Abstract
Hypothyroidism is a cause of genetic and environmentally induced deafness. The sensitivity of cochlear development and function to thyroid hormone (TH) mandates understanding TH action in this sensory organ. Prop1 df and Pou1f1 dw mutant mice carry mutations in different pituitary transcription factors, each resulting in pituitary thyrotropin deficiency. Despite the same lack of detectable serum TH, these mutants have very different hearing abilities: Prop1 df mutants are mildly affected, while Pou1f1 dw mutants are completely deaf. Genetic studies show that this difference is attributable to the genetic backgrounds. Using embryo transfer, we discovered that factors intrinsic to the fetus are the major contributor to this difference, not maternal effects. We analyzed Prop1 df mutants to identify processes in cochlear development that are disrupted in other hypothyroid animal models but protected in Prop1 df mutants by the genetic background. The development of outer hair cell (OHC) function is delayed, but Prestin and KCNQ4 immunostaining appear normal in mature Prop1 df mutants. The endocochlear potential and KCNJ10 immunostaining in the stria vascularis are indistinguishable from wild type, and no differences in neurofilament or synaptophysin staining are evident in Prop1 df mutants. The synaptic vesicle protein otoferlin normally shifts expression from OHC to IHC as temporary afferent fibers beneath the OHC regress postnatally. Prop1 df mutants exhibit persistent, abnormal expression of otoferlin in apical OHC, suggesting delayed maturation of synaptic function. Thus, the genetic background of Prop1 df mutants is remarkably protective for most functions affected in other hypothyroid mice. The Prop1 df mutant is an attractive model for identifying the genes that protect against deafness.
Keywords: thyroid hormone, deafness, dwarfism, otoferlin
Introduction
Congenital hypothyroidism (CH) occurs in 1/4,000 live births. Thyroid hormone (TH) deficiency can cause severe cognitive dysfunction and deafness (Debruyne et al. 1983; Rovet et al. 1996). The extent of hearing impairment varies among patients with CH, and the cause is unknown. Hypothyroid animals have multiple defects in cochlear development (Uziel et al. 1983, 1985a, b; O'Malley et al. 1995; Li et al. 1999; Knipper et al. 2001; Christ et al. 2004; Mustapha et al. 2009). Two dwarf mice with recessive, severe, secondary hypothyroidism Prop1 df/df and Pou1f1 dw/dw (Pit1 dw/dw) lack pituitary thyroid stimulating hormone (TSH) and TH but have very different hearing abilities (Karolyi et al. 2007). Hearing tests on the progeny of a small intercross between the two strains indicated that genetic background, or genomic variation, accounts for the differences in hearing between Prop1 df/df and Pou1f1 dw/dw mutants.
Maternal thyroid function can also affect the hearing abilities of humans and other animals. In areas with endemic cretinism, deafness is equally prevalent in euthyroid and hypothyroid patients, suggesting the maternal hypothyroidism may cause low TH levels in utero which results in auditory dysfunction in the euthyroid children (Boyages and Halpern 1993; Chan et al. 2009). A thyroid ablation study in sheep demonstrated that maternal and fetal hypothyroxinemia combine to cause neurological damage (McIntosh et al. 1983). Goitrogen treatment of pregnant and lactating rodents between the onset of fetal thyroid gland function (E17–18) and the onset of hearing at postnatal day 12 (P12) can lead to permanent hearing defects in the offspring (Deol 1973; Knipper et al. 2000). Prenatal thyroxine treatment can significantly improve the hearing of hypothyroid Tshr mutant mice (Sprenkle et al. 2001a). Elevated maternal thyroid peroxidase (TPO) autoantibodies during the third trimester are also associated with hearing deficits in children (Wasserman et al. 2008). TPO is essential for production of TH. Individuals with autoantibodies often have hypothyroidism with bouts of hyperthyroidism. Taken together, maternal effects, including maternal TH level, gestation time, and maturity of the fetus at birth, could affect the sensitivity of genetically predisposed hypothyroid animals to hearing impairment.
Pleiotropic effects of hypothyroidism on cochlear development have been demonstrated in Pou1f1 dw/dw mutants. They exhibit immature cochlear morphology, tectorial membrane abnormalities, reduced expression and function of potassium channels, hair cell loss, and strial cell deterioration (Mustapha et al. 2009). Several of these features have been reported in hypothyroid rodent models induced by thyroid-toxic drugs or other genetic lesions (Li et al. 1999; Knipper et al. 2000; Sprenkle et al. 2001b; Christ et al. 2004), suggesting that there are common effects of TH deficiency. Because of the diversity of effects, TH likely regulates multiple, critical processes of inner ear development. It still remains to be determined which processes are most sensitive to TH deficiency and to what degree the observed effects contribute to the hearing problems in the hypothyroid animals.
In this study, we report that the genetic background effects on the hearing abilities of Prop1 and Pou1f1 mutants are intrinsic to the fetuses rather than maternal. Also, we demonstrate that many of the developmental processes that are TH dependent in other animal models with hypothyroidism are rescued by the Prop1 mutant background. Thus, Prop1 df mice provide a valuable tool for us to explore the cause of variation in hearing impairment in hypothyroid mice and humans and to identify the potential modifiers that protect against hearing loss due to hypothyroidism.
Materials and methods
Mice
All experiments were approved by the University Committee on the Use and Care of Animals and conducted in accord with the principles and procedures outlined in the National Institutes of Health Guidelines.
DF/B-p/+, Prop1 df mice were obtained from Dr. Andrzej Bartke in 1988 and maintained at the University of Michigan. This stock is not inbred but has gone through population constriction. DW/J-Mlph ln/ln, Pou1f1 dw mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). The DW/J stock is inbred (Mouse Phenome Database, http://phenome.jax.org). The B6/D2 mice used as surrogate mothers are the F1 hybrids produced by breeding C57BL/6J and DBA/2J mouse strains. These hybrids were purchased from The Jackson Laboratory.
Mice were housed in specific pathogen-free conditions with automatic watering and ventilation. Previously described procedures for animal care and genotyping were used, including feeding mice a higher fat chow designed for breeding (PMI5020), delaying weaning of mutants until 35 days, and housing mutants with normal littermates to provide warmth (Karolyi et al. 2007). Genotyping detects the loss of function mutations that affect the DNA binding domains in each protein: T247C mutation in Prop1 df causing Ser83Pro and G783T in Pou1f1 dw resulting Trp261Cys (Gage et al. 1996; Douglas et al. 2001). In all experiments, at least three animals of each genotype were analyzed for each age group studied unless stated otherwise. Embryonic days gestation are counted from the time of conception with e0.5 denoting the morning after mating. Postnatal day zero (P0) is designated as the day of birth.
