Abstract
Attenuated measles virus vaccine strains have emerged as a promising oncolytic vector platform, having shown significant anti-tumor activity against a broad range of malignant neoplasms. Measles virus strains derived from the attenuated Edmonston-B (MV-Edm) vaccine lineage have been shown to selectively infect, replicate in and lyse cancer cells while causing minimal cytopathic effect on normal tissues. This review summarizes the preclinical data that led to the rapid clinical translation of oncolytic measles vaccine strains and provides an overview of early clinical data using this oncolytic platform. Furthermore, novel approaches currently under development to further enhance the oncolytic efficacy of MV-Edm strains, including strategies to circumvent immunity or modulate immune system responses, combinatorial approaches with standard treatment modalities, virus retargeting as well as strategies for in vivo monitoring of viral replication are discussed.
Keywords: cancer gene therapy, cell carriers, combination therapy, oncolytic measles, tumor targeting, virotherapy
INTRODUCTION
Although significant progress has been achieved in recent years, the majority of advanced malignancies remain incurable and in need for novel treatment modalities. Virotherapy is a novel antitumor approach that has shown considerable promise [1]. Replicating oncolytic viruses capable of infecting and destroying tumor cells can effectively propagate in cancerous tissues thus amplifying their antineoplastic effect. This ability has in large part been cultivated bymillennia of adaptive evolution which have provided viruses with diverse efficient strategies for intracellular invasion and exploitation of the target cell’s biosynthetic apparatus, resulting in viral replication followed by lysis of infected cells, release of viral progeny and spread to neighboring cells.
While safety concerns may arise when using replicating viruses, use of viral vaccine strains could facilitate the clinical translation of novel clinical oncolytic platforms by allowing use of information accumulated in a vaccination setting to expedite the development of clinical oncolytic applications. The development of oncolytic attenuated measles virus strains deriving from the Edmonston vaccine lineage (MV-Edm) represents such an example. These strains have been shown to preferentially infect, replicate, and kill cancer cells [2]. In addition, live attenuated measles vaccine strains have been administered to more than a billion people in the last 40 years with an excellent safety record [3]. These decades of experience greatly facilitated acceptance and rapid clinical translation of oncolytic MV-Edm derivatives by allowing a more accurate prediction of potential toxicities and thus guiding the conduct of preclinical toxicology studies, and the development of eligibility criteria in the clinical trials to maximize patient safety [2]. The present review aims to summarize the current advances in the preclinical and clinical investigation of oncolytic measles virus (MV) strains as well as discuss some of the novel strategies in the development of this oncolytic platform.
MECHANISMS OF MEASLES VIRUS CYTOPATHIC EFFECT AND TUMOR SELECTIVITY
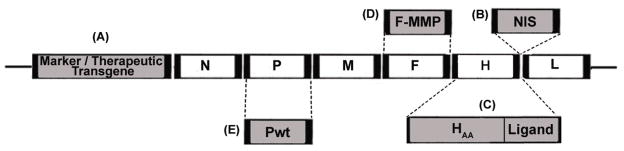
MV is a negative-strand RNA virus of the genus Morbillivirus within the Paramyxoviridae family and causes the highly contagious, common, acute, exanthemous measles disease. A number of case reports have described spontaneous remissions of leukemias, Burkitt’s lymphoma and Hodgkin’s disease following wild type measles infections [4–11]. The MV genome contains six genes encoding eight separate proteins: the nucleocapsid (N), phospho- (P), matrix (M), fusion (F), hemagglutinin (H) and large (L) proteins, as well as the two accessory proteins C and V which are encoded by the P-cistron [12] (Figure 1). Viral entry into target cells is mediated by pH-independent membrane fusion at the cell surface. H protein binding to a cellular receptor induces conformational changes to both H and F resulting in membrane fusion mediated by F [12]. The viral H and F glycoproteins are expressed on the cell surface of MV-infected cells and cell to cell fusion is triggered via H glycoprotein recognition of the viral receptor on neighboring infected or uninfected cells [13]. This results in the typical MV cytopathic effect which is the formation of giant multinucleated cell aggregates (syncytia) that ultimately undergo apoptotic death (Figure 2A). Oncolytic viral vectors are often hampered by delivery limitations. A potent bystander effect may supersede these limitations by destroying those cancer cells that the virus itself is unable to reach. Derivatives of the MV-Edm vaccine strain are capable of considerable bystander cell killing via the formation of massive syncytia [14–18]. Thus, a single cell of the glioblastoma multiforme cell line U87 transfected with the measles H and F fusogenic glycoproteins can destroy up to 80 adjacent, untransfected cells [18].
Figure 1.
The unmodified measles virus genome contains the nucleocapsid (N), phospho- (P), matrix (M), fusion (F), hemagglutinin (H) and large (L) genes. A reverse genetics system developed by Radecke et al.[33] allows the engineering of measles virus Edmonston (MV-Edm) strains. A, B: Oncolytic MV-Edm strains can be engineered to express additional transcriptional units. Examples include reporter genes such as the enhanced green fluorescent protein (eGFP) [103], the soluble extracellular N-terminal domain of the human carcinoembryonic antigen (CEA) [45] and the human lambda light immunoglobulin chain [72], as well as additional therapeutic transgenes such as the prodrug convertase purine nucleotide phosphorylase (PNP) [40, 58] and interferon beta [65] introduced upstream of the viral N gene (A) or the sodium iodide symporter (NIS) gene inserted downstream of the viral H protein [15] (B). The NIS transgene allows noninvasive monitoring of virus localization, replication and gene expression over time and may also be employed as a therapeutic transgene by allowing the intracellular concentration of toxic radioisotopes in infected cancer cells. C: MV-Edm full retargeting can be accomplished via the introduction of H mutations (HAA) that ablate natural entry via the two known measles receptors CD46 and CD150 (signaling lymphocyte-activation molecule; SLAM) [24, 36, 37, 47, 80] and the addition of large peptide sequences (ligands) on the C-terminus of H that allow viral entry and syncytial formation via a wide variety of target receptors [26, 36, 37, 39–41, 43–44, 47, 48, 50, 55, 58, 60, 78, 79, 81–83]. D: Viral retargeting may also be achieved by introducing into the F glycoprotein peptide linkers that are cleavable by specific proteases such as matrix metalloproteinases (MMPs) [66]. The modified F protein (F-MMP) can only be efficiently activated by MMPs resulting in restriction of virus tropism to sites that abundantly express theses proteases. E: The defective MV-Edm vaccine strain P gene may be replaced by a wild-type P gene (Pwt) which can more efficiently inhibit innate immune mechanisms resulting in improved oncolytic efficacy [92]. Further modifications include the replacement of the N and L genes by their wild-type counterparts to enhance innate immunity evasion [67].
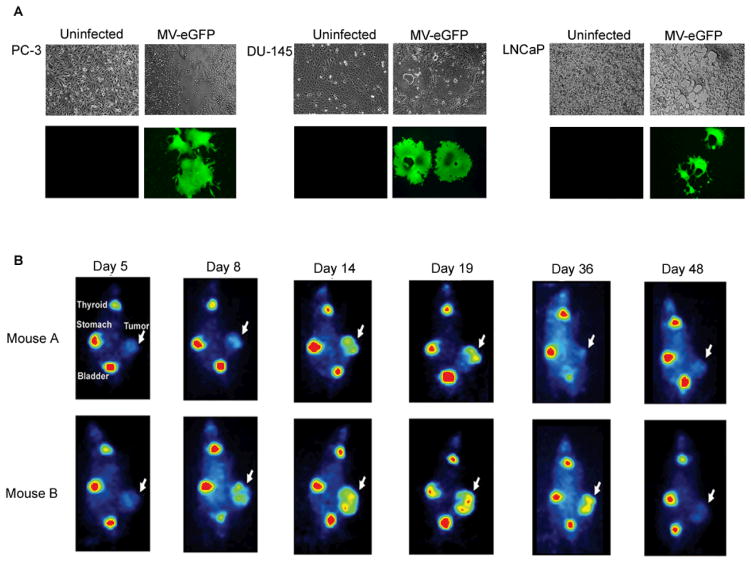
Figure 2.
