Abstract
The power and phase synchronization of the auditory steady state response (ASSR) at 40 Hz stimulation are usually reduced in schizophrenia (SZ). The sensitivity of the 40 Hz ASSR to schizophrenia spectrum phenotypes, such as schizotypal personality disorder (SPD), or to familial risk has been less well characterized. We compared the ASSR of patients with SZ, persons with schizotypal personality disorder, first degree relatives of patients with SZ, and healthy control participants. ASSRs were obtained to 20, 30, 40 and 50 Hz click trains, and assessed using measures of power (mean trial power or MTP) and phase consistency (phase locking factor or PLF). The MTP to 40 Hz stimulation was reduced in relatives, and there was a trend for MTP reduction in SZ. The 40 Hz ASSR was not reduced in SPD participants. PLF did not differ among groups. These data suggest the 40 Hz ASSR is sensitive to familial risk factors associated with schizophrenia.
Keywords: electroencephalography, auditory steady state potentials, schizophrenia, schizotypal personality disorder, first-degree relatives
1. Introduction
The electroencephalogram (EEG) and magnetoencephalogram (MEG) entrain in frequency and phase to periodic stimuli across a wide range of frequencies. The EEG response entrained to auditory stimuli is referred to as the auditory steady state response (ASSR), and is primarily generated by synchronous activity of neurons in the auditory cortex (Brenner et al., 2009; Pastor et al., 2002; Simpson et al., 2005). In humans, the largest amplitude ASSR is elicited by stimuli modulated in the 35 – 40 Hz range (Pastor et al., 2002). ASSRs are usually characterized using time-frequency analysis, such as using the Fast Fourier Transform (FFT) to isolate power at the driving frequency and harmonics in a power spectrum. More recently, time frequency measures have been used to differentiate the magnitude of the change in power from baseline at a given frequency and the reliability of phase across trials. Change in power has been referred to as mean trial power (MTP; (Krishnan et al., 2009)) or event-related spectral perturbation (Delorme and Makeig, 2004), while phase reliability or synchrony has been termed phase locking factor (PLF; (Tallon-Baudry et al., 1996)) or inter-trial coherence (Delorme and Makeig, 2004).
ASSR measures of overall power, MTP, and PLF have been reported to be reduced in schizophrenia (SZ), most consistently at 40 Hz stimulation (Kwon et al., 1999; Light et al., 2006; Spencer et al., 2008; Teale et al., 2008; Vierling-Claassen et al., 2008). Two studies using amplitude modulated tones rather than click trains have demonstrated reductions at lower frequencies of stimulation as well (Brenner et al., 2003; Krishnan et al., 2009). The neural mechanisms that produce ASSR abnormalities in schizophrenia are not well characterized, but both anatomic and neurophysiological disturbances in the auditory cortex have been implicated. Schizophrenia is associated with volumetric reductions in the posterior superior temporal gyrus (McCarley et al., 1999) as well as reductions of pyramidal neuron volume in deep layer 3 of primary and secondary auditory cortices (Sweet et al., 2003). Dysfunctional γ-aminobutyric acid (GABA) and glutamate signaling may contribute to auditory EEG abnormalities in schizophrenia due to their role in the generation, synchronization, range, and maintenance of oscillations (Uhlhaas and Singer, 2006). Medication may impact the 40 Hz ASSR. Hong et al. (2004) did not find a deficit in 40 Hz power in SZ, but reported that patients receiving conventional antipsychotic agents had reduced ASSR power compared to patients receiving novel antipsychotic agents.
Investigating individuals who share phenotypic similarities or familial risk with schizophrenia may provide insight into whether ASSR deficits occur in the absence of psychosis and associated confounds. Individuals with schizotypal personality disorder (SPD) exhibit clinical symptoms, cognitive deficits, and neurobiological abnormalities which are often similar to SZ, though usually of lesser severity (Cadenhead et al., 1999). Brenner et al. (2003) reported that ASSR power to amplitude modulated tones was unaffected across a wide range of stimulus frequencies in a small sample of persons with SPD (n=11). However, abnormalities in auditory EEG and event-related potential (ERP) response (Niznikiewicz et al., 2009; Shin et al., 2010) and reduced volume of the superior temporal gyrus (Dickey et al., 2002; Goldstein et al., 2009; Takahashi et al., 2010) in SPD suggest potential impairment in the auditory pathways. First-degree relatives of schizophrenia patients share genetic and environmental risk factors with SZ patients in the absence of psychosis and are not typically prescribed antipsychotic medications. Hong et al. (2004) found that ASSR power to 40 Hz click stimulation was reduced in a sample of first degree relatives of patients with SZ, suggesting that ASSR may be an indicator of familial risk. Notably, the relatives in Hong et al. had elevated schizotypal personality traits. Other studies indicate that relatives show disturbances in auditory pathways, evidenced by abnormalities in auditory EEG or ERP measures in discordant siblings (Karoumi et al., 2000; Winterer et al., 2003), twin pairs (Weisbrod et al., 1999), and first-degree relatives (Bramon et al., 2005; Leicht et al., 2011; Turetsky et al., 2008). Additionally, reductions in superior temporal gyrus volume have been reported in relatives (Rajarethinam et al., 2004).