Embryo transfer experiments
Three- to four-week-old Prop1 df/+ or Pou1f1 dw/+ females were super-ovulated by intraperitoneal injection of 5 U each of pregnant mare serum gonadotropin (PMSG) followed by human chorionic gonadotropin (HCG) 46–50 h later. Females were placed with heterozygous males of the same genotype, i.e., Prop1 df/+ or Pou1f1 dw/+, for overnight mating. E0.5 embryos (one-cell stage) were collected from fallopian tubes of the plugged females and cultured in M16 medium (Sigma) with penicillin and streptomycin at 37°C incubator overnight. Eight two-cell stage embryos were put into one oviduct of each pseudo-pregnant B6/D2 female, which were generated by mating with vasectomized B6/D2 males. Wild-type two-cell stage B6/D2 embryos were placed in the oviduct on the opposite side of the same pseudo-pregnant female. Pups born and weaned from surrogate mothers were genotyped and evaluated for hearing. Wild-type mice with the same coat color as the mutants were used as controls: Prop1 mice are pink-eyed fawn and Pou1f1 mice are agouti leaden. For simplicity, “_S” is added to genotype symbols to represent mice born from surrogate mother in this article.
Auditory physiology
Auditory brainstem response (ABR). Animals were anesthetized (ketamine 65 mg/kg, xylazine 3.5 mg/kg, and acepromazine 2 mg/kg). Body temperature was maintained through the use of water circulating heating pads and heat lamps. Additional anesthetic (ketamine and xylazine) was administered if needed to maintain anesthesia depth sufficient to insure immobilization and relaxation. ABRs were recorded in an electrically and acoustically shielded chamber (Acoustic Systems, Austin, TX, USA). Needle electrodes were placed at vertex (active) and the test ear (reference) and contralateral ear (ground) pinnae. Tucker Davis Technologies (TDT) System III hardware and SigGen/BioSig software (TDT, Alachua, FL, USA) were used to present the stimulus and record responses. Tones were delivered through an EC1 driver (TDT, aluminum enclosure made in-house), with the speculum placed just inside the tragus. Stimulus presentation was 15-ms tone bursts, with 1-ms rise/fall times, presented 10 per second. Up to 1,024 responses were averaged for each stimulus level. Responses were collected for stimulus levels in 10-dB steps at higher stimulus levels, with additional 5-dB steps near threshold. Thresholds were interpolated between the lowest stimulus level where a response was observed, and 5 dB lower, where no response was observed.
Distortion product otoacoustic emissions (DPOAEs) and endocochlear potential (EP) were measured as previously described (Karolyi et al. 2007).
Antibodies and immunofluorescence
The antibodies used to detect prestin (1:200), KCNJ10 (1:300), KCNJ4 (1:300), synaptophysin (1:400), TRITC-labeled secondary antibodies (1:200), and Alexa Fluor 488 conjugated secondary antibodies (1:200) have been previously described (Mustapha et al. 2009). The rabbit polyclonal anti-Neurofilament 200 antibody (1:500, Sigma) are commercially available. The rabbit polyclonal anti-otoferlin antibody (1:500) was kindly provided by Drs. Saaid Safieddine and Christine Petit (Roux et al. 2006).
Preparation of cochlear cryosections and the procedures for immunostaining those sections have been previously described (Mustapha et al. 2009).
Mouse inner ears were rapidly dissected from the temporal bones in phosphate-buffered saline (PBS). The temporal bones were immersed in 4% paraformaldehyde (PFA) for fixation. Under stereoscopic magnification, the round and oval windows were opened, and the bone from the apical tip of the cochleae was removed to allow fixative to flow throughout the tissue. One hour later, the stria vascularis and tectorial membrane were removed and the organ of Corti was exposed. After two washes in PBS, the tissue was incubated in 5% normal goat serum with 0.3% triton for 1 h, and then with the primary antibody at 4°C overnight. After three washes in PBS, samples were incubated with the secondary antibody for 2 h at room temperature, washed again three times in PBS, and mounted in ProLong Gold Antifade Reagent (Invitrogen). All fluorescent microscopy was performed on a Leica Leitz DMRB compound microscope with Leica Fiber Optic Illumination. Images were captured using a QImaging Retiga 2000R Fast 1394 camera and QCapture Pro 5.1.1.14 software. Images were processed using Adobe® Photoshop® CS2 9.0.
Statistical analysis
All statistical analyses were performed with SPSS 15.0. The p values reported for ABR were generated by independent samples t tests or Tukey’s multiple comparisons following one-way ANOVA. Error bars represent standard deviation for means.
Results
Prop1df/df mutants have a mild hearing deficit
ABR tests were used to re-assess the hearing proficiency of Prop1 df/df mutants and wild-type littermate controls because previous studies were carried out on conventionally housed mice in a quieter environment (Karolyi et al. 2007). At 4 weeks old, the ABR thresholds of Prop1 df/df mutants are elevated relative to controls by 21 dB SPL and 34 dB SPL at 4 kHz and 20 kHz, respectively (Fig. 1, P < 0.05). When the mice are 6–7 weeks old, mutant hearing has improved but is still worse than normal, with elevations of 11 and 14 dB SPL at 4 and 20 kHz, respectively (Fig. 1, P < 0.001). The hearing thresholds of the mutant mice are the same at 12 weeks as they were at 6–7 weeks (Fig. 1). This indicates that the cochlear development of Prop1 df/df mutants undergoes maturation between 4 and 7 weeks of age, but it does not achieve wild-type function even by 12 weeks. This hearing impairment is consistent with previous reports that measured different ages of conventionally housed mice at different frequencies (Karolyi et al. 2007).
FIG. 1.
Prop1 df mutants have a mild hearing deficit. ABR tests were performed on sets of normal mice (white bars) and mutant mice (black bars) at ages of 4 weeks, 6–7 weeks, and 12 weeks. N = 3 per genotype for 4- and 12-week-old mice, n = 9 for 6–7-week-old wild type, and n = 8 for 6–7-week-old mutants.