A: Prostate cancer cell lines PC-3, DU-145 and LNCaP were infected with MV-eGFP (MV-Edmonston derivative expressing the enhanced green fluorescent protein gene) [59]. Cell-cell fusion results in the formation of massive multinucleated syncytia expressing eGFP. Images were taken seventy-two hours after infection with MV-eGFP under 100x magnification. B: Nude mice harboring LNCaP xenografts were intravenously injected with a single dose of 1.5×106 TCID50 of MV-NIS. In vivo monitoring of MV-NIS tumor infection over time was achieved via 123I imaging using a micro-gamma camera system. Each mouse exhibited a distinct biological response to oncolytic treatment that correlated with 123I imaging. Following IV administration of the virus, a small focus of infection is initially detected in small, localized areas within the xenograft. Diffuse, extensive spread of the replicating virus can be visualized by day 14 after the intravenous MV-NIS injection. Persistent NIS transgene expression could be identified for as long as 36 days. Replicating MV-NIS oncolysis in mouse A resulted in substantial partial regression of the tumor which subsequently relapsed following virus clearance as evidenced by negative uptake signal. The persistent infection seen in mouse B resulted in tumor growth arrest corresponding to stable disease [61].
The H glycoprotein interacts with two known MV receptors [12, 19]: CD150 (signaling lymphocyte-activation molecule; SLAM) which is a transmembrane glycoprotein primarily expressed activated B- and T- lymphocytes, memory lymphocytes, immature thymocytes and dendritic cells, as well as CD46 (membrane cofactor protein; MCP), which is a transmembrane glycoprotein that is ubiquitously present on all nucleated primate cells and has been found to be highly expressed in virtually all cancer cells tested thus far. CD46 belongs to the membrane-associated complement regulatory protein family and acts as a cofactor in the proteolytic inactivation of C3b and C4b complement products thus protecting human cells against autologous complement destruction [19]. Cancer cells express high levels of CD46 which is thought to shield tumors from complement-mediated lysis [20, 21]. Even though the majority of wild type MV strains enter cells predominantly via SLAM [22, 23], attenuated MV-Edm vaccine strains have been adapted by serial in vitro passaging to also use CD46 as an alternative receptor [24]. Thus MV-Edm and its derivatives can infect tumor cells overexpressing CD46 while exhibiting little cytopathic effect in non-transformed cells expressing lower CD46 levels including normal ovarian surface epithelial cells, astrocytes, mesothelial cells, hepatocytes, peripheral blood lymphocytes, normal human dermal fibroblasts and coronary artery smooth muscle cells [14, 25–28]. It is also likely that other factors may additionally contribute, although to a lesser extent, to the preferential antitumor activity of MV-Edm [25, 29, 30]. For example, as is the case with most other oncolytic RNA viruses [31], tumor selectivity may be facilitated by defects in the innate antiviral responses that are commonly observed in cancer cells but not in non-malignant tissues.
ONCOLYTIC MEASLES VIRUS SAFETY
The application of replicating viral vectors in cancer therapeutics can raise important safety concerns. With regards to measles, although wild-type MV can cause potentially serious disease, the attenuated MV-Edm vaccine strains have an excellent safety record: vaccine doses having been safely administered to millions of children for more than 40 years [3]. The hypothetical scenario whereby oncolytic MV-Edm derivatives, including recombinant strains not normally found in nature, would spread in the general population is also highly unlikely since most individuals living in industrialized countries are immune to measles due to either previous infection or vaccination [32]. Furthermore, it should be noted that reversion of measles vaccine strains to pathogenicity and subsequent transmission to other individuals have never been reported [3].
MEASLES VIRUS ONCOLYTIC EFFICACY AND MONITORING IN MOUSE MODELS
The historical reports of cancer remissions following natural measles infection described above and the excellent safety record of measles vaccine strains prompted the preclinical investigation of these viruses as oncolytic agents using genetically engineered MV-Edm derivatives rescued from cloned DNA (MV-Edm tag) [33]. MV-Edm tag is the original backbone employed to construct almost all genetically engineered oncolytic MV-Edm strains tested thus far [34]. Recombinant MV-Edm derivatives can accommodate and maintain large sizes (> 6 kb) of foreign genetic sequences with exceptional genetic stability both in vitro and in vivo animal models [17, 35–37]. The potent antitumor activity of oncolytic MV-Edm strains has been investigated in wide varieties of cancer cell lines, primary cancer cells, tissues as well as murine xenograft and syngeneic models representing several malignancies including B-cell Non-Hodgkin lymphomas (NHL) [36, 38–40], multiple myeloma [15–17, 26, 41, 42], ovarian cancer [28, 37, 43–46], glioblastoma multiforme [14, 47–51], T-cell leukemia [37], erythroleukemia [37], cutaneous T-cell lymphoma [52], breast cancer [28, 53–55], hepatocellular carcinoma [27, 56, 57], colon cancer [58], prostate cancer [59–61], pancreatic cancer [37, 62, 63], mesothelioma [64, 65], fibrosarcoma [17, 39, 66], rhabdomyosarcoma [28], renal cell carcinoma [67] and medulloblastoma [68].
One of the major challenges in clinical oncolytic virotherapy pertains to implementation of convenient, non-invasive methods to monitor the in vivo replication, spread and elimination of the virus as well as to determine the gene expression profile and viral kinetics over time. Such data may be crucial in determining the optimal dose size and time intervals between repeat treatment cycles thus paving the road for individualized treatment protocols. Viruses may be modified to express clinically relevant marker peptides which ideally should have a constant circulation half-life, be easily measurable by clinically validated assays and lack immunogenicity or known biological activity. An oncolytic MV-Edm derivative (MV-CEA) has been designed to express the soluble extracellular N-terminal domain of human carcinoembryonic antigen (CEA) as an additional transcription unit upstream of the viral N gene (Figure 1A) [46]. CEA was selected because it is a well-characterized, biologically inert marker peptide that has been widely used as a tumor marker [69]. Thus, repeat CEA measurements may easily and inexpensively be performed using established sensitive and reliable assays, and can be used to follow viral replication in tumor types such as ovarian cancer and gliomas, where CEA is uncommonly expressed [2]. Infection of cancer cells by MV-CEA is followed by viral replication and gene expression resulting in CEA production and release into the extracellular space. Therefore, important feedback on MV-CEA gene expression kinetics could be obtained by CEA measurement in the serum of treated patients.