We recorded ASSRs in SZ, first degree relatives, SPD, and healthy control participants to four frequencies of stimulation (20, 30, 40 and 50 Hz), and measured MTP and PLF to evaluate entrainment at each frequency. MTP reflects the change in power relative to background activity in the baseline EEG, capturing both phase-locked and non-phase-locked activity across trials, whereas PLF measures EEG phase synchronization across trials (45). We expected to observe a pattern of deficits corresponding to symptom severity in the power and phase locking of ASSR at 40 Hz stimulation, ranging from a robust response in control participants, an intermediate deficit in SPD participants and relatives, and the most severe deficit in SZ patients. In contrast, we expected all groups to show comparable ASSRs to 20 and 30 Hz stimulation.
2. Materials and Methods
2.1. Participants
Schizophrenia and schizoaffective disorder patients (N=42), first-degree relatives of schizophrenia patients (N=35), SPD participants (N=34), and non-psychiatric comparison participants (N=56) volunteered for the present study (see Table 1 for characteristics). The patient sample was recruited through outpatient and inpatient units at community and state hospitals. Relatives were recruited through probands with SZ. Thirteen schizophrenia patients were related to one (n=6), two (n=3), three (n=3), or four (n=1) relatives in the study. An additional eight relatives were related to SZ patients who were disqualified (n=4) or did not have ASSR recorded (n=4). The relative status of two individuals was based on self-report; their exclusion did not change the outcome of the ASSR measures. SPD and control participants were recruited through newspaper and internet advertisements. All participants received detailed oral and written information about the study protocol and gave written and oral informed consent. The protocol was approved by the Indiana University–Purdue University Indianapolis Human Subjects Review Committee. Participants were paid ten dollars per hour for participation. Participants did not differ on age (F(3,163)<1) or gender (χ2=3.987, p=.263); however, control and SPD participants (F(3,163)=18.421, p<.001) and their mothers (F(3,156)=3.137, p=.027) completed more education than SZ patients and relatives (F(3,163)=18.421, p<.001) (Table 1). Paternal education did not differ among groups (F(3,140)=2.145, p=.097).
Table 1.
Characteristics and Performance on Neuropsychological Measures
| Control (N=56) |
SPD (N=34) |
SZ (N=42) |
Relatives (N=35) |
N | Sig <.05 | |
|---|---|---|---|---|---|---|
| Age | 38.75 (10.4) | 37.35 (9.2) | 36.86 (12.8) | 36.03 (12.5) | 167 | n.s. |
| Gender (M : F) | 26:30 | 20:14 | 23:19 | 13:22 | 167 | n.s. |
| Education Level | ||||||
| Participant | 4.07 (0.81) | 3.85 (1.02) | 3.10 (0.88) | 2.89 (1.01) | 167 | C, SPD > SZ, Rel |
| Mother | 3.62 (1.18) | 3.73 (1.31) | 3.13 (0.94) | 3.12 (1.13) | 160 | C, SPD > SZ, Rel |
| Father | 3.63 (1.41) | 3.59 (1.46) | 3.21 (1.48) | 2.89 (1.01) | 144 | n.s. |
| Schizotypal Personality Questionnaire | ||||||
| Cognitive Perceptual | 4.55 | 16.59 | 18.89 | 11.71 | 161 | C<Rel<SZ, SPD |
| Interpersonal | 6.44 | 15.12 | 19.68 | 14.53 | 161 | C<Rel, SPD<SZ |
| Disorganized | 2.78 | 8.44 | 8.34 | 5.56 | 152 | C<Rel<SZ, SPD |
| Wechsler Adult Intelligence Scale | ||||||
| Picture Completion | 10.78 | 10.42 | 7.31 | 8.35 | 158 | C, SPD > SZ, Rel |
| Digit Symbol | 11.16 | 9.47 | 6.93 | 8.03 | 162 | C > SPD > SZ, Rel |
| Similarities | 9.96 | 10.48 | 8.21 | 8.25 | 159 | C, SPD > SZ, Rel |
| Digit Span | 9.95 | 10.61 | 8.26 | 9.00 | 158 | C, SPD > SZ; SPD > Rel |
Note. C=control, SPD=schizotypal personality disorder; SZ=schizophrenia; Rel=relatives. Education level included self-report data on completion of grade school (1), junior high school (2), high school (3), some college (4), bachelor’s degree (5), master’s degree (6), and doctoral degree (7).
SZ patients were evaluated using the Structured Clinical Interview for Axis I disorders (SCID-I; (First et al., 2001b)), supplemented by clinical observation and medical chart review. Participants in the SPD and relative groups were diagnosed using the SCID-II for Axis II disorders (SCID-II; (First et al., 1997)) and the SCID-I. Control participants were interviewed using the non-patient version of SCID-I (First et al., 2001a) and the Schizoid, Paranoid and Schizotypal Personality Disorder modules of the SCID II to exclude psychiatric disorders. The Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987) assessed positive symptoms (M=15.4, SD=5.7), negative symptoms (M= 14.7, SD=6.8), and general function (M=30.4, SD=8.3) for 37 patients interviewed within three months of completing the study.