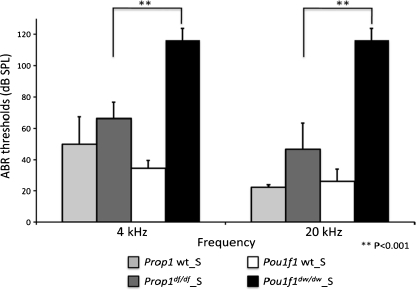
Gestational and neonatal environments do not account for variant responses to hypothyroidism in Prop1df/df and Pou1f1dw/dw mutants
To determine the degree to which maternal effects contribute to the different degrees of hearing impairment in Prop1 df/df and Pou1f1 dw/dw mutants, fertilized eggs with all genotypes from both strains were transplanted into the uteri of B6/D2 surrogate mothers, which would provide common gestation and lactation environments for both mutants. We chose the mothers of the B6/D2 strain as surrogates because they have hybrid vigor and exhibit good mothering instincts. The hearing ability of the progeny born to the surrogates was tested by ABR at 4 weeks of age, including Prop1 df/df_S and Pou1f1 dw/dw_S mutants as well as wild types from each strain. The hearing deficits of Prop1 df/df_S and Pou1f1 dw/dw_S mutants are significantly different from their normal littermates, also born to surrogate mothers, and from each other, but they are indistinguishable from the Prop1 df/df and Pou1f1 dw/dw mice born to mothers from their own backgrounds (Fig. 2, P < 0.001 for comparison of the hearing deficits between Prop1 df/df_S and Pou1f1 dw/dw_S mutants at both 4 kHz and 20 kHz). Thus, factors intrinsic to the fetus play the major roles in the different responses of Prop1 df/df and Pou1f1 dw/dw mutant cochlea to hypothyroidism, and maternal effects are minimal in this context.
FIG. 2.
Gestational and neonatal environments do not account for different hearing abilities of Prop1 df and Pou1f1 dw mutants. Pups born from surrogate mothers (designated as “Genotype_S”) were tested by ABR. The hearing deficits of Prop1 df/df_S and Pou1f1 dw/dw_S are significantly different (P < 0.001). For each group, four mice were tested (n = 4).
Mild outer hair cell (OHC) dysfunction with apparently normal expression of KCNQ4 and prestin in Prop1dfdf mutants
Cochlear OHCs are unique in their electromotility and work as a cochlear amplifier in sound processing (Ospeck et al. 2003). DPOAE is used as a standard audiometric technique to measure OHC function of amplification. At 4 weeks of age, Prop1 df/df mutants have DPOAE responses (geometric means of the primary tones) at 12 or 24 kHz that are indistinguishable from the noise floor in postmortem mutant or wild-type mice (Fig. 3A). By 7 weeks old, Prop1 df/df mutants have improved, but only have about half of the normal DPOAE at 24 kHz (Fig. 3A). At 12 weeks old, the DPOAE response of Prop1 df/df mutants are still significantly lower than the wild-type mice (data not shown). The maturation process of DPOAE in mice normally begins at 11 days old and obtains the adult-like pattern by 4 weeks (Narui et al. 2009). This demonstrates that Prop1 df/df mutant cochlea have delayed development of OHC function with persistent deficiency at 12 wks. ABR measurements improve between 4 and 7 weeks of age (Fig. 1), suggesting that the persistent OHC dysfunction could be a contributor to the mild hearing impairment in Prop1 df/df mutants.
FIG. 3.
Prop1 df mutants exhibit mild OHC dysfunction with normal expression of KCNQ4 and prestin. A DPOAEs were measured in live 4-week and 7-week-old wild-type and Prop1 df mutant mice (black circles and white squares, respectively), and compared with DPOAEs of postmortem animals (dotted and dashed line). Data are shown for the 12 and 24 kHz frequencies. N = 3 for each genotype group of 4-week-old mice and n = 6 for 7-week-old ones. B, C KCNQ4 immunoreactivity is normal in OHCs (arrows) of mutant mice relative to wild type. Frozen sections obtained from P28 wild-type and mutant mice were stained for KCNQ4 (red). Nuclei were stained with DAPI (blue). D, E Prestin expression and localization was analyzed by staining frozen sections from wild-type and Prop1 df mutants at P28 with prestin-specific antibodies (red). Nuclei were labeled using DAPI (blue). Arrows identify rows of outer hair cells. Scale bars: 10 μm.
KCNQ4 is an M-type K+ channel localized exclusively to the basal pole of the hair cells, and it is responsible for the dominant K+ conductance in mature OHCs (Marcotti and Kros 1999; Kharkovets et al. 2000). Mutations in KCNQ4 cause progressive deafness in both human and mice (Kubisch et al. 1999; Kharkovets et al. 2006). Pou1f1 dw/dw mutant mice have reduced immunohistochemical staining and function of KCNQ4 in OHCs (Mustapha et al. 2009). We examined KCNQ4 expression in Prop1 df/df mutant cochlea at 4 weeks. KCNQ4 immunoreactivity is similar in Prop1 df/df mutants and their wild-type littermates at this age (Fig. 3B, C) and at 7 weeks (data not shown), despite the hearing deficit identified by ABR and DPOAE at this age. Although immunostaining is not quantitative, KCNQ4 immunoreactivity was indistinguishable in Prop1 df/df mutants and wild types at both ages, while the staining in Pou1f1 dw/dw mutants was clearly deficient relative to their littermates at both ages. Thus, the DF/B genetic background supports apparently normal KCNQ4 immunostaining despite the severe hypothyroidism.
Prestin (SLC26A5) is one of the anion transporters in the inner ear (Lohi et al. 2000) that is expressed along the basolateral membrane of OHCs (Adler et al. 2003; Yu et al. 2006), conferring electromotility to the OHCs (Zheng et al. 2000). Prestin is transcriptionally regulated by TH during final differentiation of outer hair cells (Weber et al. 2002; Winter et al. 2006). Prestin is also required for normal OHC length (Liberman et al. 2002). We examined prestin expression in Prop1 df/df mutants by immunohistochemistry. The prestin immunostaining in OHCs of mutants and wild types at 4 weeks of age are indistinguishable, and there are no obvious differences in cell size (Fig. 3D, E). Prestin localizes at the lateral wall of Prop1 df/df mutant OHCs, which is the expected mature pattern (Fig. 3D, E). Thus, the DF/B genetic background of the Prop1 mutants supports the development of prestin expression and localization much better than observed in DW/J-Pou1f1 mutants.
Developmentally delayed expression of KCNJ10 in the stria vascularis and normal EP in Prop1df/df mutants
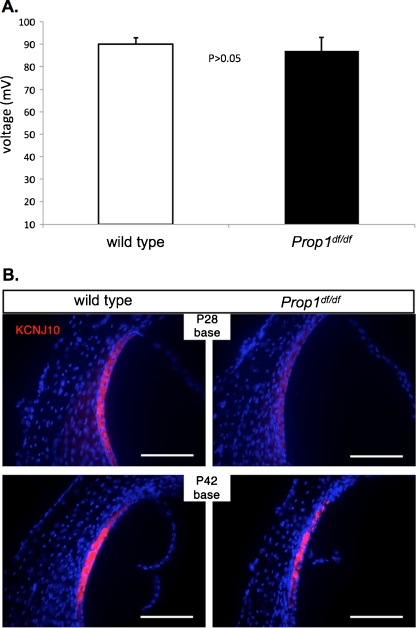
Endocochlear potential (EP) is the driving force for the transduction of ions through the channels in hair cell stereocilia. An EP level of +80 mV is essential for normal hearing. Since EP affects the amplitude of DPOAE, EP was also examined in the present study. The EP of 7-week-old Prop1 df/df mutants ranges from 81 to 93 mV (N = 3), which is indistinguishable from the EP levels (88 to 92 mV, N = 2) in wild types (Fig. 4). Thus, the abnormal DPOAE in Prop1 df/df mutants likely results from defective OHCs, not abnormal EP.