Although MV-CEA provides the opportunity for convenient real-time monitoring of viral gene expression, it does not allow determination of the anatomical localization of virus infection. Consequently, a second replicating MV-Edm derivative (MV-NIS) has been engineered to express the sodium iodide symporter (NIS) gene downstream of the viral H protein (Figure 1B) [15]. NIS is a membrane ion channel that facilitates intracellular uptake of radioisotopes such as 123I, 124I, 125I and 99mTc [70, 71] and can thus be used as a reporter gene. NIS expression on the cell membrane of MV-NIS-infected tumour cells allows radioisotope concentration which can then be non-invasively tracked by γ camera, positron emission tomography (PET) or single photon emission computed tomography combined with computed tomography (SPECT/CT). This enables the non-invasive monitoring of MV-NIS anatomical localization, spread, replication and gene expression kinetics over time (Figure 2B). The NIS transgene may also further enhance the oncolytic efficacy of MV-NIS by allowing intracellular entry and therapeutic concentration of radioisotopes such as 131I [71] that can cause direct radiation damage to neighboring uninfected cells as well as destroy tumors that are otherwise refractory to MV-NIS oncolysis [15]. The combination of an oncolytic virus with radioisotope-mediated tumor ablation via the expression of a therapeutic transgene, such as NIS, is known as radiovirotherapy [15]. The significantly enhanced antitumor activity of MV-NIS radiovirotherapy has been convincingly demonstrated in multiple myeloma [15] and prostate cancer [61] xenograft models.
The vast majority of reporter genes used in gene therapy assume, often incorrectly, that only the intended target cells may be transduced. Iankov et al. [72] recently generated a recombinant MV-Edm strain (MV-lambda) that carries the human lambda light immunoglobulin chain transcription unit upstream of the viral N gene (Figure 1A). Infection of monoclonal kappa-chain immunoglobulin-producing multiple myeloma cells by MV-lambda resulted in secretion of a unique immunoglobulin composed of one kappa and one lambda light chain, which cannot be naturally found in vivo. Therefore, detection of chimeric kappa/lambda molecules by a modified immunoassay technique can non-invasively discriminate between infected multiple myeloma and normal, non-plasma cells. This novel strategy has been termed “marker conversion” and can be used to generate unique markers of tumor-specific infection [72].
CLINICAL TESTING OF ONCOLYTIC MEASLES VIRUS STRAINS
The first clinical study of an MV vaccine strain in the treatment of cancer was an open-label non-randomized, dose-escalation phase I clinical trial that used the unmodified commercially available Edmonston-Zagreb vaccine strain (MV-EZ) in a total of five measles immune patients with ≥ stage IIb cutaneous T-cell lymphoma (CTLC) [73]. Patients were administered one or two viral treatment cycles with each cycle consisting of two intratumoral MV-EZ injections on days 4 and 17.
Subcutaneous interferon-alpha (INFα) injections were also administered 72 and 24 h before each of the two MV-EZ injections. The minimum MV-EZ dose was 102 TCID50 (50% tissue culture infective dose) per injection while the maximum dose was 103 TCID50/injection. Treatment resulted in complete regression of one CTCL tumor in one patient after the first treatment cycle and a second lesion was therefore treated in the next cycle. Four of the treated tumors showed partial regression with only one lesion demonstrating no response. Despite CTCL being an immunosuppressive disease, anti-measles antibody titers were increased in all five patients following MV-EZ therapy. No dose-limiting toxic effects were observed and treatment was well-tolerated with only minimal local irritation noted. The observed tumor responses were intriguing, especially taking into account the low viral doses used. The study design, however, did not allow assessment of the contribution of IFNα treatment on the observed antitumor effect and follow-on trials in a larger number of patients are required.
MV-CEA and MV-NIS strains are currently being tested in four Phase 1 clinical trials in recurrent ovarian carcinoma, glioblastoma multiforme, and multiple myeloma patients [2]. Among them, the phase I clinical trial of MV-CEA in patients with recurrent ovarian cancer was recently completed [74]. This represents the first clinical trial of an engineered measles virus strain in cancer treatment. MV-CEA was administered intraperitoneally in doses ranging from 103–109 TCID50 in 21 patients with platinum- and paclitaxel-refractory ovarian cancer confined to the peritoneal cavity. All patients were measles-immune and had serum CEA levels <3 ng/ml before enrollment as well as during any prior testing. Thus, any CEA elevation during oncolytic treatment could only be attributed to MV-CEA gene expression. No dose-limiting toxicity was observed at any dose level. Furthermore, no MV-CEA-induced immunosuppression, increase in anti-measles antibody titers or development of anti-CEA antibodies were observed. MV-CEA did not shed into urine or saliva while low levels of viral genomes were detected by qRT-PCR in peripheral blood mononuclear cells (PBMCs) of four patients who remained asymptomatic. Increased serum CEA was observed in all three patients treated with the highest (109 TCID50) dose. Elevated CEA levels were also detected in the peritoneal fluid of one patient treated with 108 TCID50 as well as two patients in the 109 TCID50 group. Clinical outcome was also dose-dependent with the best objective response being stable disease in fourteen patients, as assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) [75]. Furthermore, significant decreases in cancer antigen-125 (CA-125) levels were observed in five patients. The median survival (12.15 months) of patients in the trial was double the expected median survival of 6 months in this patient population, based on historical controls [76]. In order to determine the optimal monitoring strategy prior to phase II testing, a phase I trial of intraperitoneal administration of MV-NIS in patients with recurrent ovarian cancer has been initiated in order to test the clinical applicability of the NIS reporter gene, as well as set the stage for a potential future radiovirotherapy combinatory strategy to further enhance recombinant MV-Edm efficacy against ovarian cancer.
MV-CEA is also currently being tested in a phase I trial of intratumoral administration in patients with recurrent glioblastoma multiforme [2]. Doses ranging from 105 to 2×107 TCID50 are administered to measles-immune patients who are candidates for gross total or subtotal tumor resection. No dose limiting toxicity has been observed to date in doses up to 107 TCID50. A phase I clinical trial of intravenous MV-NIS administration in patients with recurrent or refractory multiple myeloma is also in progress [2]. This is the first clinical testing of a replicating oncolytic vector against multiple myeloma [77]. Patients were administered MV-NIS doses ranging from 106 to 109 TCID50 with no dose limiting toxicity being observed despite significantly lower titers or absence of neutralizing antibodies in these patients. Following determination of the maximum tolerated dose (MTD), new cohorts of three patients will be pretreated with cyclophosphamide (10 mg/kg) two days prior to MV-NIS therapy. The MV-NIS dose in this second step will range from MTD/100 to 81-fold the MTD/100. Results deriving from these studies as well as the additional preclinical work described below will serve as the basis for follow-up clinical strategies incorporating measles strains in cancer treatment.
MEASLES VIRUS RETARGETTING
Retargeting of oncolytic MV vectors was originally pursued to increase specificity against tumor cells thus making these agents safer for clinical use. However, as described in a previous section, oncolytic MV vaccine strains naturally exhibit preferential antitumor activity mediated, at least in large part, by the high CD46 expression on cancer cells. Nevertheless, although no dose limiting toxicity has to date been noted in the phase I trials of attenuated MV vaccine strains, viral retargeting can address any safety concerns that may arise when higher viral doses or more potent vectors are contemplated. It is of note that retargeted MV-Edm derivatives lack neurotoxic activity even when administered directly to the CNS of measles-naïve IFN type I receptor deficient (IFNARKO) CD46 Ge transgenic mice, a very sensitive model of viral neurotoxicity [40, 48, 50]. In addition, viral retargeting can overcome potential variability in viral receptor expression patterns which may be found on otherwise histologically identical cancer types and even within the same tumor tissue. Notably, Galanis et al. [74] found strong, diffuse CD46 expression, as assessed by immunohistochemical analysis, in ovarian tumor specimens of 13 out of the 15 patients tested during the MV-CEA against ovarian cancer trial. The low CD46 expression found in the remaining two patients points to the potential utility of viral retargeting to optimize treatment efficacy. Furthermore, broadening viral tropism to molecules expressed on the luminal endothelial surface of tumor neovasculature may increase targeted viral delivery to tumor sites following systemic administration [41, 55, 78].