The Family Interview for Genetic Studies (FIGS) was used to obtain probable diagnoses of schizophrenia in first degree relatives of all participants (Maxwell, 1992). Exclusion criteria for all participants included a history of neurological or cardiovascular disease, clinically documented hearing loss, head injury that resulted in loss of consciousness for more than 5 minutes, electroconvulsive therapy, and IQ below 70. SZ, SPD, and control participants were excluded if they had a positive drug urine screen or a current diagnosis of substance abuse or dependence. SPD participants were excluded if they had a diagnosis of bipolar disorder. Control participants were excluded if they or a diagnosis of Axis I psychiatric illness. Family members were not excluded for a diagnosis of SPD (n=5), bipolar disorder (n=4), dysthymia (n=1), current substance use (n=3 by positive drug screen) or past substance abuse or dependence (n=8), because these disorders may reflect expression of risk factors also associated with schizophrenia. Eight SZ patients and two SPD participants met criteria for past substance abuse or dependence. Table 2 describes the prescribed medication types among study participants.
Table 2.
Medication Types among Study Participants
| Control | SPD | SZ | Relatives | |
|---|---|---|---|---|
| No medications | 54 | 24 | 7 | 29 |
| Atypical antipsychotic | 0 | 1 | 28 | 3 |
| Conventional antipsychotic | 0 | 0 | 8 | 1 |
| Antidepressant | 0 | 2 | 15 | 1 |
| Anticonvulsant | 0 | 1 | 5 | 1 |
| Anticholinergic | 0 | 0 | 5 | 0 |
| Benzodiazepine | 0 | 0 | 9 | 1 |
| Sedative | 2 | 0 | 4 | 2 |
Note. Patients taking psychotropic medications typically used multiple medications. One schizophrenia patient was taking lithium. One participant with schizotypal personality disorder was taking a stimulant (ephedrine). The medication status of two SZ patients is unknown due to participation in double-blind drug trials at time of testing.
2.2. Clinical and Neuropsychological Assessment
Table 1 describes participant characteristics and performance. Dimensions of schizotypy were assessed by the Schizotypal Personality Questionnaire (SPQ; (Raine, 1991)). Subtests from the Wechsler Adult Intelligence Scale (WAIS-III; (Wechsler, 1997)) were used to assess visuospatial perception (Picture Completion), information processing speed (Digit Symbol Coding), auditory working memory (Digit Span), and abstract verbal reasoning (Similarities).
2.3. Electrophysiological Assessment
During the evaluation, participants were comfortably seated with eyes open while listening to trains of clicks presented through Etymotic insert earphones. The individual stimuli were 1 ms duration clicks (80 dB SPL), presented in trains which varied in rate of presentation (20, 30, 40 and 50 Hz) in each of four blocks, separated by a 700 ms inter-train interval. Each block had 80 trains of clicks and the order of conditions was randomized across participants. The duration of the click train was 450 ms for 20 Hz, 467 ms for 30 Hz, 475 ms for 40 Hz, and 480 ms for 50 Hz.
The EEG was continuously recorded (band pass 0.1–200 Hz, sampling rate 1000 Hz) and digitized (Neuroscan SynAmps) from the scalp, using a 32 channel electrode cap. Recordings were referenced to the nose. Electrode impedances were maintained at <10 kOhm. For each stimulus condition, the EEG was segmented into epochs with a 350 ms baseline before stimulus onset to 350 ms post-stimulus padding after the end of each 512 ms click train using Brain Vision Analyzer software (Brain Products, GMbH). Epochs were corrected for ocular artifacts using the Gratton et al. (1983) algorithm. Epochs with voltage exceeding ±150 µV at any site were automatically excluded from analyses. Participants with fewer than 30 segments remaining after artifact rejection in a frequency condition (20, 30, 40, or 50 Hz) were excluded from analysis in that condition. A mean number of 69 (SD=11), 69 (SD=11), 70 (SD=10), and 67 (SD=12) trials were included in the 20, 30, 40, and 50 Hz conditions, respectively. The number of segments accepted for signal processing did not differ significantly among groups.
2.4. Time-Frequency Analyses
Time-frequency analyses applied to single-trial EEG epochs were computed using MATLAB (The MathWorks, Natick, MA) and EEGLAB software (Delorme and Makeig, 2004; Rass et al., 2010). A 100% Hanning window was applied to the data, followed by a time-frequency spectrogram using short time windowed Fast Fourier Transform, computed for a segment of EEG for the different component frequencies. The spectrogram measured change in power from baseline (mean trial power, MTP) and phase locking factor (PLF). A 128 ms sliding window of with 10 ms time steps was used for spectrogram analysis. The frequency resolution was 1.953 Hz. Averaging the power from individual trials after subtracting the mean from the baseline period provided the MTP value. Averaging across trials for the normalized complex output for every time period and frequency provided the PLF value (Tallon-Baudry et al., 1996). For statistical analysis, mean values were obtained for the 100 to 500 ms interval after stimulus onset for the frequencies corresponding to 5 Hz below and above the stimulation frequency.
2.5. Statistical analysis
Separate ASSR analyses for MTP and PLF are reported for the FCz electrode where signal power was largest. Repeated-measures Analysis of Variance (ANOVA) for the within-subject factor of ASSR Frequency (4: 20 Hz, 30 Hz, 40 Hz, 50 Hz) and between-subjects factor of group (4: Control, SZ, SPD, Relatives) were calculated for each ASSR metric. Greenhouse-Geisser epsilon adjustments were included when appropriate. Post-hoc pair-wise ANOVAs with LSD post-hoc analyses were used for significant main effects or interactions. One-way ANOVAs were used to compare groups for demographic, clinical, and cognitive measures. Pearson correlation coefficients were used to test relationships between variables.