FIG. 4.
Endocochlear potential (EP) is normal and KCNJ10 expression is developmentally delayed in Prop1 df mutants. A EP was measured in P42 wild-type and age-matched mutant animals. Levels of EP (mV) for littermate control and Prop1 df mutants are shown. B Frozen sections of the organ of Corti of wild-type and mutant mice collected at P28 and P42 were stained for KCNJ10 (red). Nuclei are marked by DAPI (blue). KCNJ10 expression is detected in the intermediate cells of the stria vascularis.
KCNJ10 (Kir4.1) K+ channels in the intermediate cells of the stria vascularis are required for generation of a normal EP (Marcus et al. 2002). Reduction in KCNJ10 immunostaining was observed in Pou1f1 dw/dw mutants, which could account for the substantially reduced EP in those mice (Mustapha et al. 2009). We examined KCNJ10 expression in 4- and 6-week-old Prop1 df/df mutants by immunohistochemical staining. At 4 weeks of age, the KCNJ10 immunofluorescence is reduced in mutants relative to wild types (Fig. 4B). By 6 weeks of age, the KCNJ10 immunostaining in the mutants is indistinguishable from the wild types (Fig. 4B). The subcellular localization of KCNJ10 is normal in both the 4- and 6-week-old mutants. This is consistent with the normal EP observed. Thus, the DF/B-Prop1 genetic background protects against persistently reduced KCNJ10 immunostaining in other adult hypothyroid mice.
Gross neurite outgrowth and synaptogenesis of OHCs are unaffected in Prop1df/df mutant cochlea
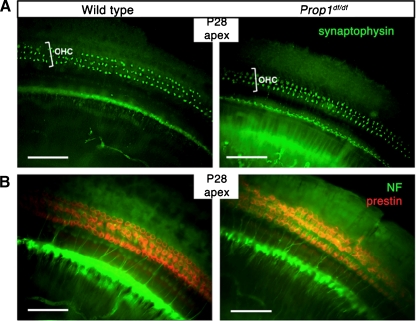
The maturation of the nervous system in the rodent cochlea takes place during the first two postnatal weeks of life, which overlaps with the critical time window of TH function (Knipper et al. 2000). TH deprivation causes abnormalities in cochlear innervation and synaptogenesis in multiple hypothyroid animal models (Uziel et al. 1983; Brandt et al. 2007; Sendin et al. 2007). Abnormal efferent fibers have been observed by dye injections in hypothyroid rats even though the olivocochlear neurons, from which the efferent fibers arise, are normal in number and distribution (Cantos et al. 2000, 2003). Neuronal marker proteins are also frequently used for examination of the innervation patterns. We used antibodies that recognize neurofilament protein NF-200, which stains both afferent and efferent fibers, to detect neurite outgrowth in Prop1 df/df mutant cochlea at 4 and 7 weeks. No significant differences were observed in neuronal fibers between mutants and wild types at 4 weeks (Fig. 5B) or 7 weeks (data not shown). Synaptophysin is a presynaptic marker of efferent fibers, which comprise 95% of the fibers innervating OHCs. A strong and normally organized pattern of synaptophysin immunostaining was observed in Prop1 df/df mutants (Fig. 5A). Thus, neither the gross neurite outgrowth nor the efferent synaptogenesis of OHCs are apparently affected by low TH levels in DF/B-Prop1 df/df mice.
FIG. 5.
Neurite growth and synaptogenesis of OHCs are grossly unaffected in Prop1 df mutants. A Synaptophysin, a presynaptic marker of efferent fibers, is stained on whole mount preparations of cochlear epithelia with an anti-synaptophysin antibody (green). B Neurofilament protein (NF-200) immunostaining was used to detect the neurite outgrowth in cochlea whole mounts of P28 mutants as well as wild-type controls. Prestin immunostaining was used to indicate the position of OHCs (red).
Prolonged presence of otoferlin in apical OHCs of Prop1df/df mutants
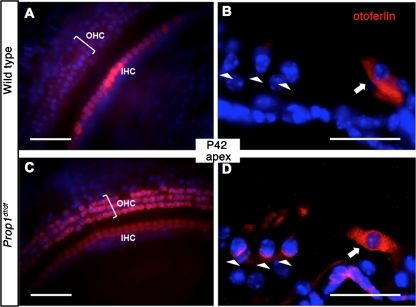
Otoferlin is thought to be the major calcium sensor and essential for exocytosis at both inner hair cell (IHC) and immature OHC ribbon synapses (Roux et al. 2006; Beurg et al. 2008). Expression of otoferlin begins prenatally in both IHCs and OHCs and vanishes from OHCs by P6 (Roux et al. 2006). The disappearance of otoferlin immunostaining in OHCs occurs around the same time as the retraction of afferent fibers from OHCs (Beurg et al. 2008). Together with myosin VI, otoferlin is involved in the maintenance of the basolateral synaptic structure of IHCs (Heidrych et al. 2009; Roux et al. 2009). We examined the expression of otoferlin by immunostaining whole-mount and cryosectioned tissues. Similar expression levels of otoferlin were seen in IHCs of Prop1 df/df mutant cochlea as the wild type. Abnormally strong otoferlin immunostaining persists in the OHCs in the apical coil of 6-week-old Prop1 df/df mutant cochlea (Fig. 6C, D) and none was observed in the wild-type littermates. Weak otoferlin immunostaining is reported in the OHCs in the apical region of mature cochlea in wild-type animals (Roux et al. 2006), but this may represent a strain difference in maturation or sensitivity of detection. Nevertheless, the abnormal persistence of otoferlin expression in apical OHCs of Prop1 df/df mutant cochlea may represent some remaining immaturity of the cells.
FIG. 6.
Prolonged presence of otoferlin at apical OHCs in Prop1 df mutants. A, C Otoferlin immunostaining (red) was done on whole mount preparations of sensory epithelia from the apical turn of Prop1 df mutants and wild type. B, D Frozen sections of organ of Corti were collected and stained by anti-Otoferlin antibody (red). Arrowheads indicate the rows of OHCs. Arrows point to the IHCs. Nuclei are blue in all stainings.