As it pertains to retargeting, a particularly convenient characteristic of MV vectors is that viral attachment and fusion are mediated by two separate glycoproteins, H and F respectively. It is the H protein that determines viral binding tropism [12]. Thus, retargeting procedures can focus on H protein modifications without significantly affecting the considerable fusogenic capacity of the virus. Accordingly, a large number of studies have shown that fully retargeted MV-Edm strains demonstrate no significant attenuation of oncolytic efficacy against cells or xenografts expressing adequate levels of target receptors [43, 45, 47, 48, 50, 55, 79]. Following identification of aminoacid residues necessary for H interaction with CD46 and SLAM [24] it was determined that full retargeting may be optimally accomplished by a single CD46-ablating substitution of tyrosine by alanine at position 481 (Y481A) and a single SLAM-ablating substitution of arginine by alanine at position 533 [24, 36, 47, 80] in combination with display of targeting ligands on the C-terminus of the H protein [36, 37, 81] (Figure 1C). Construction of a wide variety of retargeted oncolytic MV-Edm derivatives has been successfully accomplished, including viruses displaying single chain antibodies against CD38 [26, 36, 37], EGFR [36, 37, 48], EGFRvIII [36, 47], alpha-folate receptor [45], HER2/neu [43], CD20 [39, 40], CEA [58, 82], PSMA [60] and the Wue-1 ligand [79]. Retargeted oncolytic MVs have also been constructed to display the snake venom peptide echistatin [41], cyclic arginine-glycine-aspartate (RGD) [78], single-chain T cell receptors [83] as well as cytokines such as IL-13 [50] and peptide ligands to the urokinase receptor (uPAR) [55].
Maturation of the MV F protein is normally achieved via proteolytic cleavage of a precursor protein (F0) into the large F1 and the smaller F2 subunits. F0 cleavage is normally mediated by the ubiquitous cellular protease furin. Selective activation of mutated F proteins can thus restrict MV tissue tropism [84]. Springfeld et al. [66] accordingly developed an MV-Edm targeting strategy which achieves tumor selectivity by inserting into the F glycoprotein peptide sequences that are sensitive to tumor-specific proteases (Figure 1D). This restricts proteolytic maturation of F at sites where the target protease is abundantly secreted. This alternative targeting approach can potentiallybe used in combination with retargeting by ligand displayon chimeric H as these two strategies are not expected to be mutually exclusive. It has therefore now become possible to reprogram MV-Edm tropism at different levels.
IMMUNE EVASION STRATEGIES
Further developmental strategies of oncolytic MVs will be guided by data on safety, pharmacokinetics and efficacy generated by phase I clinical testing. The results of the first completed trials [73, 74] as well as preliminary data from the ongoing trials strongly suggest the safety of oncolytic MV vaccine strains. Consequently, efforts should be concentrated on enhancing oncolytic efficacy. Most candidates for measles virotherapy will have prior immunity to the virus and this may significantly impact the therapeutic efficacy, especially if systemic virotherapy delivery is contemplated. Three main strategies to modulate or circumvent anti-measles immunity are currently being explored. The first is the use of cells as delivery vehicles for oncolytic MV. Ideally these cell carriers should be highly permissive and susceptible to measles infection and be capable of trafficking to tumor sites as well as efficiently deliver their cargo to tumor cells while also protecting the virus from antibody neutralization. Cell carriers should additionally be safe, non-tumorigenic and easily available for clinical applications. The virus may also propagate inside cell carriers resulting in targeted release of additional oncolytic virus progeny in cancer tissues. Notably, cell-associated viremia is a natural part of the MV life cycle and has been shown to protect the virus from anti-measles antibodies [85, 86]. Another important advantage of MV oncolytic vectors for cell carrier use is the natural fusogenicity of these viruses, which allows transfer of viral progeny via heterofusion of infected cell carriers with cancer cells. More specifically, the MV H and F envelope glycoproteins expressed on the cell surface of infected cell carriers interact with the CD46 receptor on the cell membrane of target tumor cells resulting in cell-to-cell heterofusion and syncytia formation. Thus, no naked virions are exposed to antibodies in the extracellular environment during viral spread.
Various cell types have been investigated as potential carriers for oncolytic MV-Edm strains including immune cells such as the U-937 monocytic cell line [53, 56], immature and mature primary dendritic cells [53], PMBCs [56], activated T cells [87] and primary CD14+ cells [88]. Additional potential MV carriers cells include stem cells such as mesenchymal progenitor cells [89], the multiple myeloma MM1 cell line [90] and other cell types such as blood outgrowth endothelial cells [56, 91]. Each cell carrier type may display different tumor homing properties as well as variability in virus protection, propagation and delivery. Therefore further detailed preclinical studies will be required to determine the optimal cell carrier depending on the tumor type.
A second strategy to modulate anti-measles immune responses is combination therapy with the immunosuppressive drug cyclophosphamide. Cyclophosphamide treatment of squirrel monkeys prior to intravenous administration of MV-NIS resulted in decreased humoral immune response to the virus as well as prolonged viral gene expression [42]. Ungerechts et al. [58] additionally showed that cyclophosphamide administration significantly delayed anti-measles antibody production and enhanced the oncolytic activity of a CEA-retargeted MV-Edm derivative that was also armed with the prodrug convertase purine nucleotide phosphorylase (PNP). Allen et al. [51] further demonstrated that cyclophosphamide combination with either MV-CEA or MV-NIS significantly increased viral replication and antitumor efficacy in orthotopic glioblastoma multiforme xenograft models. The ongoing phase I clinical trial of systemic MV-NIS administration for multiple myeloma treatment with or without cyclophosphamide pretreatment is expected to provide crucial data on the effectiveness of this approach.
Another novel approach is to suppress the innate immune response to virus infection by arming the MV-Edm tag strains with wild-type MV genes that are capable of inhibiting intracellular pathways associated with innate immunity [67, 92]. MV vaccine strains induce substantially higher levels of type I IFN compared to wild-type MV [92]. This is in large part due to mutational defects in the P gene which also encodes two additional proteins, C and V, from overlapping open reading frames. All three proteins have been shown to inhibit type I IFN-induced intracellular pathways [93–95]. Haralambieva et al. [92] generated an MV-Edm derivative expressing enhanced green fluorescent protein (eGFP; MV-eGFP-Pwt virus) whereby the defective vaccine strain P gene was replaced by a wild-type P gene derived from the MV IC-B strain (Figure 1E). In vitro infection of cancer cell lines and primary cancer cells by the chimeric virus induced significantly lower levels of type I IFN compared to the unmodified MV-eGFP. Furthermore, MV-eGFP-Pwt showed substantially improved oncolytic efficacy compared to MV-eGFP in multiple myeloma xenograft models. It should be noted that while wild-type MV is capable of completely shutting down type I IFN induction, MV-eGFP-Pwt markedly suppressed but did not completely nullify IFN production [92]. This implies that other proteins such as the wild-type measles N protein, which has been shown to activate the interferon regulatory factor 3 (IRF3) [96], may play a role in innate immunity evasion by wild-type MV strains. Meng et al. [67] recently followed on this approach by generating a chimeric MV-Edm derivative armed with wild-type measles N, P and L genes (MV-NPL virus). This virus demonstrated significantly more efficient replication and enhanced killing efficacy in renal cancer cells in vitro and in vivo compared to an MV-Edm strain armed only with the wild-type P gene (MV-P) and to the unmodified MV-Edm tag strain. Notably, MV-NPL induced minimal cytopathic effects on the normal human skin fibroblast cell line BJ-1. However, additional in vitro as well as in vivo toxicity studies in animals will be required to confirm that this strategy does not compromise safety.