3. Results
3.1. Clinical and Neuropsychological Assessment
Performance on the SPQ and WAIS measures can be found in Table 1. One-way ANOVAs of group (4) for the cognitive-perceptual (F(3,157)=33.191, p<.001), interpersonal (F(3,157)=27.644, p<.001), and disorganized (F(3,157)=21.540, p<.001) dimensions of the SPQ revealed that control participants had fewer schizotypal symptoms than all other groups, and relatives had fewer cognitive-perceptual and disorganized symptoms than SZ and SPD participants (Table 1). SPQ differences for relatives did not change when relatives diagnosed with SPD were excluded from analysis. WAIS-III subtest performance scores were age scaled and analyzed for Picture Completion, Digit Symbol Coding, Similarities, and Digit Span measures. One-way ANOVA for Group (4) on each measure were used to assess cognitive function across groups. Group differences were found for Picture Completion (F(3,154)=10.701, p<.001), Digit Symbol (F(3,158)=19.703, p<.001), Similarities (F(3,155)=6.334, p<.001), and Digit Span (F(3,154)=4.693, p=.004). Control and SPD participants showed superior performance to SZ and relatives on most measures.
3.2. Electrophysiological Measures
3.2.1. Mean Trial Power
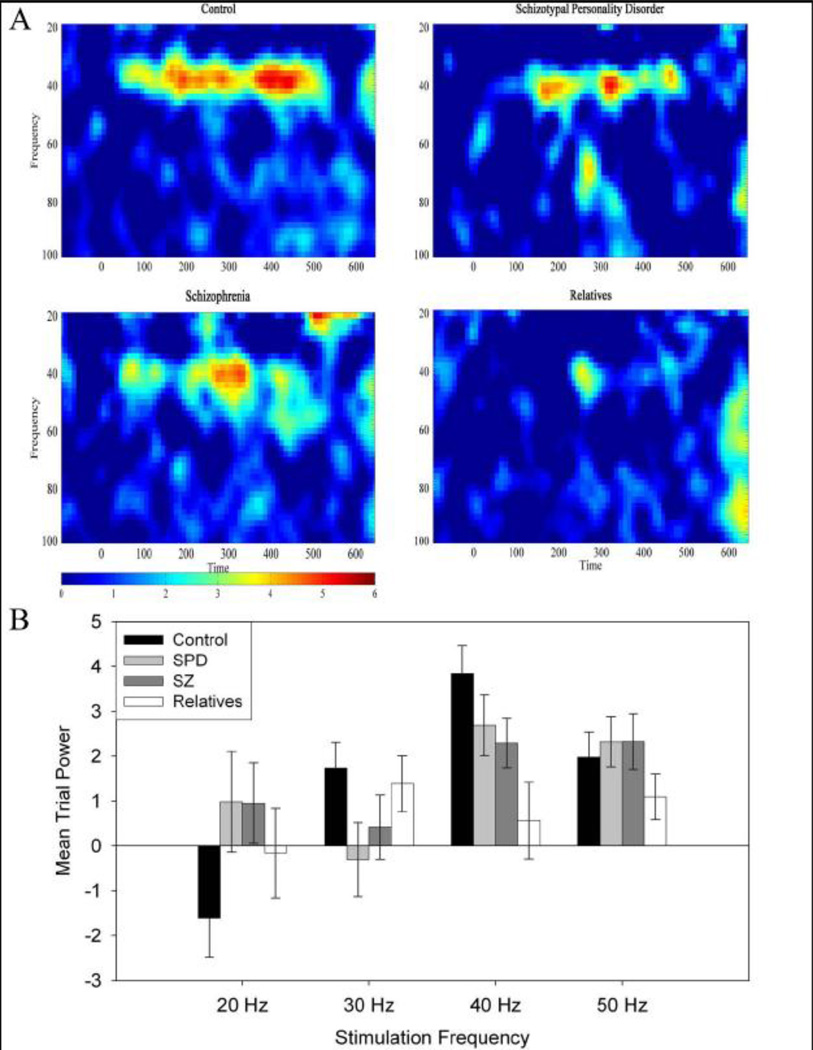
Full group and condition ANOVAs revealed that relatives had reduced MTP response at 40 Hz compared to the other groups. A Group (4) × Frequency (4) Repeated-Measures ANOVA revealed a main effect of frequency (F(3,462)=7.523, p<.001) and a Group×Frequency interaction (F(9,462)=2.495, p=.014). The largest response for all groups was at the 40 Hz stimulation frequency. Post-hoc ANOVAs revealed decreased 40 Hz MTP (F(3,161)=4.404, p=.008) for relatives compared to control (LSD p=.001) and SPD participants (LSD p=.046) and a reduction at trend level compared to SZ (LSD p=.087) (Figure 1). A trend for reduced 40 Hz response was also found for SZ patients compared to control participants (LSD p=.082). Attenuation of 40 Hz MTP persisted between control participants and relatives when the relatives group was restricted to individuals without Axis-I disorders, a positive drug screen, or a diagnosis of SPD, resulting in a significant (F(1,77)=9.179, p=.003). Excluding patients withdrawn from medication (n=7) and having an unknown medication status (n=2) increased the difference between SZ patients and control participants (LSD p=.062) and decreased the difference between SZ and relatives (LSD p=.124).