Discussion
Maternal effects are minimal for variation of hearing deficits between Prop1df/df and Pou1f1dw/dw mutants
Maternal effects are defined as the causal influence of the maternal genotype or phenotype on the phenotype of the offspring (Wolf and Wade 2009). Based on this definition, maternal effects may include direct or indirect consequences of maternal traits, such as nesting behavior, gene transcription, hormone levels, antibodies, placental permeability, and the particular environments in which mothers lay eggs (Rhees et al. 1999; Wolf and Wade 2009). In mammals, the roles of maternal effects exist at two distinct maternal stages: prenatal uterine and postnatal nursing and nurturing. By transferring embryos between two inbred mouse strains with large body size (C3H) and small body size (SWR), both uterine and postnatal maternal effects were proven to contribute to the prenatal and early postnatal development of offspring, and no obvious donor genotype effects were observed (Cowley et al. 1989; Pomp et al. 1989; Rhees et al. 1999).
The hearing abilities of progeny are substantially affected by maternal TH levels in both human and rodents (Boyages and Halpern 1993; Knipper et al. 2001). In our breeding scheme, the mothers of Prop1 df/df and Pou1f1 dw/dw mutants are heterozygous for the recessive Prop1 and Pou1f1 mutations, respectively. Thus, maternal TH levels are in the normal range. We suspected that strain differences in normal basal TH levels transferred to the fetus or neonate through the placenta or milk, respectively, could contribute to the different levels of hearing impairment characteristic of Prop1 df/df and Pou1f1 dw/dw mutant mice. In addition, it is possible that strain differences in gestation time or maturity at birth could influence the effects of fetal hypothyroidism on hearing deficit. For example, longer gestation time might allow a hypothyroid fetus to benefit from maternal TH long enough to protect it during the critical period for TH-dependent cochlear development.
Our embryo transfer experiments demonstrated that a consistent mothering environment does not significantly change the hearing deficits in Prop1 df/df and Pou1f1 dw/dw mutants from the ones they exhibit when born from mothers on the original backgrounds. This contrasts with the changes in hearing abilities observed for Prop1 df/df and Pou1f1 dw/dw mutants when the original backgrounds were mixed (Karolyi et al. 2007). From these results, we conclude that the strain differences intrinsic to the fetus play the major role in inducing different hearing deficits between Prop1 df/df and Pou1f1 dw/dw mutants. Other strain combinations might reveal strong maternal effects, however, because there are compelling data to support the importance of maternal thyroid hormone for development (Deol 1973; Knipper et al. 2000). Future genetic studies with DF/B-Prop1 and DW/J-Pou1f1 mutants may identify loci that enhance or suppress the ability of hypothyroid mice to develop normal hearing, and the results of the embryo transfer studies direct the focus to factors intrinsic to the fetus and/or neonate. A protective locus on Chr 2 called Modifier of dw hearing, Mdwh, has been mapped in a cross between DW/J-Pou1f1 mice and Mus castaneus (Fang et al. 2011).
The effects of genetic background on hearing ability in mice are noted in many inbred strains. The most familiar example is that C57BL/6 J mice carry a mutation in cadherin 23 (Cdh23) known as Age-related hearing locus (Ahl) and develop progressive deafness beginning at about 1 to 2 months at the highest frequencies. Quantitative trait loci (QTL) analysis has identified several modifiers of hearing impairment (Ikeda et al. 1999, 2002; Drayton and Noben-Trauth 2006; Mashimo et al. 2006; Noguchi et al. 2006; Van Eyken et al. 2006; Ohlemiller et al. 2010). We expect that QTL analysis of DF/B and/or DW/J strains could identify genes that confer protection or susceptibility to hypothyroidism-induced hearing impairment (Fang et al. 2011). These modifiers could also be genes involved in Mendelian hearing defects, as mutations in Cdh23 also account for nonsyndromic autosomal recessive deafness DFNB12. Alternatively, they could be genes that regulate the transport and bioactivity of thyroid hormone within the cochlea.
Delayed maturation may contribute the mild hearing impairment in Prop1df/df mutants
The Prop1 df genetic background protected against most abnormalities reported in deaf, hypothyroid mice. Because the persistent hearing impairment in Prop1 df/df mutants represents less than 20 dB threshold elevation, very subtle differences in maturation that are not obvious may be contributing factors. The effects of hypothyroidism on the cochlear nervous system are expected to be mostly associated with OHC wiring (Uziel et al. 1983). This may be because the pattern of synapses at OHC normally undergoes profound changes within the first two postnatal weeks, which is also the critical time window of TH functioning for normal hearing. We observed an immature pattern of otoferlin expression in apical regions of Prop1 df/df mutant cochlea. During normal cochlear development, otoferlin initially is expressed in IHCs and OHCs. By P6, the disappearance of otoferlin from OHCs parallels the retraction of afferent dendrites and formation of efferent synapses (Roux et al. 2006). Thus, abnormal persistence of otoferlin in Prop1 df/df mutant OHCs implies an immature innervation pattern at the level of OHCs. In Prop1 df/df mutant cochlea, the prolonged persistence of otoferlin staining in OHCs was only observed at apical turn, tuned for low frequencies. Although the delayed maturation of otoferlin staining in the apical OHCs may contribute to the mild degree of hearing impairment in Prop1 df/df mutants, it is not consistent with high frequency OHC dysfunction, for which the underlying factor(s) remains to be uncovered.
Usually, IHC dysfunction corresponds to severe hearing loss because IHCs are the primary sensory receptors within the cochlea. The maturation of ribbon synapses in IHCs is affected by hypothyroidism in rodents (Brandt et al. 2007; Sendin et al. 2007). In those studies, the expression of otoferlin in IHCs was completely absent or substantially reduced in drug-treated rats and Pax8 knockout mice, respectively. This is quite different from the Prop1 df/df mutants, which preserved almost normal otoferlin expression level in IHCs. The Prop1 df/df genetic background is remarkably protective of otoferlin expression in IHCs.
