed] [Google Scholar]
- Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biological Psychiatry. 2009;65:770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Gorman J. The psychobiology of anxiety. Clinical Neuroscience Research. 2005:335–347. [Google Scholar]
- Kinnally EL, Karere GM, Lyons LA, Mendoza SP, Mason WA, Capitanio JP. Serotonin pathway gene-gene and gene-environment interactions influence behavioral stress response in infant rhesus macaques. Dev Psychopathol. 2010;22:35–44. doi: 10.1017/S0954579409990241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MH, Gluck JP, Petitto JM, Hensley LL, Ozer H. Early social deprivation in nonhuman primates: Long-term effects on survival and cell-mediated immunity. Biological Psychiatry. 2000;47:119–126. doi: 10.1016/s0006-3223(99)00238-3. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: The promise and the limitations. Journal of Child Psychology and Psychiatry. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): Generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Novak MA, Bowman RE, Harlow HF. Behavioral and hormonal effects of attachment object separation in surrogate-peer-reared and mother-reared infant rhesus monkeys. Developmental Psychobiology. 1975;8:425–435. doi: 10.1002/dev.420080507. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Winslow JT. Non-human primates: Model animals for developmental psychopathology. Neuropsychopharmacology. 2009;34:90–105. doi: 10.1038/npp.2008.150. [DOI] [PubMed] [Google Scholar]
- Novak MA, Sackett GP. The effects of rearing experiences: The Early Years. In: Sackett GP, Ruppenthal G, Elias K, editors. Nursery rearing of nonhuman primates in the 21st century. New York: Springer Science+Business Media, Inc; 2006. pp. 5–19. [Google Scholar]
- O’Connor TG, Rutter M. Attachment disorder behavior following early severe deprivation: Extension and longitudinal follow-up. English and romanian adoptees study team. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:703–712. doi: 10.1097/00004583-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: implications for the pathophysiology of depression and anxiety. Arch Women Ment Health. 2003;6:15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- Rogers J, Shelton SE, Shelledy W, Garcia R, Kalin NH. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes, Brain, and Behavior. 2008;7:463–469. doi: 10.1111/j.1601-183X.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum LA, Forger C, Noland S, Trost RC, Coplan JD. Response of adolescent bonnet macaques to an acute fear stimulus as a function of early rearing conditions. Developmental Psychobiology. 2001;39:40–45. doi: 10.1002/dev.1026. [DOI] [PubMed] [Google Scholar]
- Rutter M. The long-term effects of early experience. Developmental Medicine & Child Neurology. 1980;22:800–815. doi: 10.1111/j.1469-8749.1980.tb03751.x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Developmental catch-up, and deficit, following adoption after severe global early privation. The English and Romanian adoptees (ERA) study team. Journal of Child Psychology and Psychiatry. 1998;39:465–476. [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. American Journal of Primatology. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Struble RG, Riesen AH. Changes in cortical dendritic branching subsequent to partial social isolation in stumptailed monkeys. Developmental Psychobiology. 1978;11:479–486. doi: 10.1002/dev.420110511. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30:3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Willette AA, Lubach GR, Coe CL. Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain Behav Immun. 2007;21:807–815. doi: 10.1016/j.bbi.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DE, Coleman K, Bacanu SA, Devlin BJ, Rogers J, Ryan ND, Cameron JL. Heritability of fearful-anxious endophenotypes in infant rhesus macaques: A preliminary report. Biological Psychiatry. 2003;53:284–291. doi: 10.1016/s0006-3223(02)01601-3. [DOI] [PubMed] [Google Scholar]
- Young LD, Suomi SS, Harlow HF, McKinney WT., Jr Early stress and later response to separation in rhesus monkeys. American Journal of Psychiatry. 1973;130:400–5. doi: 10.1176/ajp.130.4.400. [DOI] [PubMed] [Google Scholar]