Figure 1.
A. Mean Trial Power spectrogram at 40 Hz stimulation for control, schizotypal personality disorder, schizophrenia, and first-degree relatives, showing magnitude of response across time. B. Mean trial power as a function of stimulation frequency for control, schizotypal personality disorder, schizophrenia, and first-degree relatives recorded at site FCz. Error bars represent ± 1 standard error.
3.2.2. Phase Locking Factor
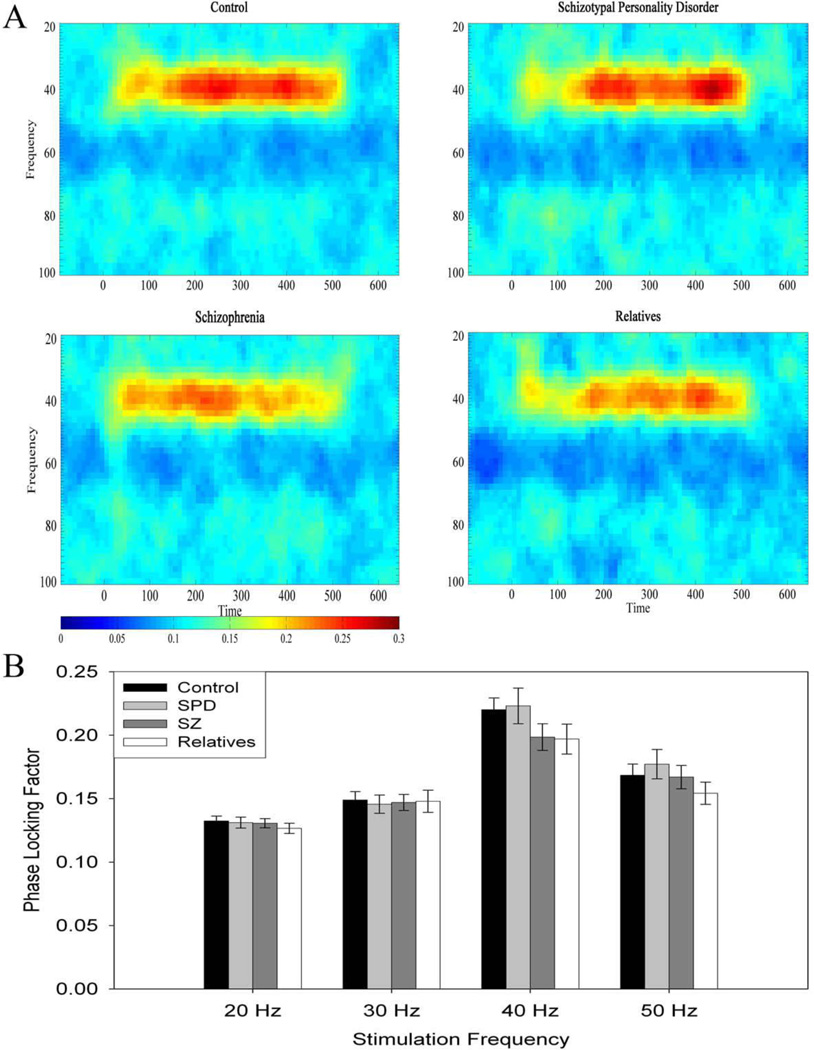
The greatest response for PLF was at the 40 Hz stimulation frequency for all groups. A Group (4) × Frequency (4) Repeated-Measures ANOVA revealed a main effect of Frequency (F(9,462)=85.088, p<.001) (Figure 2). There was no significant main effect of Group or Group × Frequency interaction. Excluding patients withdrawn from medication and having an unknown medication status did not significantly affect the results.
Figure 2.
A. Phase Locking Factor spectrogram at 40 Hz stimulation for control, schizotypal personality disorder, schizophrenia, and first-degree relatives, showing synchrony across time. B. Phase Locking Factor as a function of stimulation frequency for control, schizotypal personality disorder, schizophrenia, and first-degree relatives, recorded at site FCz. Error bars represent ± 1 standard error.
3.2.3 Correlation between ASSR and Neuropsychological Measures
Exploratory correlation analyses between the ASSR measurements (20, 30, 40, and 50 Hz PLF and MTP) and neuropsychological tests (Similarities, Picture Completion, Digit Span, and Digit Symbol) were computed, and the following significant correlation coefficients were obtained. Among the control participants, Similarities was correlated with 40 Hz PLF (r=.38, p<.01) and Digit Symbol was correlated with 50 Hz MTP (r=.26, p=.03). Among the SZ patients, Similarities was correlated with 40 Hz MTP (r=.34, p=.04), 40 Hz PLF (r=.34, p=.04); Digit Symbol was negatively correlated with 20 Hz MTP (r=−.33 p=.04); and Digit Span was correlated with 50 Hz PLF (r=.38, p=.02). Among SPD participants, Similarities was correlated with 40 Hz PLF (r=.39, p=.03) and 50 Hz PLF (r=.45, p=.01). Among relatives, Similarities was correlated with 40 Hz MTP (r=.34, p=.04) and 50 Hz PLF (r=.40, p=.02); while Digit Symbol was negatively correlated with 20 Hz MTP (r=−.33, p=.04).