Gene regulation by TH may be substantially affected by genetic background
We examined expression of several cochlear genes that are affected in other hypothyroid animal models. In Pou1f1 dw/dw mutants, prestin expression and localization are developmentally delayed, but they become indistinguishable from normal littermates by 6 weeks (Mustapha et al. 2009). The capacitance levels in mutants this age are compatible with levels in young hearing mice, consistent with adequate prestin function for hearing. In Prop1 df/df mutants, however, both expression and localization of prestin are not obviously affected by the absence of TH, suggesting prestin function is not strictly TH dependent. TH response elements (TREs) exist within the Prestin gene and are regulated by binding of TH receptor (TR), retinoid X receptor (RXR) heterodimers (Weber et al. 2002). The Prop1 df genetic background may support either RXR or an as yet uncharacterized heterodimer partner of TRs interacting with TREs to compensate the absence of TH to activate prestin gene. We cannot rule out the possibility that there is a biologically significant level of TH produced in Prop1 df/df mice, even though it is not detectable in serum, that contributes to the protective effect (Gage et al. 1996). Alternatively, completely independent transcriptional control elements or factors may compensate.
KCNQ4 expression is significantly reduced in Pou1f1 dw/dw mutants from weaning to adulthood (Mustapha et al. 2009), but Prop1 df/df mutants, in contrast, have apparently normal KCNQ4 immunostaining. Unliganded TRalpha1 receptors exert a repressive influence on KCNQ4 expression during final differentiation of OHCs (Winter et al. 2006). TRalpha1 knockout mice, however, do not exhibit hearing impairment, which implies that the activation of KCNQ4 gene cannot solely depend on TH/TR pathway (Rusch et al. 1998). A novel TH-signaling pathway can bypass TRs to mediate TH regulation (Shibusawa et al. 2003). Genetic studies may reveal the strain-specific modifier genes that lead to differential expression of KCNQ4 in hypothyroid mice.
Reduction of KCNJ10 expression in the stria vascularis likely contributes to the reduction of EP level in Pou1f1 dw/dw mutants (Mustapha et al. 2009). In contrast, the EP level of Prop1 df/df mutants is normal at 6 weeks, and expression of KCNJ10 is developmentally delayed but reaches apparently normal levels by 6 weeks. The mechanism whereby TH regulates Kcnj10 gene expression in susceptible strains is not known. It could be a direct or indirect transcriptional regulator, or influence the stability of the RNA or protein. In fact, differences in scaffolding protein expression can have profound, pleiotropic effects on protein stability (Heydemann and McNally 2007).
The roles of the TH, TR complex in gene regulation have been widely documented in many physiological fields including development, homeostasis, cell proliferation and differentiation, etc. The downstream effects of TH/TRs on gene expression can be either activation or repression. A comparison of the cochlea gene expression profiles from Pou1f1 dw/dw mutants and wild types revealed that half of the genes are up-regulated and half are down-regulated in Pou1f1 dw/dw mutants (Tzy-wen Gong, unpublished data). This proves the complexity of gene regulation by TH. It is intriguing that the genetic background of Prop1 mice can rescue expression of so many genes despite the existence of profound hypothyroidism. We favor a model involving genetic variation in a TH responsive transcription factor in the cochlea or alteration that boosts the effective level of thyroid hormone in the cochlea (van der Deure et al. 2010). This could account for improvement in many TH-dependent processes in the presence of the DF/B background. Alternatively, there could be a complex set of genes that contribute to the protective effects. Sorting out this difference could be very important for us to identify the genes and pathways that are the most sensitive to TH regulation in inner ear development.
In conclusion, the Prop1 df/df mutant mice lack TH, yet they exhibit only a mild hearing deficit. The genetic background of Prop1 mice can compensate for many cochlear developmental processes that are apparently dependent on TH in other strains. Identification of the protective factor(s) for hypothyroidism-induced hearing loss by genetic mapping would help us understand the mechanism of gene regulation by TH in the inner ear and potentially identify novel genes involved in the normal auditory function.
Acknowledgments
Funding for this study came from the March of Dimes #6-FY08-262 (S.A.C.), P30 DC05188 (D.F.D.), and R01DC009590 (M.M.). We thank the following individuals for their important contributions to this work: Yehoash Raphael and Ken Johnson for helpful discussion and suggestions, Maggie Van Keuren and the Transgenic Animal Model Core of the University of Michigan for performing the embryo transfer, the grants that support that Core (CA46592, AR20557, DK34933, DK20572, P30DK08194), Jennifer Benson and Kärin Halsey for ABR tests and EP measurements at Kresge Hearing Research Institute, and other members of the Camper Lab including Thomas Jones and Michelle Fleming. A.G. and Q.F. did the animal breeding and genotyping and immunostaining for their undergraduate honor’s thesis and Ph.D. thesis, respectively. D.F.D. oversaw the cochlear physiology tests: ABR, DPOAE, and EP. Concept and oversight were shared by M.M. and S.A.C. The manuscript was written by A.G., Q.F., and S.A.C.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Qing Fang and Alicia M. Giordimaina contributed equally to this work.
References
- Adler HJ, Belyantseva IA, Merritt RC, Jr, Frolenkov GI, Dougherty GW, Kachar B. Expression of prestin, a membrane motor protein, in the mammalian auditory and vestibular periphery. Hear Res. 2003;184:27–40. doi: 10.1016/S0378-5955(03)00192-8. [DOI] [PubMed] [Google Scholar]
- Beurg M, Safieddine S, Roux I, Bouleau Y, Petit C, Dulon D. Calcium- and otoferlin-dependent exocytosis by immature outer hair cells. J Neurosci. 2008;28:1798–1803. doi: 10.1523/JNEUROSCI.4653-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyages SC, Halpern JP. Endemic cretinism: toward a unifying hypothesis. Thyroid. 1993;3:59–69. doi: 10.1089/thy.1993.3.59. [DOI] [PubMed] [Google Scholar]
