Abstract
The amino terminal domain (NT) of the connexins consists of their first 22–23 amino acids. Site-directed mutagenesis studies have demonstrated that NT amino acids are determinants of gap junction channel properties including unitary conductance, permeability/selectivity, and gating in response to transjunctional voltage. The importance of this region has also been emphasized by the identification of multiple disease-associated connexin mutants affecting amino acid residues in the NT region. The first part of the NT is α-helical. The structure of the Cx26 gap junction channel shows that the NT α-helix localizes within the channel, and lines much of the wall of the pore. Interactions of the amino acid residues in the NT with those in the transmembrane helices may be critical for holding the channel open. The predicted sites of these interactions and the applicability of the Cx26 structure to the NT of other connexins are considered.
1. Sequence of the amino terminal domain
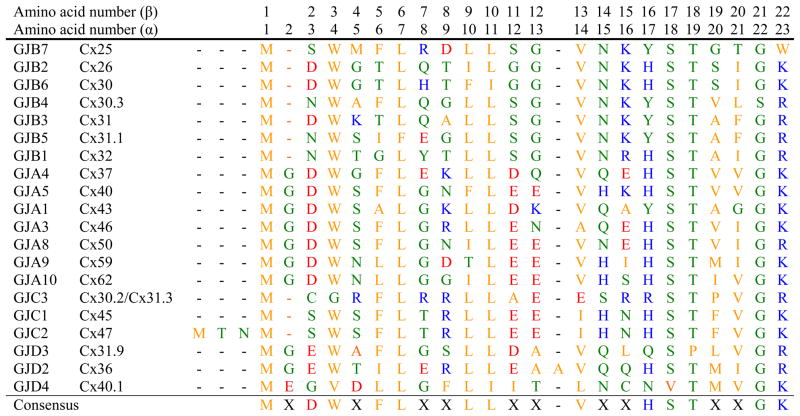
The connexin proteins have been divided into different domains based on their topologies within the membrane. These topologies and the demarcation of the domains were initially predicted from hydropathy plots of the connexin polypeptide sequences derived from their cloned cDNAs [1–3]. The amino terminal domain (NT)1 of the connexins consists of 22–23 amino acids in all members of the family except Cx36 and Cx47 (Fig. 1).2 This region begins with the methionine encoded by the ATG translational start codon. The NT ends just before the first transmembrane domain (TM1), with a positively charged amino acid (lysine or arginine) at position 22 (β-connexins) or 23 (α-connexins).
Figure 1.
Comparison of the amino acid sequences of the amino terminal domains of the 20 human connexins. The connexins are grouped according to sub-families (GJA, GJB, etc.) and then ordered by increasing molecular weight. Gaps were allowed to maximize the identities among sequences. Numbers across the top indicate the amino acid number for the β-connexins (GJB) and for the α-connexins (GJA). Polar amino acids are shown in green; hydrophobic amino acids are shown in orange, negatively charged amino acids are shown in red and positively charged amino acids are shown in blue. The consensus sequence requires ≥50% identity at a given position [6].
The sequence of amino acids is substantially conserved within the NT regions of different connexins. As shown by the sequence comparison in Fig. 1, identical amino acids in more than 10 of the 20 members include Trp3(4), Leu6(7), Leu9(10), Leu10(11), Val13(14), Ser17(18), Thr18(19), and Gly21(22) (numbered according to the β-connexins with α-connexin numbering in parentheses).
Hydropathy plots predicted that the NT was oriented towards the cytoplasmic side of the junctional membrane [1–3]. This hypothesis was supported by topology studies that mapped the binding of sequence-specific antibodies or protease-sensitive sites [7–12]. However, in isolated gap junctions, this region showed limited sensitivity to proteases [8,11,13] and accessibility of
2. Modifications of NT amino acids and protein-protein interactions
A few papers have provided evidence for modifications of amino acid residues in the NT regions of several connexins. Edman degradation studies of isolated gap junctions from heart and lens have suggested that the first methionine is post-translationally removed from at least some α-connexins, including Cx43, Cx46, and Cx50 [14,15]. A recent study using mass spectrometry has shown removal of the N-terminal methionines from bovine Cx46 and Cx50 [16]. A second group of investigators [17] also demonstrated cleavage to remove the N-terminal methionine from Cx46, but they only identified this truncated form in membrane protein samples prepared from nuclear lens fiber cells, not in those prepared from the lens cortex. Both groups of investigators observed that some (but not all) of the new N-terminal glycine residues were acetylated [16,17]. N-terminal acetylation of proteins is a process that usually occurs co-translationally and is catalyzed by the large N-α-acetyltransferase complexes (reviewed in [18,19]). Following removal of the N-terminal methionine by methionine aminopeptidase, the extent of N-acetylation depends on the identity of the amino acid at position 2. As noted by Shearer et al. [16], the unusual data obtained for the lens connexins suggest that N-terminal acetylation of Gly2 in these proteins may be post-translational and may have a regulatory role. N-terminal acetylation of Met1 in Cx26 has also been demonstrated [20].
Other identified modifications of NT amino acids include acetylation of Lys15 in Cx26 [20], hydroxylation of Asn14 in Cx26 [20], and deamidation of Asn13 in Cx46 [17]. Locke et al. [21] presented mass spectrometry data suggesting phosphorylation within the NTs of Cx26 and Cx32, but they did not identify the modified amino acids. The presence of both unmodified and modified NT peptides isolated from Cx26 and Cx32 imply that these modifications also are not “all or none” and may occur post-translationally.. (Indeed, they may occur after oligomerization of connexins into hemichannels.)
The connexin NT domain may also be involved in interactions with other cytoplasmic proteins. The binding between Cx32 and calmodulin involves the Cx32 NT [22], and Trp3 has been identified as a crucial amino acid for this interaction [23].