COMBINATION TREATMENT APPROACHES
Oncolytic virotherapy agents are a novel drug class with unique mechanisms of antitumor action. Thus, no cross-resistance with existing conventional therapeutic approaches is expected. Furthermore, a rapidly growing amount of data demonstrate enhanced or synergistic anticancer activity of MV-Edm strains when combined with other treatment modalities including the immunosuppressive alkylating agent cyclophosphamide discussed in previous sections [42, 51, 58]. Thus, combination of MV-Edm derivatives with cancer treatments capable of augmenting MV fusogenicity is currently being explored. Heat shock protein 90 (HSP90) inhibitors are a novel class of antitumor drugs that are currently undergoing phase I and II clinical investigation [97]. Liu et al. [28] showed that these small molecule drugs significantly enhanced the cytopathic effect of MV-CEA by increasing fusion susceptibility of various cancer cell lines without affecting non-transformed human dermal fibroblast cells. This effect is likely achieved via RhoA-mediated cytoskeletal modulation [28].
Radiation therapy has also shown strong synergistic interaction with MV-CEA in vitro against both radiation-sensitive and radiation-resistant malignant glioma cells as well as in in vivo xenograft models of this brain tumor using a total external beam radiation dose of 12 Gy per animal [49]. MV-NIS radiovirotherapy with 131I has been shown to significantly improve oncolytic activity against multiple myeloma [15] and prostate cancer xenografts [61]. While NIS is by far the most widely studied therapeutic transgene in MV vectors [15, 61], recent experiments have tested the applicability of PNP, a prodrug convertase that metabolizes the prodrugs fludarabine phosphate and 6-methylpurine-2′-deoxyriboside into the highly cytotoxic 2-fluoroadenine and 6-methylpurine respectively. MV-Edm derivatives retargeted to CD20 (MV-PNP HblindantiCD20) and CEA (MV-PNP-antiCEA) were engineered to additionally carry the PNP gene (Figure 1A). Co-administration of either fludarabine phosphate or 6-methylpurine-2′-deoxyriboside significantly enhanced the oncolytic efficacy of the PNP-armed viruses (chemovirotherapy) against human lymphoma xenograft and syngeneic murine colon adenocarcinoma models respectively [40, 58]. Type I interferons are known to elicit antitumor immune responses and are routinely used in the therapy of various cancers [98]. Li et al.[65] recently generated recombinant MV-Edm derivatives carrying a gene encoding mouse INFβ (Figure 1A). These viruses were found to be significantly more effective in orthotopic (peritoneal) mesothelioma models by inhibiting tumor angiogenesis as well as increasing CD68+ immune cell infiltration [65].
MATHEMATICAL MODELING AND PREDICTIVE TEST SYSTEMS OF MEASLES VIROTHERAPY
Recent efforts have attempted to construct and validate mathematical models of measles oncolytic behavior [99–102]. The first such model developed by Dingli et al.[99] explored the population dynamics of radiovirotherapy and the model parameters were fitted to prior experimental data of MV-NIS on multiple myeloma tumors. Bajzer et al. [100] followed on this study by constructing and validating a more detailed model that accounted for the effects of cell-cell fusion as well as the fact that virus particles that have already infected tumor cells are no longer capable of spreading to additional cells. An interesting finding of that study was that MV-Edm strains with weaker cytopathic capacity may induce greater tumor growth retardation as compared to strains that more rapidly lyse cells [100]. A later model [101] further explored the population dynamics of syncytia and was validated using data from a large preclinical efficacy study of MV-NIS administration in KAS-6/1 myeloma xenografts [42].
The measles virotherapy model of Bajzer et al. [100] was combined with methodological procedures derived from optimization theory resulting in a number of intriguing findings [102]. It was thus shown that variable but correctly timed viral administration schemes maximize oncolytic benefit compared to either regularly scheduled delivery or continuous infusion. Moreover, it was demonstrated that, in the absence of an immune response, treating larger tumors is more cost-effective [102]. Despite limitations, including the fact that none of the above models explicitly take into account the effects of potential immune responses against virus-infected cancer cells and/or against the virus itself, mathematical analysis provides greatly welcomed insights into the various potential behaviors and dynamic interplays occurring during oncolytic measles virotherapy.
ADVANTAGES AND LIMITATIONS OF MEASLES ONCOLYTIC VECTORS
As discussed in previous sections, the measles oncolytic platform has a number of advantages. Significant oncolytic activity following natural measles infection has been documented by several case reports in the past [4–11]. Derivatives of the MV-Edm strain have an excellent safety record [3] and exhibit natural oncolytic specificity which is largely mediated by high expression levels of CD46 in tumor cells [14, 25–28]. In addition, the oncolytic potency and tumor-selectivity of the attenuated MV-Edm strains has been well-documented in in vitro and in vivo models [14–17, 25–28, 36, 38–45, 47–68]. Notably, measles is an RNA virus with a strictly cytoplasmic replication cycle that lacks an intermediate DNA step, thus eliminating the possibility of insertional DNA mutagenesis. Powerful reverse genetics systems have been established for engineering MV-Edm strains [33, 36, 37] and recombinant viruses [17, 35, 36]. These viruses are highly stable with very low recombination rates [35]. In addition, full transductional retargeting of MV-Edm is feasible using a wide variety of targets [26, 36, 39–41, 43, 44, 47, 48, 50, 55, 58, 60, 66, 78, 79, 81–83]. The natural fusogenicity of measles strains induces a potent additional bystander effect [14–18] and may also be utilized in immune evasion strategies using cell carriers [53, 56, 87–91]. Furthermore, robust technology exists to manufacture clinical grade, high titer lots of MV-Edm derivatives that are essential for clinical applications of measles-based therapeutics at low production costs [2, 74].
Although pre-existing immunity to measles provides a considerable safety advantage, it could also compromise oncolytic MV efficacy, especially following repeat systemic administration. Immune evasion strategies are developed to address this issue, and discussed in previous sections. Variability in viral receptor expression, although not clinically documented yet, could represent another potential challenge that could be overcome with the use of targeting strategies.
CONCLUSIONS AND OUTLOOK
Attenuated measles vaccine strains are considerably promising oncolytic vectors having demonstrated significant antitumor efficacy in a very wide range of human malignancies in vitro and in vivo. Completed and ongoing phase I clinical trials are providing important insights with regards to safety, kinetics and efficacy of measles vaccine strains as well as the potential benefits of real-time monitoring strategies using the CEA and NIS reporter genes. So far, data produced by clinical testing point to the excellent safety profile of MV vaccine derivatives in addition to promising evidence of biological oncolytic activity. Thus, a number of approaches are currently being developed aimed at augmenting the antitumor activity of these agents. Many of these strategies hope to effectively shield the virus from innate and measles-specific immunity without sacrificing safety and efficacy. In addition, a number of methodologic discoveries and biotechnology innovations have allowed the efficient and complete retargeting of MV-Edm strains at different levels. These breakthroughs coupled with the ongoing development of predictive test systems for the oncolytic effectiveness of different MV vaccine strains as well as the advancement of biologically coherent mathematical models can lead to the development of individualized measles virotherapy strategies.