- Brandt N, Kuhn S, Munkner S, Braig C, Winter H, Blin N, Vonthein R, Knipper M, Engel J. Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells. J Neurosci. 2007;27:3174–3186. doi: 10.1523/JNEUROSCI.3965-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantos R, Lopez DE, Sala ML, Rueda J. Study of the olivocochlear neurons using two different tracers, fast blue and cholera toxin, in hypothyroid rats. Anat Embryol (Berl) 2000;201:245–257. doi: 10.1007/s004290050315. [DOI] [PubMed] [Google Scholar]
- Cantos R, Lopez DE, Merchan JA, Rueda J. Olivocochlear efferent innervation of the organ of Corti in hypothyroid rats. J Comp Neurol. 2003;459:454–467. doi: 10.1002/cne.10620. [DOI] [PubMed] [Google Scholar]
- Chan SY, Vasilopoulou E, Kilby MD. The role of the placenta in thyroid hormone delivery to the fetus. Nat Clin Pract Endocrinol Metab. 2009;5:45–54. doi: 10.1038/ncpendmet1026. [DOI] [PubMed] [Google Scholar]
- Christ S, Biebel UW, Hoidis S, Friedrichsen S, Bauer K, Smolders JW. Hearing loss in athyroid pax8 knockout mice and effects of thyroxine substitution. Audiol Neurootol. 2004;9:88–106. doi: 10.1159/000076000. [DOI] [PubMed] [Google Scholar]
- Cowley DE, Pomp D, Atchley WR, Eisen EJ, Hawkins-Brown D. The impact of maternal uterine genotype on postnatal growth and adult body size in mice. Genetics. 1989;122:193–203. doi: 10.1093/genetics/122.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne F, Vanderschueren-Lodeweyckx M, Bastijns P. Hearing in congenital hypothyroidism. Audiology. 1983;22:404–409. doi: 10.3109/00206098309072800. [DOI] [PubMed] [Google Scholar]
- Deol MS. Congenital deafness and hypothyroidism. Lancet. 1973;2:105–106. doi: 10.1016/S0140-6736(73)93310-2. [DOI] [PubMed] [Google Scholar]
- Douglas KR, Brinkmeier ML, Kennell JA, Eswara P, Harrison TA, Patrianakos AI, Sprecher BS, Potok MA, Lyons RH, Jr, MacDougald OA, Camper SA. Identification of members of the Wnt signaling pathway in the embryonic pituitary gland. Mamm Genome. 2001;12:843–851. doi: 10.1007/s00335-001-2076-0. [DOI] [PubMed] [Google Scholar]
- Drayton M, Noben-Trauth K. Mapping quantitative trait loci for hearing loss in Black Swiss mice. Hear Res. 2006;212:128–139. doi: 10.1016/j.heares.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Fang Q, Longo-Guess C, Gagnon LH, Mortensen AH, Dolan DF, Camper SA, Johnson KR (2011) A modifier gene alleviates hypothyroidism-induced hearing impairment in Pou1f1dw dwarf mice. Genetics 189:665–673 [DOI] [PMC free article] [PubMed]
- Gage PJ, Roller ML, Saunders TL, Scarlett LM, Camper SA. Anterior pituitary cells defective in the cell-autonomous factor, df, undergo cell lineage specification but not expansion. Development. 1996;122:151–160. doi: 10.1242/dev.122.1.151. [DOI] [PubMed] [Google Scholar]
- Heidrych P, Zimmermann U, Kuhn S, Franz C, Engel J, Duncker SV, Hirt B, Pusch CM, Ruth P, Pfister M, Marcotti W, Blin N, Knipper M. Otoferlin interacts with myosin VI: implications for maintenance of the basolateral synaptic structure of the inner hair cell. Hum Mol Genet. 2009;18:2779–2790. doi: 10.1093/hmg/ddp213. [DOI] [PubMed] [Google Scholar]
- Heydemann A, McNally EM. Consequences of disrupting the dystrophin–sarcoglycan complex in cardiac and skeletal myopathy. Trends Cardiovasc Med. 2007;17:55–59. doi: 10.1016/j.tcm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Zheng QY, Zuberi AR, Johnson KR, Naggert JK, Nishina PM. Microtubule-associated protein 1A is a modifier of tubby hearing (moth1) Nat Genet. 2002;30:401–405. doi: 10.1038/ng838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Zheng QY, Rosenstiel P, Maddatu T, Zuberi AR, Roopenian DC, North MA, Naggert JK, Johnson KR, Nishina PM. Genetic modification of hearing in tubby mice: evidence for the existence of a major gene (moth1) which protects tubby mice from hearing loss. Hum Mol Genet. 1999;8:1761–1767. doi: 10.1093/hmg/8.9.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolyi IJ, Dootz GA, Halsey K, Beyer L, Probst FJ, Johnson KR, Parlow AF, Raphael Y, Dolan DF, Camper SA. Dietary thyroid hormone replacement ameliorates hearing deficits in hypothyroid mice. Mamm Genome. 2007;18:596–608. doi: 10.1007/s00335-007-9038-0. [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006;25:642–652. doi: 10.1038/sj.emboj.7600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Zinn C, Maier H, Praetorius M, Rohbock K, Kopschall I, Zimmermann U. Thyroid hormone deficiency before the onset of hearing causes irreversible damage to peripheral and central auditory systems. J Neurophysiol. 2000;83:3101–3112. doi: 10.1152/jn.2000.83.5.3101. [DOI] [PubMed] [Google Scholar]
- Knipper M, Richardson G, Mack A, Muller M, Goodyear R, Limberger A, Rohbock K, Kopschall I, Zenner HP, Zimmermann U. Thyroid hormone-deficient period prior to the onset of hearing is associated with reduced levels of beta-tectorin protein in the tectorial membrane: implication for hearing loss. J Biol Chem. 2001;276:39046–39052. doi: 10.1074/jbc.M103385200. [DOI] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/S0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- Li D, Henley CM, O'Malley BW., Jr Distortion product otoacoustic emissions and outer hair cell defects in the hyt/hyt mutant mouse. Hear Res. 1999;138:65–72. doi: 10.1016/S0378-5955(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics. 2000;70:102–112. doi: 10.1006/geno.2000.6355. [DOI] [PubMed] [Google Scholar]
- Marcotti W, Kros CJ. Developmental expression of the potassium current IK, n contributes to maturation of mouse outer hair cells. J Physiol. 1999;520(Pt 3):653–660. doi: 10.1111/j.1469-7793.1999.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DC, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol. 2002;282:C403–C407. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- Mashimo T, Erven AE, Spiden SL, Guenet JL, Steel KP. Two quantitative trait loci affecting progressive hearing loss in 101/H mice. Mamm Genome. 2006;17:841–850. doi: 10.1007/s00335-004-2438-5. [DOI] [PubMed] [Google Scholar]
- McIntosh GH, Potter BJ, Mano MT, Hua CH, Cragg BG, Hetzel BS. The effect of maternal and fetal thyroidectomy on fetal brain development in the sheep. Neuropathol Appl Neurobiol. 1983;9:215–223. doi: 10.1111/j.1365-2990.1983.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Mustapha M, Fang Q, Gong TW, Dolan DF, Raphael Y, Camper SA, Duncan RK. Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants. J Neurosci. 2009;29:1212–1223. doi: 10.1523/JNEUROSCI.4957-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narui Y, Minekawa A, Iizuka T, Furukawa M, Kusunoki T, Koike T, Ikeda K. Development of distortion product otoacoustic emissions in C57BL/6 J mice. Int J Audiol. 2009;48:576–581. doi: 10.1080/14992020902858959. [DOI] [PubMed] [Google Scholar]