3. Role of NT in formation of functional gap junction channels
A few studies have examined the roles of the NT domain or individual amino acids within it in various steps in the formation of gap junction channels and plaques. The studies of Zoidl et al. [24] suggested that at least part of the NT may be required for proper trafficking and formation of gap junction plaques. These authors observed that a truncated mutant of Cx36 (missing the first 20 amino acids) was retained in the cytoplasm when expressed in transfected neuronal cells. Kyle et al. [25] created a series of mutants of Cx37 containing deletions of different stretches of amino acids within the NT. When tested by transient transfection of HeLa cells, a mutant with a deletion of most of the NT (amino acids 2–21) was found only in the cytoplasm. However, all constructs containing at least 9 amino acids formed gap junction plaques, implying that the absolute length of the NT and the identities of specific amino acids are not requirements for normal biosynthesis of gap junctions. In agreement with these findings, Kyle et al. [26] found that expression of a Cx37 mutant containing prolines at positions 10 and 15 (instead of the normal Leu15 and Gln15) also formed plaques. However, all of these mutations disrupted hemichannel and channel function.
The positively charged amino acid at the carboxyl end of the NT (position 23 in the α-connexins or 22 in the β-connexins) may have a significant role in connexin biosynthesis. Positively charged amino acids in the hydrophilic intracellular domains of membrane proteins can influence the orientation and insertion of hydrophobic transmembrane elements by acting as a “membrane anchor”. Thomas et al. [27] studied a cataract-associated mutant of Cx50 (R23T) and a series of other amino acid substitutions for Arg23. They found that mutants containing negatively-charged amino acids completely failed to form plaques; most other substitutions allowed formation of some plaques, but disrupted function.
NT amino acids may have a role in hetero-oligomerization of different connexins into hexamers. Falk et al. [28] studied Cx32 and Cx43, two connexins that do not normally make heteromeric channels. When they replaced Asp12 and Lys13 in Cx43 with the corresponding amino acid residues in Cx32, Ser and Gly, the mutant interacted with Cx43 (based on co-localization within the secretory pathway and inhibition of function). However, when Gemel et al. [29] made comparable substitutions of the corresponding residues in Cx40 or Cx43, they saw no evidence of hetero-oligomerization with Cx26.
4. Roles of NT amino acids in channel gating, unitary conductance, and permeability
Although multiple domains of the connexin polypeptides contribute to the biophysical properties of gap junction channels and hemichannels, several of the amino acids within the NT have important roles in voltage-dependent gating, unitary conductance, and permeability. NT amino acids may also be involved in the modulation of channel activity by some cytoplasmic components. Most of these amino acids have been identified through studies in which site-directed mutants were generated and examined in in vitro expression systems. Several groups of investigators have conducted extensive series of experiments on this subject.
Verselis, Bargiello, and colleagues have conducted a series of studies focused on elucidating the basis of transjunctional voltage (Vj) dependent gating [30–32]. Vj-gating corresponds to rapid transitions (observed in single-channel recordings) between a fully open and one of several subconductance states that reduce the conductance of the open state by ~80%. These studies began with the observation that Cx32 and Cx26 showed opposite polarity of Vj-gating. Using a chimeric approach, they showed that the difference in gating polarity was due to the electrostatic effect of a charge difference in the second amino acid [30]. They proposed that the Cx32 and Cx26 voltage sensors are oppositely charged. The voltage sensor of Cx26 hemichannels has a net negative charge due to the presence of Asp2 while the Cx32 sensor has a positive charge contributed by the unmodified N-terminal methionine residue. Purnick et al. [31] reported that the polarity of Vj gating in Cx32 hemichannels could also be reversed by negative charge substitutions at the 5th, 8th, 9th and 10th positions. They suggested that the first 10 amino acid residues of Cx32 were located in close proximity to the aqueous pore and could sense changes in the voltage field allowing the NT to move as a single unit in response to changes in voltage. Oh et al. [32] extended the studies of substitutions of the 2nd, 5th, and 8th residues and concluded that the charge at the 2nd position was also critical for determining the polarity of Vj-gating.
In a related series of experiments, Oh et al. [33] studied positive or negative charge substitutions at various positions of the NT in a chimeric connexin (Cx32 containing the first extracellular loop of Cx43, Cx32*43E1) hemichannel and demonstrated the importance of the 2nd, 5th, and 8th amino acids in determining charge selectivity. In this study, negative charge substitutions at the 2nd, 5th and 8th positions increased unitary conductance and cation selectivity while positive charge substitutions at the 5th position decreased unitary conductance and produced a non-selective channel [33]. They also found that a construct with a cysteine substitution at position 8 could be modified by a methanethiosulfonate reagent indicating that this residue lines the aqueous pore (near the intracellular entrance of the channel). These results suggest that the charge selectivity of a connexin hemichannel is determined by charges dispersed over the permeation pathway (including several charged residues in the NT). (Some of these and related studies are described in more detail in the accompanying article by Bargiello [34].)
Studies from other laboratories have suggested the general importance of the N-terminal charge in determining the polarity of Vj gating in other connexins. For example, replacement of Asp3 with asparagine in Cx50 also resulted in an inversion of the polarity of Vj gating [35]. Interestingly, this substitution also affects the sensitivity of Cx50 channels to CO2 [35].
Ebihara and colleagues [36–38] have used chimeric strategies to examine the different physiological properties of the connexins expressed in the lens (including Cx43, Cx46, and Cx50), and found that several of these properties were strongly influenced by amino acids within the NT. Tong et al. [36] found that introducing the NT of Cx46 into Cx45.6 (the chicken ortholog of mammalian Cx50) slowed the inactivation kinetics of the macroscopic junctional currents, reduced the voltage sensitivity of the steady-state junctional conductance, and decreased the conductance of single gap junctional channels so that they all more closely resembled those of Cx46. Their studies of individual amino acid substitutions identified Arg9 of Cx46 as the main determinant for the differences in voltage-dependent gating. Similarly, Tong and Ebihara [37] found that Arg9 in the NT of Cx56 (the chicken ortholog of mamalian Cx46) was one of two critical determinants (along with E43) for the difference in its voltage-dependent gating from Cx45.6. They also found that Arg9 influenced single channel conductance and rectification. Dong et al. [38] showed that replacement of the NT of Cx45.6 with the corresponding domain of Cx43 increased permeability to Lucifer yellow, reduced the Vj-gating sensitivity, and reduced the unitary conductance.