4. Discussion
Reduced gamma band ASSR was found for first-degree relatives of SZ patients. MTP at the 40 Hz stimulation frequency for relatives differed from all other groups. In contrast, SZ patients differed from control participants at trend level for MTP, which differed from multiple studies showing an effect on the 40 Hz ASSR. Hong et al. (2004) also found that relatives, but not patients with schizophrenia, demonstrated a 40 Hz ASSR deficit. Thus, the Hong et al. present data indicating that first-degree relatives may actually show a more robust deficit than that observed in medicated SZ patients. No group differences were found across the other stimulation frequencies. The only neuropsychological test which showed consistent positive correlations with ASSR measures was Similarities, a test of verbal concept formation.
It is possible that anti-psychotic medications may affect the 40 Hz ASSR response in this and other studies. Hong et al (2004) found no differences between control participants and SZ patients and instead found that atypical antipsychotic medications enhanced 40 Hz response compared to conventional antipsychotic medications. SZ patients in our study were predominantly taking atypical antipsychotics, along with other medications, and excluding patients not taking medications did not result in a significant group difference. To our knowledge, ASSRs have not been evaluated in the context of a pharmacological trial which would allow causal inferences regarding possible anti-psychotic effects.
The pathophysiological basis for the marked reduction of ASSR power found in this present data and by Hong et al (2004) would be of great interest because these individuals do not experience the severe psychotic symptoms, psychosocial disability, and antipsychotic medication treatment associated with a diagnosis of schizophrenia. The integrity of oscillatory activity of auditory cortex neurons is sensitive to factors influencing GABA and glutamate circuitry. Pathophysiology of these circuits is commonly found in schizophrenia and associated with a reduced ASSR (Uhlhaas et al., 2009; Uhlhaas and Singer, 2010). Genetic similarity to SZ increases the risk for psychopathology, including bipolar disorder, SPD, and drug use. Abnormalities in GABA and glutamate circuits may extend to relatives and contribute to a reduced ASSR. Previous studies have found elevations of glutamate/ glutamine (Purdon et al., 2008; Tibbo et al., 2004) and changes in glutamate/ glutamine metabolism (Keshavan et al., 2009) and striatal dopamine (Huttunen et al., 2008) in first degree relatives. Antipsychotic medications commonly prescribed to SZ patients may influence the structural and chemical components of the oscillatory response by targeting GABA and glutamate circuits. However, medication effects alone cannot explain the striking ASSR reduction in the relatives group since 83% of the group was not taking medication.
Gamma range synchronization deficits in schizophrenia and in relatives revealed by EEG and MEG studies have increased interest in the cellular mechanisms that enable oscillations within neural ensembles. In vitro studies and biophysical modeling suggest that GABAergic interneurons are critical for generation of oscillatory activity at high frequencies within cortical circuits (Uhlhaas and Singer, 2010). Specifically, fast-spiking, parvablumin-expressing GABAergic neurons serve as pacemakers for networks of pyramidal neurons. Further, alterations in GABAergic neurotransmission are shown to reduce evoked steady state gamma oscillation in modeling studies (Vierling-Claassen et al, 2008). Neuropathological studies, animal models, and risk genes for schizophrenia, such as NRG1 and ERBB4, have implicated abnormalities of GABAergic function (Fazzari et al., 2010; Lewis et al., 2005). These cellular alterations may therefore point to a pathophysiological basis for altered synchronization in schizophrenia and relatives. The present findings additionally argue for the possibility that disturbances in gene expression and GABAergic neurotransmission may be present in first-degree relatives who do not have psychotic symptoms and have not been treated with conventional or atypical antipsychotic medications.
The absence of ASSR abnormalities in the SPD group replicates findings from a previous study of ASSRs in SPD participants that used amplitude modulated tones (Brenner et al., 2003). Negative findings are consistent with the hypothesis that some SPD participants, particularly those without evidence of genetic risk, may be “pseudo-schizotypal” (Raine, 2006) and do not share the pathophysiology of schizophrenia. This results in smaller effect sizes in SPD samples for endophenotypic measures. For example, one of the most robust ERP biomarkers of schizophrenia, auditory P300 amplitude reduction, appears to be less reliably affected in SPD: Previous studies have shown moderate (Mannan et al., 2001), mild (Niznikiewicz et al., 2000) or non-significant reductions of auditory P300 amplitude (Salisbury et al., 1996; Shin et al., 2010; Trestman et al., 1996). Moreover, familial SPD participants have been reported to be more impaired than non-familial SPD participants on a variety of schizophrenia biomarkers, including smooth pursuit eye movement abnormalities (Thaker et al., 1996), grammatical comprehension errors (Condray & Steinhauer, 1992) and P300 amplitude (Kimble et al., 2000).
Further studies are needed to resolve the contribution of familial or genetic risk factors to abnormalities in neural synchrony and oscillatory activity. Correlating the structural or functional abnormalities of relevant regions, especially of the superior temporal gyrus, with ASSR would reveal its contribution to this response across populations. Additionally, experimental manipulation of pharmacological treatment in patients or animal models could clarify whether anti-psychotic medication has a direct effect on ASSRs. Understanding the functional significance of the ASSR deficit could enhance its utility as a biomarker in studying etiology, mechanisms, and intervention strategies in the schizophrenia spectrum and related disorders.
Acknowledgments
We thank Colleen Merrill and Misty Bodkins for their assistance with collecting the data presented in this report.