References
- 1.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4(2):101–17. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 2.Msaouel P, Dispenzieri A, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther. 2009;11(1):43–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin DE, Pan CH. Measles: old vaccines, new vaccines. Curr Top Microbiol Immunol. 2009;330:191–212. doi: 10.1007/978-3-540-70617-5_10. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez SA. Observacion de un caso de enfermedad de Hodgkin, con regresion de los sintomas e infartos ganglionares, post-sarampion. Arch Cubanos Cancer. 1949;8:26–31. [Google Scholar]
- 5.Bluming AZ, Ziegler JL. Regression of Burkitt’s lymphoma in association with measles infection. Lancet. 1971;2(7715):105–6. doi: 10.1016/s0140-6736(71)92086-1. [DOI] [PubMed] [Google Scholar]
- 6.Gross S. Measles and leukaemia. Lancet. 1971;1(7695):397–8. doi: 10.1016/s0140-6736(71)92232-x. [DOI] [PubMed] [Google Scholar]
- 7.Mota HC. Infantile Hodgkin’s disease: remission after measles. Br Med J. 1973;2(5863):421. doi: 10.1136/bmj.2.5863.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquinucci G. Possible effect of measles on leukaemia. Lancet. 1971;1(7690):136. doi: 10.1016/s0140-6736(71)90869-5. [DOI] [PubMed] [Google Scholar]
- 9.Zygiert Z. Hodgkin’s disease: remissions after measles. Lancet. 1971;1(7699):593. doi: 10.1016/s0140-6736(71)91186-x. [DOI] [PubMed] [Google Scholar]
- 10.Taqi AM, Abdurrahman MB, Yakubu AM, Fleming AF. Regression of Hodgkin’s disease after measles. Lancet. 1981;1(8229):1112. doi: 10.1016/s0140-6736(81)92286-8. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler JL. Spontaneous remission in Burkitt’s lymphoma. Natl Cancer Inst Monogr. 1976;44:61–5. [PubMed] [Google Scholar]
- 12.Yanagi Y, Takeda M, Ohno S. Measles virus: cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87(Pt 10):2767–79. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- 13.Wild TF, Malvoisin E, Buckland R. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991;72(Pt 2):439–42. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]
- 14.Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ, Mishra PK, Macura SI, Russell SJ, Galanis EC. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63(10):2462–9. [PubMed] [Google Scholar]
- 15.Dingli D, Peng KW, Harvey ME, Greipp PR, O’Connor MK, Cattaneo R, Morris JC, Russell SJ. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103(5):1641–6. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 16.Peng KW, Ahmann GJ, Pham L, Greipp PR, Cattaneo R, Russell SJ. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98(7):2002–7. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- 17.Peng KW, Facteau S, Wegman T, O’Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8(5):527–31. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 18.Galanis E, Bateman A, Johnson K, Diaz RM, James CD, Vile R, Russell SJ. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum Gene Ther. 2001;12(7):811–21. doi: 10.1089/104303401750148766. [DOI] [PubMed] [Google Scholar]
- 19.Dhiman N, Jacobson RM, Poland GA. Measles virus receptors: SLAM and CD46. Rev Med Virol. 2004;14(4):217–29. doi: 10.1002/rmv.430. [DOI] [PubMed] [Google Scholar]
- 20.Jurianz K, Ziegler S, Garcia-Schuler H, Kraus S, Bohana-Kashtan O, Fishelson Z, Kirschfink M. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36(13–14):929–39. doi: 10.1016/s0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 21.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40(2–4):109–23. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 22.Ono N, Tatsuo H, Hidaka Y, Aoki T, Minagawa H, Yanagi Y. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J Virol. 2001;75(9):4399–401. doi: 10.1128/JVI.75.9.4399-4401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider U, von Messling V, Devaux P, Cattaneo R. Efficiency of measles virus entry and dissemination through different receptors. J Virol. 2002;76(15):7460–7. doi: 10.1128/JVI.76.15.7460-7467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vongpunsawad S, Oezgun N, Braun W, Cattaneo R. Selectively receptor-blind measles viruses: Identification of residues necessary for SLAM- or CD46-induced fusion and their localization on a new hemagglutinin structural model. J Virol. 2004;78(1):302–13. doi: 10.1128/JVI.78.1.302-313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64(14):4919–26. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 26.Peng KW, Donovan KA, Schneider U, Cattaneo R, Lust JA, Russell SJ. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood. 2003;101(7):2557–62. doi: 10.1182/blood-2002-07-2195. [DOI] [PubMed] [Google Scholar]
- 27.Blechacz B, Splinter PL, Greiner S, Myers R, Peng KW, Federspiel MJ, Russell SJ, LaRusso NF. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology. 2006;44(6):1465–77. doi: 10.1002/hep.21437. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Erlichman C, McDonald CJ, Ingle JN, Zollman P, Iankov I, Russell SJ, Galanis E. Heat shock protein inhibitors increase the efficacy of measles virotherapy. Gene Ther. 2008;15(14):1024–34. doi: 10.1038/gt.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–41. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong HT, Timm MM, Greipp PR, Witzig TE, Dispenzieri A, Russell SJ, Peng KW. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp Hematol. 2006;34(6):713–20. doi: 10.1016/j.exphem.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther. 2009;9(9):1163–76. doi: 10.1517/14712590903170653. [DOI] [PubMed] [Google Scholar]
- 32.McQuillan GM, Kruszon-Moran D, Hyde TB, Forghani B, Bellini W, Dayan GH. Seroprevalence of measles antibody in the US population, 1999–2004. J Infect Dis. 2007;196(10):1459–64. doi: 10.1086/522866. [DOI] [PubMed] [Google Scholar]
- 33.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter MA. Rescue of measles viruses from cloned DNA. Embo J. 1995;14(23):5773–84. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blechacz B, Russell SJ. Measles virus as an oncolytic vector platform. Curr Gene Ther. 2008;8(3):162–75. doi: 10.2174/156652308784746459. [DOI] [PubMed] [Google Scholar]
- 35.Billeter MA, Naim HY, Udem SA. Reverse genetics of measles virus and resulting multivalent recombinant vaccines: applications of recombinant measles viruses. Curr Top Microbiol Immunol. 2009;329:129–62. doi: 10.1007/978-3-540-70523-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura T, Peng KW, Harvey M, Greiner S, Lorimer IA, James CD, Russell SJ. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol. 2005;23(2):209–14. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 37.Hadac EM, Peng KW, Nakamura T, Russell SJ. Reengineering paramyxovirus tropism. Virology. 2004;329(2):217–25. doi: 10.1016/j.virol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 38.Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA, Fielding AK. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97(12):3746–54. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- 39.Bucheit AD, Kumar S, Grote DM, Lin Y, von Messling V, Cattaneo RB, Fielding AK. An oncolytic measles virus engineered to enter cells through the CD20 antigen. Mol Ther. 2003;7(1):62–72. doi: 10.1016/s1525-0016(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 40.Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Johnston PB, Parker WB, Sorscher EJ, Cattaneo R. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 2007;67(22):10939–47. doi: 10.1158/0008-5472.CAN-07-1252. [DOI] [PubMed] [Google Scholar]