Veenstra and colleagues have studied the inhibitory effects of polyamines on some connexin channels and the influence of amino acids near the cytoplasmic mouth of the pore (especially ones in the NT) on this block. Musa and Veenstra [39] observed that spermine was a potent inhibitor of gap junctional conductance in cells expressing Cx40, but not Cx43. By replacing Glu9 and Glu13 in Cx40 with the corresponding amino acids from Cx43 (both lysines), Musa et al. [40] eliminated the block by spermine. Interestingly, channels formed from these substitution mutants also showed reduced Vj gating sensitivity and unitary conductance. Channels formed by the reciprocal glutamate substituted Cx43 mutant were less Vj sensitive, but did not become sensitive to spermine suggesting that the determinants of this property are more complex than just these two residues. Subsequent studies have demonstrated that mutagenesis of other NT residues in Cx40 (including His15, Lys16, and probably Glu12) also affect spermine block [29,41]. Neutralization of His15 and Lys16 reduced the Vj sensitivity of Cx40 channels without affecting their unitary junctional conductance or relative cation/anion permeability ratios. This suggested that these residues contributed to the voltage sensor and spermine receptor of Cx40, without significantly altering the electrostatic surface charge potentials that contribute to the ion selectivity of this gap junction channel [41].
The penultimate glycine (Gly21(22)) in the NT may also contribute to channel properties. Xia et al. [42] studied a glycine to arginine substitution in Cx50, Cx50G22R, that does not produce functional channels when expressed alone. However, when co-expressed with Cx46, the channels exhibit reduced junctional conductance and altered voltage sensitivity [42].
It is interesting that the critical residues implicated in determining these various physiological properties are among the most divergent amino acids within the NT domain (see [26, 32, 35]). Although overall the sequence of this domain is quite conserved, the substitutions confer many of the characteristic properties that differentiate channels formed of different connexins.
5. Mutations within the NT domain identified in patients with genetic diseases
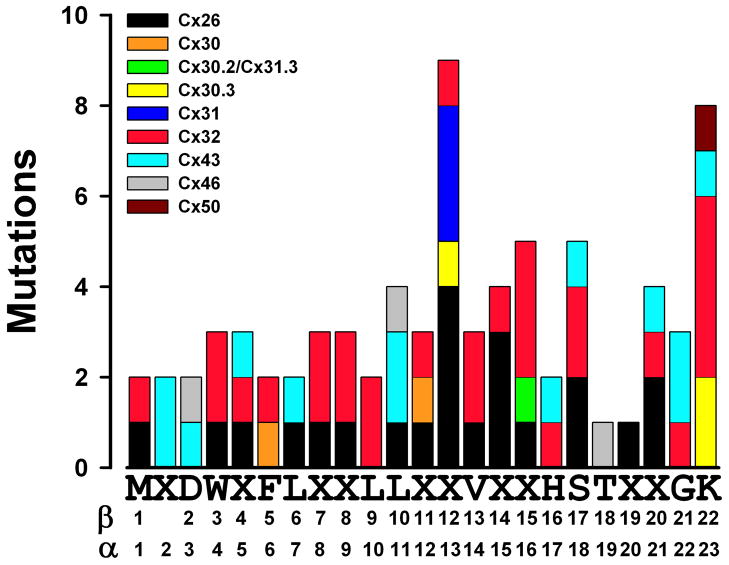
Mutations of connexin genes have been identified in patients with a variety of inherited pathologies including sensorineural deafness (non-syndromic and syndromic, especially associated with skin diseases), X-linked Charcot-Marie-Tooth disease, cataracts, oculodentodigital dysplasia, and keratodermas. Disease-associated mutations have been identified in all domains of the connexin polypeptide including the NT of Cx26, Cx30, Cx30.2/Cx31.3, Cx31, Cx32, Cx43, Cx46, and Cx50. The broad distribution of these amino acid substitutions across this region is illustrated in Fig. 2; details of the mutations and references for their descriptions are included in Supplementary Table 1.
Figure 2.
Distribution of mutations at different positions within the connexin NT identified in hereditary human diseases. The number of mutations corresponds to the different mutations identified at a given position (details and references are shown in Supplementary Table 1). The sequence on the X axis represents the NT consensus sequence as shown in Fig. 1. The numbers below the NT consensus sequence indicate the positions of the amino acid residues for β- and α-connexins.
Some of these mutations have been studied in expression systems to examine their cellular or physiological properties. Many mutants (including Cx30G11R, Cx31G12R, Cx31G12D, Cx32W3S, Cx32G12S, Cx50R23T) are absent from the cell surface or gap junction plaques (and sometimes have been localized to specific subcellular compartments of the secretory pathway) and are non-functional [27,43–45]. Some others (including Cx26S17F, Cx30T5M, Cx43Y17S, and Cx43G21R) localize to gap junction plaques, but do not form functional channels [43,46,47]. Cx32R15W has no conductance above baseline at least partly due to a severely reduced open probability [48]. Interestingly, some of the non-functional mutants inhibit their co-expressed wild type counterparts (like Cx43G21R) [49,50] potentially explaining autosomal dominant inheritance of the disease. Unusual mutants, like Cx32R15Q, show junctional conductances that are not significantly different from those of their wild type counterparts, but may have alterations of channel properties (like alterations in steady-state conductance-voltage relationships) [48]. A few mutations (including Cx26G11E, Cx26G11R, and Cx26N14K) cause the death of cells in which they are expressed [51,52]; mechanisms invoked for these findings include increased hemichannel activity and dysregulation of intracellular calcium levels.
6. Structure of the amino terminal domain
The structure of the NT domain was initially investigated by studying synthetic peptides using circular dichroism and nuclear magnetic resonance (NMR) [26,53–55]. Based on the studies of a 15 amino acid peptide, Purnick et al. [53] proposed a model for the NT of Cx26 with an α-helix extending from position 1 to 10 and a critical bend at positions 11 and 12 that was suggested to act as a “hinge” allowing the first 10 amino acids to swing into the pore and block the channel. Subsequent studies of longer peptides corresponding to the NT domains in Cx26 [54] and Cx32 [55] generally supported this model. Studies of a 23 amino acid peptide corresponding to the NT of Cx37 (an α-connexin) suggested that it is also primarily α-helical, especially between amino acids 5 and 15 [26]. Furthermore, mutagenesis shows that this structure is required for formation of functional channels, but not gap junctional plaques [26].