Role of Funding Source
We are grateful for support from NIMH RO1 MH62150 (BFO), NIMH R21 MH091774, and IUSM/CTR, NIH/NCRR Grant Number RR025761 to BFO; NIMH R01 MH074983 to WPH; and NIDA T32 DA024628-01 (OR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest.
There are no conflicts of interest for any of the authors of this article. No author has any possible financial gain for the findings presented here.
Contributors
Olga Rass (rasso@indiana.edu) was the primary author of this manuscript.
Jennifer Forsyth (jenforsyth@gmail.com) and Mallory Klaunig (mjklaunig@gmail.com) assisted with the patient recruitment and assessment, database management, literature searches, questionnaire and WAIS scoring, statistical analyses, and writing the manuscript.
Giri Krishnan (girik@ucr.edu) assisted with the electrophysiological and time-frequency analysis; and contributed to the interpretation of these analysis.
William P. Hetrick (whetrick@indiana.edu) and Brian F. O’Donnell (bodonnel@indiana.edu) were responsible for the design of this study and supervised subject recruitment, diagnostic procedures, symptom assessment, neuropsychological testing; and contributed to the interpretation of the statistical analysis.
Alan Breier (abreier@iupui.edu) assisted in recruitment, diagnosis, and interpretation of the pharmacological data.
Colleen A. Brenner (cbrenner@psych.ubc.ca) assisted with training and supervision of the research technicians, assisted with diagnostic procedures, carried out initial data analysis and contributed to the interpretation of the statistical analysis.
All authors contributed to and have approved the final manuscript.
References
- Bramon E, McDonald C, Croft RJ, Landau S, Filbey F, Gruzelier JH, Sham PC, Frangou S, Murray RM. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27(4):960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, O'Donnell BF. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35(6):1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner CA, Sporns O, Lysaker PH, O'Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160(12):2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Perry W, Shafer K, Braff DL. Cognitive functions in schizotypal personality disorder. Schizophr Res. 1999;37(2):123–132. doi: 10.1016/s0920-9964(98)00147-9. [DOI] [PubMed] [Google Scholar]
- Condray R, Steinhauer SR. Schizotypal personality disorder in individuals with and without schizophrenic relatives: similarities and contrasts in neurocognitive and clinical functioning. Schizophr Res. 1992;7(1):33–41. doi: 10.1016/0920-9964(92)90071-c. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Frumin M, Niznikiewicz MA, Hirayasu Y, Fraone S, Seidman LJ, Shenton ME. Smaller left Heschl's gyrus volume in patients with schizotypal personality disorder. Am J Psychiatry. 2002;159(9):1521–1527. doi: 10.1176/appi.ajp.159.9.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, D.C.: American Psychiatric Press, Inc.; 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Non-patient Edition (SCID-I/NP, 2/2001 revision) New York: Biometrics Research Department, New York State Psychiatric Institute; 2001a. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition (SCID-I/P, 2/2001 revision) New York: Biometrics Research Department, New York State Psychiatric Institute; 2001b. [Google Scholar]
- Goldstein KE, Hazlett EA, New AS, Haznedar MM, Newmark RE, Zelmanova Y, Passarelli V, Weinstein SR, Canfield EL, Meyerson DA, Tang CY, Buchsbaum MS, Siever LJ. Smaller superior temporal gyrus volume specificity in schizotypal personality disorder. Schizophr Res. 2009;112(1–3):14–23. doi: 10.1016/j.schres.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, Buchanan RW, Thaker GK. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70(2–3):293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Huttunen J, Heinimaa M, Svirskis T, Nyman M, Kajander J, Forsback S, Solin O, Ilonen T, Korkeila J, Ristkari T, McGlashan T, Salokangas RK, Hietala J. Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol Psychiatry. 2008;63(1):114–117. doi: 10.1016/j.biopsych.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Karoumi B, Laurent A, Rosenfeld F, Rochet T, Brunon AM, Dalery J, d'Amato T, Saoud M. Alteration of event related potentials in siblings discordant for schizophrenia. Schizophr Res. 2000;41(2):325–334. doi: 10.1016/s0920-9964(99)00062-6. [DOI] [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Dick RM, Diwadkar VA, Montrose DM, Prasad KM, Stanley JA. Striatal metabolic alterations in non-psychotic adolescent offspring at risk for schizophrenia: a (1)H spectroscopy study. Schizophr Res. 2009;115(1):88–93. doi: 10.1016/j.schres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Kimble M, Lyons M, O'Donnell B, Nestor P, Niznikiewicz M, Toomey R. The effect of family status and schizotypy on electrophysiologic measures of attention and semantic processing. Biol Psychiatry. 2000;47(5):402–412. doi: 10.1016/s0006-3223(99)00184-5. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O'Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47(4):1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56(11):1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht G, Karch S, Karamatskos E, Giegling I, Moller HJ, Hegerl U, Pogarell O, Rujescu D, Mulert C. Alterations of the early auditory evoked gamma-band response in first-degree relatives of patients with schizophrenia: Hints to a new intermediate phenotype. J Psychiatr Res. 2011;45(5):699–705. doi: 10.1016/j.jpsychires.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60(11):1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Mannan MR, Hiramatsu KI, Hokama H, Ohta H. Abnormalities of auditory event-related potentials in students with schizotypal personality disorder. Psychiatry and clinical neurosciences. 2001;55(5):451–457. doi: 10.1046/j.1440-1819.2001.00889.x. [DOI] [PubMed] [Google Scholar]