- 41.Hallak LK, Merchan JR, Storgard CM, Loftus JC, Russell SJ. Targeted measles virus vector displaying echistatin infects endothelial cells via alpha(v)beta3 and leads to tumor regression. Cancer Res. 2005;65(12):5292–300. doi: 10.1158/0008-5472.CAN-04-2879. [DOI] [PubMed] [Google Scholar]
- 42.Myers RM, Greiner SM, Harvey ME, Griesmann G, Kuffel MJ, Buhrow SA, Reid JM, Federspiel MJ, Ames MM, Dingli D, Schweikart K, Welch A, Dispenzieri A, Peng KW, Russell SJ. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin Pharmacol Ther. 2007;82(6):700–710. doi: 10.1038/sj.clpt.6100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasegawa K, Hu C, Nakamura T, Marks JD, Russell SJ, Peng KW. Affinity thresholds for membrane fusion triggering by viral glycoproteins. J Virol. 2007;81(23):13149–57. doi: 10.1128/JVI.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasegawa K, Pham L, O’Connor MK, Federspiel MJ, Russell SJ, Peng KW. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res. 2006;12(6):1868–75. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- 45.Hasegawa K, Nakamura T, Harvey M, Ikeda Y, Oberg A, Figini M, Canevari S, Hartmann LC, Peng KW. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6170–8. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 46.Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62(16):4656–62. [PubMed] [Google Scholar]
- 47.Allen C, Vongpunsawad S, Nakamura T, James CD, Schroeder M, Cattaneo R, Giannini C, Krempski J, Peng KW, Goble JM, Uhm JH, Russell SJ, Galanis E. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66(24):11840–50. doi: 10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- 48.Paraskevakou G, Allen C, Nakamura T, Zollman P, James CD, Peng KW, Schroeder M, Russell SJ, Galanis E. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol Ther. 2007;15(4):677–86. doi: 10.1038/sj.mt.6300105. [DOI] [PubMed] [Google Scholar]
- 49.Liu C, Sarkaria JN, Petell CA, Paraskevakou G, Zollman PJ, Schroeder M, Carlson B, Decker PA, Wu W, James CD, Russell SJ, Galanis E. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin Cancer Res. 2007;13(23):7155–65. doi: 10.1158/1078-0432.CCR-07-1306. [DOI] [PubMed] [Google Scholar]
- 50.Allen C, Paraskevakou G, Iankov I, Giannini C, Schroeder M, Sarkaria J, Puri RK, Russell SJ, Galanis E. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol Ther. 2008;16(9):1556–64. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen C, Msaouel P, Aderca I, Penheiter AR, Iankov I, Federspiel MJ, Opyrchal M, Carlson SK, Russell SJ, Galanis E. Optimizing the Antitumor Activity of Engineered Measles Virus Strains in the Treatment of Gliomas. Mol Ther. 2009;17(Supplement 1):S108. [Google Scholar]
- 52.Kunzi V, Oberholzer PA, Heinzerling L, Dummer R, Naim HY. Recombinant measles virus induces cytolysis of cutaneous T-cell lymphoma in vitro and in vivo. J Invest Dermatol. 2006;126(11):2525–32. doi: 10.1038/sj.jid.5700529. [DOI] [PubMed] [Google Scholar]
- 53.Iankov ID, Msaouel P, Allen C, Federspiel MJ, Bulur PA, Dietz AB, Gastineau D, Ikeda Y, Ingle JN, Russell SJ, Galanis E. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonald CJ, Erlichman C, Ingle JN, Rosales GA, Allen C, Greiner SM, Harvey ME, Zollman PJ, Russell SJ, Galanis E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res Treat. 2006;99(2):177–84. doi: 10.1007/s10549-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 55.Jing Y, Tong C, Zhang J, Nakamura T, Iankov I, Russell SJ, Merchan JR. Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor. Cancer Res. 2009;69(4):1459–68. doi: 10.1158/0008-5472.CAN-08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iankov ID, Blechacz B, Liu C, Schmeckpeper JD, Tarara JE, Federspiel MJ, Caplice N, Russell SJ. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15(1):114–22. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 57.Zimmermann M, Armeanu S, Smirnow I, Kupka S, Wagner S, Wehrmann M, Rots MG, Groothuis GM, Weiss TS, Konigsrainer A, Gregor M, Bitzer M, Lauer UM. Human precision-cut liver tumor slices as a tumor patient-individual predictive test system for oncolytic measles vaccine viruses. Int J Oncol. 2009;34(5):1247–56. [PubMed] [Google Scholar]
- 58.Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Parker WB, Sorscher EJ, Cattaneo R. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol Ther. 2007;15(11):1991–7. doi: 10.1038/sj.mt.6300291. [DOI] [PubMed] [Google Scholar]
- 59.Msaouel P, Iankov ID, Allen C, Morris JC, von Messling V, Cattaneo R, Koutsilieris M, Russell SJ, Galanis E. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate. 2009;69(1):82–91. doi: 10.1002/pros.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu C, Hasegawa K, Russell SJ, Sadelain M, Peng KW. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate. 2009;69(10):1128–41. doi: 10.1002/pros.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Msaouel P, Iankov ID, Allen C, Aderca I, Federspiel MJ, Tindall DJ, Morris JC, Koutsilieris M, Russell SJ, Galanis E. Noninvasive imaging and radiovirotherapy of prostate cancer using an oncolytic measles virus expressing the sodium iodide symporter. Mol Ther. 2009;17(12):2041–8. doi: 10.1038/mt.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carlson SK, Classic KL, Hadac EM, Bender CE, Kemp BJ, Lowe VJ, Hoskin TL, Russell SJ. In vivo quantitation of intratumoral radioisotope uptake using micro-single photon emission computed tomography/computed tomography. Mol Imaging Biol. 2006;8(6):324–32. doi: 10.1007/s11307-006-0058-z. [DOI] [PubMed] [Google Scholar]
- 63.Carlson SK, Classic KL, Hadac EM, Dingli D, Bender CE, Kemp BJ, Russell SJ. Quantitative molecular imaging of viral therapy for pancreatic cancer using an engineered measles virus expressing the sodium-iodide symporter reporter gene. AJR Am J Roentgenol. 2009;192(1):279–87. doi: 10.2214/AJR.08.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gauvrit A, Brandler S, Sapede-Peroz C, Boisgerault N, Tangy F, Gregoire M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68(12):4882–92. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Peng KW, Dingli D, Kratzke RA, Russell SJ. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010 doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Springfeld C, von Messling V, Frenzke M, Ungerechts G, Buchholz CJ, Cattaneo R. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 2006;66(15):7694–700. doi: 10.1158/0008-5472.CAN-06-0538. [DOI] [PubMed] [Google Scholar]
- 67.Meng X, Nakamura T, Okazaki T, Inoue H, Takahashi A, Miyamoto S, Sakaguchi G, Eto M, Naito S, Takeda M, Yanagi Y, Tani K. Enhanced antitumor effects of an engineered measles virus Edmonston strain expressing the wild-type N, P, L genes on human renal cell carcinoma. Mol Ther. 2010;18(3):544–51. doi: 10.1038/mt.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Studebaker AW, Kreofsky CR, Pierson CR, Russell SJ, Galanis E, Raffel C. Treatment of medulloblastoma with a modified measles virus. Neuro Oncol. 2010 doi: 10.1093/neuonc/noq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol. 2009;18(1):15–24. doi: 10.1016/j.suronc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 70.Dingli D, Bergert ER, Bajzer Z, O’Connor MK, Russell SJ, Morris JC. Dynamic iodide trapping by tumor cells expressing the thyroidal sodium iodide symporter. Biochem Biophys Res Commun. 2004;325(1):157–66. doi: 10.1016/j.bbrc.2004.09.219. [DOI] [PubMed] [Google Scholar]
- 71.Dingli D, Russell SJ, Morris JC. 3rd, In vivo imaging and tumor therapy with the sodium iodide symporter. J Cell Biochem. 2003;90(6):1079–86. doi: 10.1002/jcb.10714. [DOI] [PubMed] [Google Scholar]