A few years ago, Oshima et al. [56] published structural studies of a “permeability” mutant (M34A) of Cx26 after crystallization of Cx26M34A that had been expressed in Sf9 insect cells. Although these studies were not of adequate resolution to identify individual amino acids or to assign specific connexin domains within the structure, they did identify a density within the pore of the channel which they suggested might represent a bundle of N-termini acting as a “plug” to close the channel. This suggestion was supported by the absence of this “plug” in a subsequent study of a site-directed mutant with a deletion of much of the NT [57]
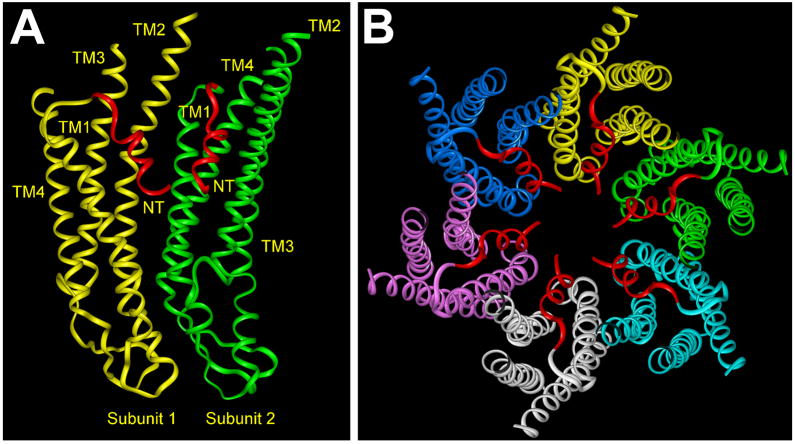
Recently Maeda et al. [58] have determined the structure of a Cx26 channel at 3.5 Å resolution. (This structure is discussed in detail in the accompanying manuscript by Tsukihara [59].) This structure does show that the N-terminal regions of the six subunits line the pore entrance and form a “funnel”, which restricts the diameter at the entrance of the pore to 14 Å. Within the channel, TM1 and TM2 face the pore, but TM2 and the cytoplasmic half of TM1 are not exposed to its lumen, because they are covered by the first part of the NT which is α-helical (Fig. 3). The beginning of the NT is located deep within the pore. The NT helix extends beyond the cytoplasmic side of the membrane and then forms a loop (including the highly conserved amino acids Ser17 and Thr18) that bends back to the membrane where TM1 begins. The bend may be quite flexible accounting for the observation that the NT helices were the most mobile domains in the Cx26 structure [58], and potentially allowing rapid movements necessary for gating of the channel.
Figure 3.
Models illustrating the relationships between subunits (protomers) and their NT domains within a gap junction hemichannel based on the structure of the Cx26 gap junction channel [58]. A. Ribbon model of two adjacent subunits. Subunit 1 is shown in yellow, and Subunit 2 is shown in green. The regions of the NT domains that face the pore are indicated in red. The four transmembrane helices are identified (TM1–TM4). This structural model is oriented with the cytoplasmic side of the channel at the top and the extracellular side at the bottom. B. Ribbon model representations of all six subunits (illustrated in different colors except for their NT domains that are shown in red) within a hemichannel viewed from the cytoplasmic aspect.
The structure determined by Maeda et al. [58] represents an open configuration of the channel. These authors have endorsed the hypothesis that interactions between amino acids within the NT helix and TM helices along the wall of the pore help to hold the channel open. These authors identified two critical interactions: (1) a circular hydrogen bond network between Asp2 and Thr5 of the neighboring monomer at the narrowest portion of the funnel and (2) a hydrophobic interaction between Trp3 (which is conserved in almost all connexins) and a hydrophobic segment in TM1 from the neighboring monomer (including Met34 in the case of Cx26) [58]. Additional hydrophobic interactions stabilize the binding between the NT and TM2 of the same subunit as detailed in Table 1.
Table 1.
Intra- and inter-subunit interactions of NT amino acids
| Cx26 | Interacting amino acid | Cx37 | Interacting amino acid | ||
|---|---|---|---|---|---|
| Subunit 1 | Subunit 2 | Subunit 1 | Subunit 2 | ||
| Asp2 | Leu6 (side) | Thr5 (NH) | Asp3 | Leu7 (side) | Phe6 (NH) Lys9 (side) |
| Trp3 | Met34 | Trp4 | Leu35 | ||
| Gly4 | Gly5 | ||||
| Thr5 | Phe6 | ||||
| Leu6 | Asp2 (side) Ile9 (side) |
Thr5 (side) | Leu7 | Asp3 (side) Leu10 (side) |
Phe6 (side) |
| Gln7 | Leu89 | Glu8 | Gly5 (NH) Glu8 (NH) Leu90 (side) |
Lys9 (side) | |
| Thr8 | Lys9 | Ile31 (side) | |||
| Ile9 | Leu6 (side) | Leu10 | Phe6 (side) Leu7 (side) |
||
| Leu10 | Ala92 Ala96 |
Leu11 | Leu93 (side) Ile97 (side) |
||
| Gly11 | Asp12 | Thr27 (OH, side) Lys162 (side) |
|||
| Gly12 | Gln13 | ||||
| Val13 | Ala92 Val95 (side) |
Val14 | Leu93 (side) Val96 (side) |
||
The first column lists the amino acid residues within the portion of the NT that lines the pore (indicated in red in Fig. 3). Interactions with amino acid residues from the same connexin (intra-subunit) are listed in the column labeled Subunit 1 (yellow in Figs. 3, 4). Interactions with amino acid residues in the adjacent connexin (inter-subunit) are listed in the column labeled Subunit 2 (green in Figs. 3, 4). Amino acid residue interactions within Cx26 are based on the structure determined by Maeda et al. [58]. Amino acid interactions within Cx37 are predicted based on homology modeling after substitution of its sequence into the Cx26 structure. The amino acid regions or chemical groups involved in intra- and inter-molecular interaction between the amino acids are indicated in parenthesis.
The general features of the Cx26 structure likely apply to the other members of the family. The alignment of the NT sequences of different connexins (Fig. 1) shows that some of the residues most critical for structural features are highly conserved, including Trp3(4), Leu6(7), Leu9(10), Leu10(11), Val13(14), Ser17(18), Thr18(19),
To predict critical interactions for amino acids within the NT domain of another connexin (Cx37), we developed a homology model by substituting the sequence of Cx37 into the coordinates determined for the Cx26 structure and applying energetic optimization to eliminate bad non-bonded contacts (as described in Supplementary Methods).