- Maxwell ME. Family Interview for Genetic Studies (FIGS): A Manual for FIGS. Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; 1992. [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45(9):1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niznikiewicz MA, Spencer KM, Dickey C, Voglmaier M, Seidman LJ, Shenton ME, McCarley RW. Abnormal pitch mismatch negativity in individuals with schizotypal personality disorder. Schizophr Res. 2009;110(1–3):188–193. doi: 10.1016/j.schres.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niznikiewicz MA, Voglmaier MM, Shenton ME, Dickey CC, Seidman LJ, Teh E, Van Rhoads R, McCarley RW. Lateralized P3 deficit in schizotypal personality disorder. Biol Psychiatry. 2000;48(7):702–705. doi: 10.1016/s0006-3223(00)00938-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Penuelas I, Masdeu JC. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci. 2002;22(23):10501–10506. doi: 10.1523/JNEUROSCI.22-23-10501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon SE, Valiakalayil A, Hanstock CC, Seres P, Tibbo P. Elevated 3T proton MRS glutamate levels associated with poor Continuous Performance Test (CPT-0X) scores and genetic risk for schizophrenia. Schizophr Res. 2008;99(1–3):218–224. doi: 10.1016/j.schres.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17(4):555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annual review of clinical psychology. 2006;2:291–326. doi: 10.1146/annurev.clinpsy.2.022305.095318. [DOI] [PubMed] [Google Scholar]
- Rajarethinam R, Sahni S, Rosenberg DR, Keshavan MS. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. Am J Psychiatry. 2004;161(6):1121–1124. doi: 10.1176/appi.ajp.161.6.1121. [DOI] [PubMed] [Google Scholar]
- Rass O, Krishnan G, Brenner CA, Hetrick WP, Merrill CC, Shekhar A, O'Donnell BF. Auditory steady state response in bipolar disorder: relation to clinical state, cognitive performance, medication status, and substance disorders. Bipolar Disord. 2010;12(8):793–803. doi: 10.1111/j.1399-5618.2010.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Voglmaier MM, Seidman LJ, McCarley RW. Topographic abnormalities of P3 in schizotypal personality disorder. Biol Psychiatry. 1996;40(3):165–172. doi: 10.1016/0006-3223(95)00373-8. [DOI] [PubMed] [Google Scholar]
- Shin YW, Krishnan G, Hetrick WP, Brenner CA, Shekhar A, Malloy FW, O'Donnell BF. Increased temporal variability of auditory event-related potentials in schizophrenia and Schizotypal Personality Disorder. Schizophr Res. 2010;124(1–3):110–118. doi: 10.1016/j.schres.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson MI, Hadjipapas A, Barnes GR, Furlong PL, Witton C. Imaging the dynamics of the auditory steady-state evoked response. Neurosci Lett. 2005;385(3):195–197. doi: 10.1016/j.neulet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64(5):369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Pierri JN, Auh S, Sampson AR, Lewis DA. Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharmacology. 2003;28(3):599–609. doi: 10.1038/sj.npp.1300120. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Zhou SY, Tanino R, Nakamura K, Kawasaki Y, Seto H, Kurachi M. A follow-up MRI study of the superior temporal subregions in schizotypal disorder and first-episode schizophrenia. Schizophr Res. 2010;119(1–3):65–74. doi: 10.1016/j.schres.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16(13):4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage. 2008;42(4):1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker GK, Cassady S, Adami H, Moran M, Ross DE. Eye movements in spectrum personality disorders: comparison of community subjects and relatives of schizophrenic patients. Am J Psychiatry. 1996;153(3):362–368. doi: 10.1176/ajp.153.3.362. [DOI] [PubMed] [Google Scholar]
- Tibbo P, Hanstock C, Valiakalayil A, Allen P. 3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am J Psychiatry. 2004;161(6):1116–1118. doi: 10.1176/appi.ajp.161.6.1116. [DOI] [PubMed] [Google Scholar]
- Trestman RL, Horvath T, Kalus O, Peterson AE, Coccaro E, Mitropoulou V, Apter S, Davidson M, Siever LJ. Event-related potentials in schizotypal personality disorder. The Journal of neuropsychiatry and clinical neurosciences. 1996;8(1):33–40. doi: 10.1176/jnp.8.1.33. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Calkins ME. Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry. 2008;64(12):1051–1059. doi: 10.1016/j.biopsych.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolic D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52(1):155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99(5):2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Administration and Scoring Manual: Wechsler Adult Intelligence Scale. 3 ed. San Antonio, TX: Psychological Corp; 1997. [Google Scholar]
- Weisbrod M, Hill H, Niethammer R, Sauer H. Genetic influence on auditory information processing in schizophrenia: P300 in monozygotic twins. Biol Psychiatry. 1999;46(5):721–725. doi: 10.1016/s0006-3223(99)00022-0. [DOI] [PubMed] [Google Scholar]
- Winterer G, Egan MF, Raedler T, Sanchez C, Jones DW, Coppola R, Weinberger DR. P300 and genetic risk for schizophrenia. Arch Gen Psychiatry. 2003;60(11):1158–1167. doi: 10.1001/archpsyc.60.11.1158. [DOI] [PubMed] [Google Scholar]