- Noguchi Y, Kurima K, Makishima T, de Angelis MH, Fuchs H, Frolenkov G, Kitamura K, Griffith AJ. Multiple quantitative trait loci modify cochlear hair cell degeneration in the Beethoven (Tmc1Bth) mouse model of progressive hearing loss DFNA36. Genetics. 2006;173:2111–2119. doi: 10.1534/genetics.106.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley BW, Jr, Li D, Turner DS. Hearing loss and cochlear abnormalities in the congenital hypothyroid (hyt/hyt) mouse. Hear Res. 1995;88:181–189. doi: 10.1016/0378-5955(95)00111-G. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Rosen AD, Gagnon PM. A major effect QTL on chromosome 18 for noise injury to the mouse cochlear lateral wall. Hear Res. 2010;260:47–53. doi: 10.1016/j.heares.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospeck M, Dong XX, Iwasa KH. Limiting frequency of the cochlear amplifier based on electromotility of outer hair cells. Biophys J. 2003;84:739–749. doi: 10.1016/S0006-3495(03)74893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomp D, Cowley DE, Eisen EJ, Atchley WR, Hawkins-Brown D. Donor and recipient genotype and heterosis effects on survival and prenatal growth of transferred mouse embryos. J Reprod Fertil. 1989;86:493–500. doi: 10.1530/jrf.0.0860493. [DOI] [PubMed] [Google Scholar]
- Rhees BK, Ernst CA, Miao CH, Atchley WR. Uterine and postnatal maternal effects in mice selected for differential rate of early development. Genetics. 1999;153:905–917. doi: 10.1093/genetics/153.2.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Hosie S, Johnson SL, Bahloul A, Cayet N, Nouaille S, Kros CJ, Petit C, Safieddine S. Myosin VI is required for the proper maturation and function of inner hair cell ribbon synapses. Hum Mol Genet. 2009;18:4615–4628. doi: 10.1093/hmg/ddp429. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Rovet J, Walker W, Bliss B, Buchanan L, Ehrlich R. Long-term sequelae of hearing impairment in congenital hypothyroidism. J Pediatr. 1996;128:776–783. doi: 10.1016/S0022-3476(96)70329-3. [DOI] [PubMed] [Google Scholar]
- Rusch A, Erway LC, Oliver D, Vennstrom B, Forrest D. Thyroid hormone receptor beta-dependent expression of a potassium conductance in inner hair cells at the onset of hearing. Proc Natl Acad Sci U S A. 1998;95:15758–15762. doi: 10.1073/pnas.95.26.15758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendin G, Bulankina AV, Riedel D, Moser T. Maturation of ribbon synapses in hair cells is driven by thyroid hormone. J Neurosci. 2007;27:3163–3173. doi: 10.1523/JNEUROSCI.3974-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibusawa N, Hashimoto K, Nikrodhanond AA, Liberman MC, Applebury ML, Liao XH, Robbins JT, Refetoff S, Cohen RN, Wondisford FE. Thyroid hormone action in the absence of thyroid hormone receptor DNA-binding in vivo. J Clin Invest. 2003;112:588–597. doi: 10.1172/JCI18377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenkle PM, McGee J, Bertoni JM, Walsh EJ. Prevention of auditory dysfunction in hypothyroid Tshr mutant mice by thyroxin treatment during development. J Assoc Res Otolaryngol. 2001;2:348–361. doi: 10.1007/s101620010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenkle PM, McGee J, Bertoni JM, Walsh EJ. Consequences of hypothyroidism on auditory system function in Tshr mutant (hyt) mice. J Assoc Res Otolaryngol. 2001;2:312–329. doi: 10.1007/s101620010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel A, Legrand C, Rabie A. Corrective effects of thyroxine on cochlear abnormalities induced by congenital hypothyroidism in the rat. I. Morphological study. Brain Res. 1985;351:111–122. doi: 10.1016/0165-3806(85)90236-6. [DOI] [PubMed] [Google Scholar]
- Uziel A, Marot M, Rabie A. Corrective effects of thyroxine on cochlear abnormalities induced by congenital hypothyroidism in the rat. Brain Res. 1985;351:123–127. doi: 10.1016/0165-3806(85)90237-8. [DOI] [PubMed] [Google Scholar]
- Uziel A, Pujol R, Legrand C, Legrand J. Cochlear synaptogenesis in the hypothyroid rat. Brain Res. 1983;283:295–301. doi: 10.1016/0165-3806(83)90186-4. [DOI] [PubMed] [Google Scholar]
- van der Deure WM, Peeters RP, Visser TJ. Molecular aspects of thyroid hormone transporters, including MCT8, MCT10, and OATPs, and the effects of genetic variation in these transporters. J Mol Endocrinol. 2010;44:1–11. doi: 10.1677/JME-09-0042. [DOI] [PubMed] [Google Scholar]
- Van Eyken E, Van Laer L, Fransen E, Topsakal V, Lemkens N, Laureys W, Nelissen N, Vandevelde A, Wienker T, Van De Heyning P, Van Camp G. KCNQ4: a gene for age-related hearing impairment? Hum Mutat. 2006;27:1007–1016. doi: 10.1002/humu.20375. [DOI] [PubMed] [Google Scholar]
- Wasserman EE, Nelson K, Rose NR, Eaton W, Pillion JP, Seaberg E, Talor MV, Burek L, Duggan A, Yolken RH. Maternal thyroid autoantibodies during the third trimester and hearing deficits in children: an epidemiologic assessment. Am J Epidemiol. 2008;167:701–710. doi: 10.1093/aje/kwm342. [DOI] [PubMed] [Google Scholar]
- Weber T, Zimmermann U, Winter H, Mack A, Kopschall I, Rohbock K, Zenner HP, Knipper M. Thyroid hormone is a critical determinant for the regulation of the cochlear motor protein prestin. Proc Natl Acad Sci U S A. 2002;99:2901–2906. doi: 10.1073/pnas.052609899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Braig C, Zimmermann U, Geisler HS, Franzer JT, Weber T, Ley M, Engel J, Knirsch M, Bauer K, Christ S, Walsh EJ, McGee J, Kopschall I, Rohbock K, Knipper M. Thyroid hormone receptors TRalpha1 and TRbeta differentially regulate gene expression of Kcnq4 and prestin during final differentiation of outer hair cells. J Cell Sci. 2006;119:2975–2984. doi: 10.1242/jcs.03013. [DOI] [PubMed] [Google Scholar]
- Wolf JB, Wade MJ. What are maternal effects (and what are they not)? Philos Trans R Soc Lond B Biol Sci. 2009;364:1107–1115. doi: 10.1098/rstb.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N, Zhu ML, Zhao HB. Prestin is expressed on the whole outer hair cell basolateral surface. Brain Res. 2006;1095:51–58. doi: 10.1016/j.brainres.2006.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]