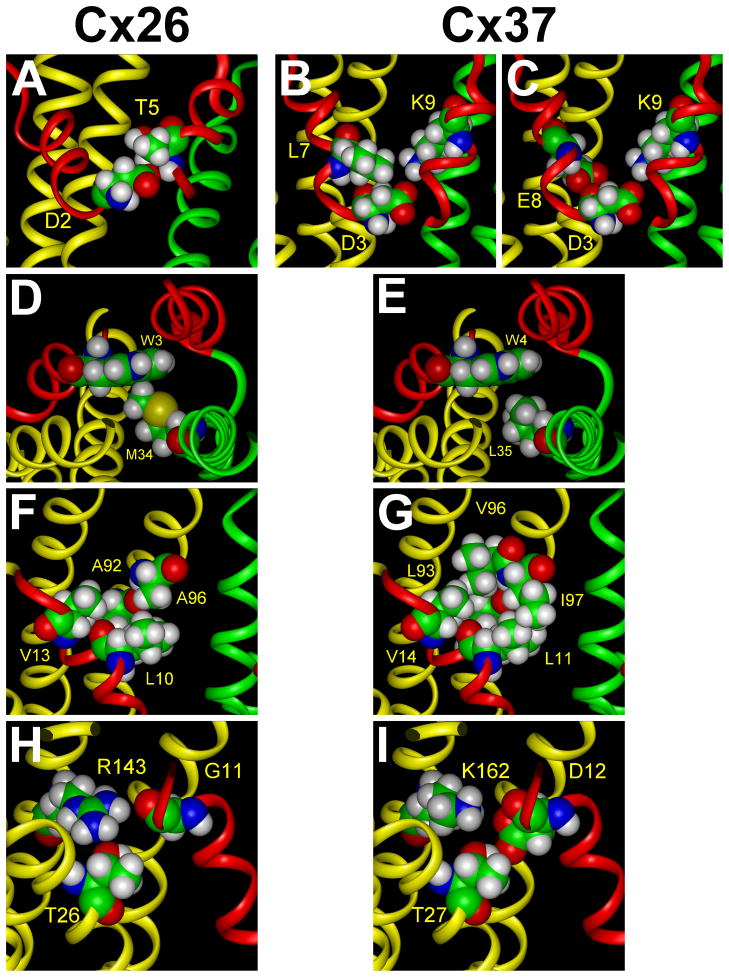
The predicted Cx37 structure preserves some of the interactions identified in Cx26. The Cx37 structure appears to be stabilized by some hydrophobic interactions that are strengthened in Cx37 and by several additional electrostatic interactions. Asp2 of Cx26 has critical van der Waals contacts with the nonpolar atoms of the side chains of Leu6 (not shown) in the same connexin subunit, and its side chain carboxyl group forms a hydrogen bond with the amide group of Thr5 in the adjacent connexin subunit (Fig. 4A); similar interactions occur in Cx37 between the corresponding Asp3 and Leu7 (Fig. 4B) and Asp3 and Phe6 (not shown). However, the model predicts that Asp3 (together with Glu8 in the same subunit) of Cx37 has additional electrostatic interactions with Lys9 in the adjacent monomer (Fig. 4B,C). The van der Waals interaction between Trp3 and Met34 (of the adjacent subunit) inside of the hydrophobic core may be much more important in Cx26 (Fig. 4D) than the corresponding relationship between Trp4 and Leu35 in Cx37 which are predicted to be farther apart (Fig. 4E). Hydrophobic interactions between the NT and TM2 appear important for both connexins. In Cx26, Leu10 and Val13 interact with hydrophobic residues including Ala92 and Ala96 (Fig. 4F); however, in Cx37 the corresponding interactions of Leu11 and Val14 would lead to the formation of a tighter hydrophobic knot due to the longer side chains of Leu93 and Ile97 (Fig. 4G). The cytoplasmic part of the NT of Cx26 (Gly11) has limited interactions with the adjacent transmembrane helices from the same subunit (Fig. 4H), but the homology model predicts that Asp12 of Cx37 has strong electrostatic interactions with Lys162 at the base of TM3 and forms a hydrogen bond with Thr27 of TM1 (Fig. 4I). Overall, it is predicted that the structure of the open Cx37 channel may be more stable than that of the open Cx26 channel.
Figure 4.
Comparison of the interactions of Cx26 NT amino acids with those predicted for the Cx37 NT based on homology modeling. A. In Cx26, Asp2 forms a hydrogen bond with Thr5 of the adjacent monomer. B. In Cx37, Asp3 similarly forms a hydrogen bond with Phe6 of the adjacent monomer (not shown) and additionally participates in hydrophobic interactions with Leu7 in the same monomer. C. In Cx37, both Asp3 and Glu8 interact electrostatically with Lys9 of the adjacent subunit. D. In Cx26, the side chain of Trp3 participates in van der Waals contacts with Met34 in TM1 of the adjacent subunit. E. In Cx37, there is a weaker interaction between the corresponding amino acid residues, Trp4 and Leu35, because their side chains are farther apart. F. In Cx26, Leu10 participates in hydrophobic interactions with Ala92 and Ala96 in TM2 of the same subunit, and Val13 interacts with Ala92 and Val95 (not shown). G. In Cx37, Leu11 has more extensive interactions with the corresponding Leu93 and Ile97 because of their longer side chains. Val14 has significant hydrophobic interactions with Leu93 and Val96. H. In Cx26, Gly11 does not have significant electrostatic or hydrogen bonding interactions with Thr26 and Arg143 although their side chains are in proximity. I. In Cx37, Asp12 (the substitution for Gly11) interacts through a hydrogen bond with Thr27 in TM1 and electrostatically with Lys162 in TM3 of the same subunit.