- 72.Iankov ID, Hillestad ML, Dietz AB, Russell SJ, Galanis E. Converting tumor-specific markers into reporters of oncolytic virus infection. Mol Ther. 2009;17(8):1395–403. doi: 10.1038/mt.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heinzerling L, Kunzi V, Oberholzer PA, Kundig T, Naim H, Dummer R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106(7):2287–94. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- 74.Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, Kaur JS, Haluska PJ, Jr, Aderca I, Zollman PJ, Sloan JA, Keeney G, Atherton PJ, Podratz KC, Dowdy SC, Stanhope CR, Wilson TO, Federspiel MJ, Peng KW, Russell SJ. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70(3):875–82. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 76.Markman M, Webster K, Zanotti K, Peterson G, Kulp B, Belinson J. Survival following the documentation of platinum and taxane resistance in ovarian cancer: a single institution experience involving multiple phase 2 clinical trials. Gynecol Oncol. 2004;93(3):699–701. doi: 10.1016/j.ygyno.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 77.Stief AE, McCart JA. Oncolytic virotherapy for multiple myeloma. Expert Opin Biol Ther. 2008;8(4):463–73. doi: 10.1517/14712598.8.4.463. [DOI] [PubMed] [Google Scholar]
- 78.Ong HT, Trejo TR, Pham LD, Oberg AL, Russell SJ, Peng KW. Intravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol Ther. 2009;17(6):1012–21. doi: 10.1038/mt.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hummel HD, Kuntz G, Russell SJ, Nakamura T, Greiner A, Einsele H, Topp MS. Genetically engineered attenuated measles virus specifically infects and kills primary multiple myeloma cells. J Gen Virol. 2009;90(Pt 3):693–701. doi: 10.1099/vir.0.007302-0. [DOI] [PubMed] [Google Scholar]
- 80.Nakamura T, Peng KW, Vongpunsawad S, Harvey M, Mizuguchi H, Hayakawa T, Cattaneo R, Russell SJ. Antibody-targeted cell fusion. Nat Biotechnol. 2004;22(3):331–6. doi: 10.1038/nbt942. [DOI] [PubMed] [Google Scholar]
- 81.Schneider U, Bullough F, Vongpunsawad S, Russell SJ, Cattaneo R. Recombinant measles viruses efficiently entering cells through targeted receptors. J Virol. 2000;74(21):9928–36. doi: 10.1128/jvi.74.21.9928-9936.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hammond AL, Plemper RK, Zhang J, Schneider U, Russell SJ, Cattaneo R. Single-chain antibody displayed on a recombinant measles virus confers entry through the tumor-associated carcinoembryonic antigen. J Virol. 2001;75(5):2087–96. doi: 10.1128/JVI.75.5.2087-2096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng KW, Holler PD, Orr BA, Kranz DM, Russell SJ. Targeting virus entry and membrane fusion through specific peptide/MHC complexes using a high-affinity T-cell receptor. Gene Ther. 2004;11(15):1234–9. doi: 10.1038/sj.gt.3302286. [DOI] [PubMed] [Google Scholar]
- 84.Maisner A, Mrkic B, Herrler G, Moll M, Billeter MA, Cattaneo R, Klenk HD. Recombinant measles virus requiring an exogenous protease for activation of infectivity. J Gen Virol. 2000;81(Pt 2):441–9. doi: 10.1099/0022-1317-81-2-441. [DOI] [PubMed] [Google Scholar]
- 85.Ordman CW, Jennings CG, Janeway CA. Chemical Clinical Immunological Studies on the Products of Human Plasma Fractionation Xii. The Use of Concentrated Normal Human Serum Gamma Globulin (Human Immune Serum Globulin) in the Prevention and Attenuation of Measles. J Clin Invest. 1944;23(4):541–9. doi: 10.1172/JCI101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zingher A, Mortimer P. Convalescent whole blood, plasma and serum in the prophylaxis of measles: JAMA, 12 April, 1926; 1180–1187. Rev Med Virol. 2005;15(6):407–18. doi: 10.1002/rmv.480. discussion 418–21. [DOI] [PubMed] [Google Scholar]
- 87.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14(4):324–33. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 88.Peng KW, Dogan A, Vrana J, Liu C, Ong HT, Kumar S, Dispenzieri A, Dietz AB, Russell SJ. Tumor-associated macrophages infiltrate plasmacytomas and can serve as cell carriers for oncolytic measles virotherapy of disseminated myeloma. Am J Hematol. 2009;84(7):401–7. doi: 10.1002/ajh.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mader EK, Maeyama Y, Lin Y, Butler GW, Russell HM, Galanis E, Russell SJ, Dietz AB, Peng KW. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15(23):7246–55. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu C, Russell SJ, Peng KW. Systemic Therapy of Disseminated Myeloma in Passively Immunized Mice Using Measles Virus-infected Cell Carriers. Mol Ther. 2010 doi: 10.1038/mt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei J, Wahl J, Nakamura T, Stiller D, Mertens T, Debatin KM, Beltinger C. Targeted release of oncolytic measles virus by blood outgrowth endothelial cells in situ inhibits orthotopic gliomas. Gene Ther. 2007;14(22):1573–86. doi: 10.1038/sj.gt.3303027. [DOI] [PubMed] [Google Scholar]
- 92.Haralambieva I, Iankov I, Hasegawa K, Harvey M, Russell SJ, Peng KW. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther. 2007;15(3):588–97. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shaffer JA, Bellini WJ, Rota PA. The C protein of measles virus inhibits the type I interferon response. Virology. 2003;315(2):389–97. doi: 10.1016/s0042-6822(03)00537-3. [DOI] [PubMed] [Google Scholar]
- 94.Takeuchi K, Kadota SI, Takeda M, Miyajima N, Nagata K. Measles virus V protein blocks interferon (IFN)-alpha/beta but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 2003;545(2–3):177–82. doi: 10.1016/s0014-5793(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 95.Devaux P, von Messling V, Songsungthong W, Springfeld C, Cattaneo R. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology. 2007;360(1):72–83. doi: 10.1016/j.virol.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 96.ten Oever BR, Servant MJ, Grandvaux N, Lin R, Hiscott J. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. J Virol. 2002;76(8):3659–69. doi: 10.1128/JVI.76.8.3659-3669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hwang M, Moretti L, Lu B. HSP90 inhibitors: multi-targeted antitumor effects and novel combinatorial therapeutic approaches in cancer therapy. Curr Med Chem. 2009;16(24):3081–92. doi: 10.2174/092986709788802999. [DOI] [PubMed] [Google Scholar]
- 98.Bracarda S, Eggermont AM, Samuelsson J. Redefining the role of interferon in the treatment of malignant diseases. Eur J Cancer. 2010;46(2):284–97. doi: 10.1016/j.ejca.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 99.Dingli D, Cascino MD, Josic K, Russell SJ, Bajzer Z. Mathematical modeling of cancer radiovirotherapy. Math Biosci. 2006;199(1):55–78. doi: 10.1016/j.mbs.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Bajzer Z, Carr T, Josic K, Russell SJ, Dingli D. Modeling of cancer virotherapy with recombinant measles viruses. J Theor Biol. 2008;252(1):109–22. doi: 10.1016/j.jtbi.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 101.Dingli D, Offord C, Myers R, Peng KW, Carr TW, Josic K, Russell SJ, Bajzer Z. Dynamics of multiple myeloma tumor therapy with a recombinant measles virus. Cancer Gene Ther. 2009;16(12):873–82. doi: 10.1038/cgt.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Biesecker M, Kimn JH, Lu H, Dingli D, Bajzer Z. Optimization of virotherapy for cancer. Bull Math Biol. 2010;72(2):469–89. doi: 10.1007/s11538-009-9456-0. [DOI] [PubMed] [Google Scholar]
- 103.Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999;73(11):9568–75. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]