We chose Cx37 for our comparison to Cx26, in part because of our previous interests in the importance of the NT domain for its behavior [25,26], but also because structural similarities identified through comparison to a member of a different connexin sub-family might increase the likelihood of generalizable conclusions. Nevertheless, several caveats apply to our predictions of critical interactions of NT amino acids. An obvious issue is that the Cx26 structure does not include Met1; similarly, our homology model does not contain Met1 or Gly2 of Cx37 (much less account for the possible removal of the methionine and acetylation of the glycine). Because Asp3 in Cx37 has a similar location to Asp2 in Cx26, the model predicts a similar radius of the pore at its narrowest point; however, this similarity may be hard to reconcile with the unitary conductance and and permeability differences between Cx26 and Cx37 channels. There are several bad contact points in regions of the Cx26 crystal structure that may be corrected in subsequent studies; in our modeling, we have eliminated prohibited contacts. Certainly, the actual residue interactions present in different connexins may not always correspond to those predicted by the homology modeling. Many of the α- and β-group connexins do not form either heterotypic or heteromeric channels with each other, suggesting that they have differences in structure and amino acid interactions (in at least some domains). Indeed, our own NMR studies suggest that the α-helical portion of the NT in Cx37 may be longer than that in Cx26 [26].
7. Unanswered questions and directions for future studies
The substantial sequence conservation among connexins and the predictions from homology modeling suggest that gross features of the Cx26 structure will be shared by other connexins. However, some details of the structure of the NT and especially the amino acid residues involved in the relationships between the NT and the other connexin domains within a hemichannel (like those that line the wall of the channel) will not be the same for all connexins. While Maeda et al. [58] emphasized the importance of Asp2 for the Cx26 structure, the amino acid at this position is not absolutely conserved in all connexins (e.g., it is an asparagine in Cx32) (Fig. 1). Some connexins have longer NT domains; Cx36 has an interposed amino acid (Ala14), and Cx47 has additional amino acids at the beginning of the molecule (Fig. 1). The residue corresponding to Met34 (of Cx26) may not be implicated in forming critical interactions in all connexins; our homology modeling does not predict an interaction of the corresponding residues in Cx37 (Fig. 4E). Furthermore, in Cx46 (another α-connexin), a cysteine substitution of the corresponding position (Leu35) is accessible to cysteine modifying agents from the extracellular side of the hemichannel indicating that it lines the hemichannel pore [60].
Resolution of these issues will require further structural and functional studies on wild type and mutated members of the connexin family. They may lead to the identification of compensatory changes in the connexin sequences that maintain interactions among subunits to form hemichannels as well as interactions involved in holding channels open or closed. Future studies should provide a structural basis for understanding how differences in NT domains contribute to connexin specific physiological properties like gating by voltage, conductance, and permeability.
Since modifications of amino acids within the connexin NT region may affect their intra- and inter-subunit interactions, the extent and functional consequences of the modifications also need to be examined. N-terminal acetylation (as observed in some α-connexins) would neutralize the positive charge of the amine group of Gly2 and increase its volume. These alterations might affect gating or its polarity, charge selectivity and/or pore diameter. Similarly, a relative change in the negative charge of the NT region by phosphorylation, hydroxylation or deamidation of amino acid residues may affect gating, permeability and single channel conductance. A modification that decreased or blocked the interaction of the NT with the TM helices along the pore wall should result in decreased or absent function.
One important unresolved question is the timing of modifications of NT amino acids during the lifecycle of connexins. These modifications require interaction with cytoplasmic modifying enzymes like kinases or the N-terminal acetyltransferase complexes. It seems likely that some modifications occur co-translationally or post-translationally prior to oligomerization when the NT is accessible. Interactions should only occur post-translationally (or within oligomeric hemichannels) if the NT had a different conformation than that determined in the structural studies of Cx26, since the protein interactions would otherwise be prevented by steric hindrance. For similar reasons, further investigation of interactions of the NT domain with other cellular proteins (like between Cx32 and calmodulin) seems warranted.
Supplementary Material
Highlights.
The NT domain of the connexins consists of their first 22–23 amino acids.
Many NT amino acids are conserved in different connexins
The first part of the NT is α-helical, and it lines much of the wall of the pore.
NT amino acids are determinants of several channel properties.
Disease-associated mutations have been identified throughout the connexin NT
Acknowledgments
Supported by NIH grants EY08368 and HL59199 (to ECB).
Footnotes
Abbreviations used: amino terminal domain, NT; Connexin, Cx; transmembrane domain, TM; transjunctional voltage, Vj; nuclear magnetic resonance, NMR
Several years ago, the known connexins were divided into sub-families, designated by Greek letters, based on sequence similarities [4]. Determination of many more connexin sequences and their phylogenetic analysis expanded the number of subdivisions [5]. The current connexin gene nomenclature incorporates sub-families indicated by GJA, GJB, etc. corresponding to α, β, etc. (reviewed in [6]). The comparison of connexin NT sequences in Fig. 1 is organized according to these sub-families. antibodies [10], suggesting that NT was protected by its own folding, interaction with some other regions, or close association with the membrane.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paul DL. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986;103:123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987;105:2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang JT, Nicholson BJ. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989;109:3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar NM, Gilula NB. Molecular biology and genetics of gap junction channels. Semin Cell Biol. 1992;3:3–16. doi: 10.1016/s1043-4682(10)80003-0. [DOI] [PubMed] [Google Scholar]
- 5.Cruciani V, Mikalsen SO. The vertebrate connexin family. Cell Mol Life Sci. 2006;63:1125–1140. doi: 10.1007/s00018-005-5571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer EC, Berthoud VM. The Family of Connexin Genes. In: Harris A, Locke D, editors. Connexins: A Guide. Humana Press; New York: 2009. pp. 3–26. [Google Scholar]
- 7.Goodenough DA, Paul DL, Jesaitis L. Topological distribution of two connexin32 antigenic sites in intact and split rodent hepatocyte gap junctions. J Cell Biol. 1988;107:1817–1824. doi: 10.1083/jcb.107.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertzberg EL, Disher RM, Tiller AA, Zhou Y, Cook RG. Topology of the Mr 27,000 liver gap junction protein. Cytoplasmic localization of amino- and carboxyl termini and a hydrophilic domain which is protease-hypersensitive. J Biol Chem. 1988;263:19105–19111. [PubMed] [Google Scholar]
- 9.Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yancey SB, John SA, Lal R, Austin BJ, Revel JP. The 43-kD polypeptide of heart gap junctions: immunolocalization, topology, and functional domains. J Cell Biol. 1989;108:2241–2254. doi: 10.1083/jcb.108.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman S, Evans WH. Topography of connexin32 in rat liver gap junctions. Evidence for an intramolecular disulphide linkage connecting the two extracellular peptide loops. J Cell Sci. 1991;100:567–578. doi: 10.1242/jcs.100.3.567. [DOI] [PubMed] [Google Scholar]
- 12.Zhang JT, Nicholson BJ. The topological structure of connexin 26 and its distribution compared to connexin 32 in hepatic gap junctions. J Membr Biol. 1994;139:15–29. doi: 10.1007/BF00232671. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer DB, Green CR, Evans WH, Gilula NB. Topological analysis of the major protein in isolated intact rat liver gap junctions and gap junction-derived single membrane structures. J Biol Chem. 1987;262:7751–7763. [PubMed] [Google Scholar]
- 14.Manjunath CK, Nicholson BJ, Teplow D, Hood L, Page E, Revel JP. The cardiac gap junction protein (Mr 47,000) has a tissue-specific cytoplasmic domain of Mr 17,000 at its carboxy-terminus. Biochem Biophys Res Commun. 1987;142:228–234. doi: 10.1016/0006-291x(87)90475-x. [DOI] [PubMed] [Google Scholar]
- 15.Kistler J, Christie D, Bullivant S. Homologies between gap junction proteins in lens, heart and liver. Nature. 1988;331:721–723. doi: 10.1038/331721a0. [DOI] [PubMed] [Google Scholar]
- 16.Shearer D, Ens W, Standing K, Valdimarsson G. Posttranslational modifications in lens fiber connexins identified by off-line-HPLC MALDI-quadrupole time-of-flight mass spectrometry. Invest Ophthalmol Vis Sci. 2008;49:1553–1562. doi: 10.1167/iovs.07-1193. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Schey KL. Phosphorylation and truncation sites of bovine lens connexin 46 and connexin 50. Exp Eye Res. 2009;89:898–904. doi: 10.1016/j.exer.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starheim KK, Gromyko D, Velde R, Varhaug JE, Arnesen T. Composition and biological significance of the human Nα-terminal acetyltransferases. BMC Proc. 2009;3(Suppl 6):S3. doi: 10.1186/1753-6561-3-S6-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnesen T. Towards a functional understanding of protein N-terminal acetylation. PLoS Biol. 2011;9:e1001074. doi: 10.1371/journal.pbio.1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locke D, Bian S, Li H, Harris AL. Post-translational modifications of connexin26 revealed by mass spectrometry. Biochem J. 2009;424:385–398. doi: 10.1042/BJ20091140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locke D, Koreen IV, Harris AL. Isoelectric points and post-translational modifications of connexin26 and connexin32. FASEB J. 2006 doi: 10.1096/fj.05-5309fje. [DOI] [PubMed] [Google Scholar]
- 22.Dodd R, Peracchia C, Stolady D, Török K. Calmodulin association with connexin32 derived peptides suggests trans-domain interaction in chemical gating of gap junction channels. J Biol Chem. 2008:26911–26920. doi: 10.1074/jbc.M801434200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Török K, Stauffer K, Evans WH. Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem J. 1997;326:479–483. doi: 10.1042/bj3260479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoidl G, Meier C, Petrasch-Parwez E, Zoidl C, Habbes HW, Kremer M, Srinivas M, Spray DC, Dermietzel R. Evidence for a role of the N-terminal domain in subcellular localization of the neuronal connexin36 (Cx36) J Neurosci Res. 2002;69:448–465. doi: 10.1002/jnr.10284. [DOI] [PubMed] [Google Scholar]
- 25.Kyle JW, Minogue PJ, Thomas BC, Lopez-Domowicz D, Berthoud VM, Hanck DA, Beyer EC. An intact connexin N-terminus is required for function, but not for gap junction formation. J Cell Sci. 2008;121:2744–2750. doi: 10.1242/jcs.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyle JW, Berthoud VM, Kurutz J, Minogue PJ, Greenspan M, Hanck DA, Beyer EC. The N terminus of connexin37 contains an α-helix that is required for channel function. J Biol Chem. 2009;284:20418–20427. doi: 10.1074/jbc.M109.016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas BC, Minogue PJ, Valiunas V, Kanaporis G, Brink PR, Berthoud VM, Beyer EC. Cataracts are caused by alterations of a critical N-terminal positive charge in connexin50. Invest Ophthalmol Vis Sci. 2008;49:2549–2556. doi: 10.1167/iovs.07-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagree V, Brunschwig K, Lopez P, Gilula NB, Richard G, Falk MM. Specific amino-acid residues in the N-terminus and TM3 implicated in channel function and oligomerization compatibility of connexin43. J Cell Sci. 2003;116:3189–3201. doi: 10.1242/jcs.00604. [DOI] [PubMed] [Google Scholar]
- 29.Gemel J, Lin X, Veenstra RD, Beyer EC. N-terminal residues in Cx43 and Cx40 determine physiological properties of gap junction channels, but do not influence heteromeric assembly with each other or with Cx26. J Cell Sci. 2006;119:2258–2268. doi: 10.1242/jcs.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 31.Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys J. 2000;79:2403–2415. doi: 10.1016/S0006-3495(00)76485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys J. 2004;87:912–928. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh S, Verselis VK, Bargiello TA. Charges dispersed over the permeation pathway determine the charge selectivity and conductance of a Cx32 chimeric hemichannel. J Physiol. 2008;586:2445–2461. doi: 10.1113/jphysiol.2008.150805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bargiello TA, Tang Q, Oh S, Kwon T. Voltage-dependent conformational changes in connexin channels. Biochim Biophys Acta Biomemb. doi: 10.1016/j.bbamem.2011.09.019. http://dx.doi.org/10.1016/j.bbamem.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peracchia C, Peracchia LL. Inversion of both gating polarity and CO2 sensitivity of voltage gating with D3N mutation of Cx50. Am J Physiol Cell Physiol. 2005;288:C1381–C1389. doi: 10.1152/ajpcell.00348.2004. [DOI] [PubMed] [Google Scholar]
- 36.Tong JJ, Liu X, Dong L, Ebihara L. Exchange of gating properties between rat Cx46 and chicken Cx45.6. Biophys J. 2004;87:2397–2406. doi: 10.1529/biophysj.104.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong JJ, Ebihara L. Structural determinants for the differences in voltage gating of chicken Cx56 and Cx45.6 gap-junctional hemichannels. Biophys J. 2006;91:2142–2154. doi: 10.1529/biophysj.106.082859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong L, Liu X, Li H, Vertel BM, Ebihara L. Role of the N-terminus in permeability of chicken connexin45.6 gap junctional channels. J Physiol. 2006;576:787–799. doi: 10.1113/jphysiol.2006.113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musa H, Veenstra RD. Voltage-dependent blockade of connexin40 gap junctions by spermine. Biophys J. 2003;84:205–219. doi: 10.1016/S0006-3495(03)74843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musa H, Fenn E, Crye M, Gemel J, Beyer EC, Veenstra RD. Amino terminal glutamate residues confer spermine sensitivity and affect voltage gating and channel conductance of rat connexin40 gap junctions. J Physiol. 2004;557:863–878. doi: 10.1113/jphysiol.2003.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin X, Fenn E, Veenstra RD. An amino-terminal lysine residue of rat connexin40 that is required for spermine block. J Physiol. 2006;570:251–269. doi: 10.1113/jphysiol.2005.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia CH, Cheung D, Derosa AM, Chang B, Lo WK, White TW, Gong X. Knock-in of α3 connexin prevents severe cataracts caused by an α8 point mutation. J Cell Sci. 2006;119:2138–2144. doi: 10.1242/jcs.02940. [DOI] [PubMed] [Google Scholar]
- 43.Common JEA, Becker D, Di WL, Leigh IM, O'Toole EA, Kelsell DP. Functional studies of human skin disease- and deafness-associated connexin 30 mutations. Biochem Biophys Res Commun. 2002;298:651–656. doi: 10.1016/s0006-291x(02)02517-2. [DOI] [PubMed] [Google Scholar]
- 44.Richard G, Smith LE, Bailey RA, Itin P, Hohl D, Epstein EHJ, DiGiovanna JJ, Compton JG, Bale SJ. Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nat Genet. 1998;20:366–369. doi: 10.1038/3840. [DOI] [PubMed] [Google Scholar]
- 45.Deschenes SM, Walcott JL, Wexler TL, Scherer SS, Fischbeck KH. Altered trafficking of mutant connexin32. J Neurosci. 1997;17:9077–9084. doi: 10.1523/JNEUROSCI.17-23-09077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richard G, Rouan F, Willoughby CE, Brown N, Chung P, Ryynanen M, Jabs EW, Bale SJ, DiGiovanna JJ, Uitto J, Russell L. Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet. 2002;70:1341–1348. doi: 10.1086/339986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shibayama J, Paznekas W, Seki A, Taffet S, Wang JE, Delmar M, Musa H. Functional characterization of Connexin43 mutations found in patients with Oculodentodigital Dysplasia (ODDD) Circ Res. 2005:e83–e91. doi: 10.1161/01.RES.0000168369.79972.d2. [DOI] [PubMed] [Google Scholar]
- 48.Abrams CK, Freidin MM, Verselis VK, Bennett MV, Bargiello TA. Functional alterations in gap junction channels formed by mutant forms of connexin 32: evidence for loss of function as a pathogenic mechanism in the X-linked form of Charcot-Marie-Tooth disease. Brain Res. 2001;900:9–25. doi: 10.1016/s0006-8993(00)03327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roscoe W, Veitch GI, Gong XQ, Pellegrino E, Bai D, McLachlan E, Shao Q, Kidder G, Laird DW. Oculodentodigital dysplasia-causing connexin43 mutants are non-functional and exhibit dominant effects on wild-type connexin43. J Biol Chem. 2005;280:11456–11466. doi: 10.1074/jbc.M409564200. [DOI] [PubMed] [Google Scholar]
- 50.Gong XQ, Shao Q, Langlois S, Laird DW, Bai D. Differential potency of dominant negative connexin43 mutations in oculodentodigital dysplasia. J Biol Chem. 2007:19190–19202. doi: 10.1074/jbc.M609653200. [DOI] [PubMed] [Google Scholar]
- 51.Terrinoni A, Codispoti A, Serra V, Didona B, Bruno E, Nistico R, Giustizieri M, Alessandrini M, Campione E, Melino G. Connexin 26 (GJB2) mutations, causing KID Syndrome, are associated with cell death due to calcium gating deregulation. Biochem Biophys Res Commun. 2010;394:909–914. doi: 10.1016/j.bbrc.2010.03.073. [DOI] [PubMed] [Google Scholar]
- 52.Lazic T, Horii KA, Richard G, Wasserman DI, Antaya RJ. A report of GJB2 (N14K) Connexin 26 mutation in two patients--a new subtype of KID syndrome? Pediatr Dermatol. 2008;25:535–540. doi: 10.1111/j.1525-1470.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 53.Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL. Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys. 2000;381:181–190. doi: 10.1006/abbi.2000.1989. [DOI] [PubMed] [Google Scholar]
- 54.Arita K, Akiyama M, Aizawa T, Umetsu Y, Segawa I, Goto M, Sawamura D, Demura M, Kawano K, Shimizu H. A novel N14Y mutation in Connexin26 in keratitis-ichthyosis-deafness syndrome: analyses of altered gap junctional communication and molecular structure of N terminus of mutated Connexin26. Am J Pathol. 2006;169:416–423. doi: 10.2353/ajpath.2006.051242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalmatsky BD, Bhagan S, Tang Q, Bargiello TA, Dowd TL. Structural studies of the N-terminus of Connexin 32 using 1H NMR spectroscopy. Arch Biochem Biophys. 2009;490:9–16. doi: 10.1016/j.abb.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE. Three-dimensional structure of a human connexin26 gap junction channel reveals a plug in the vestibule. Proc Natl Acad Sci U S A. 2007;104:10034–10039. doi: 10.1073/pnas.0703704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE. Projection structure of a N-terminal deletion mutant of connexin 26 channel with decreased central pore density. Cell Commun Adhes. 2008;15:85–93. doi: 10.1080/15419060802013588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 59.Tsukihara T. Structural organization of hemichannels. Biochim Biophys Acta Biomemb. 2011 in press. [Google Scholar]
- 60.Zhou XW, Pfahnl A, Werner R, Hudder A, Llanes A, Luebke A, Dahl G. Identification of a pore lining segment in gap junction hemichannels. Biophys J. 1997;72:1946–1953. doi: 10.1016/S0006-3495(97)78840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.