Summary
Background
Localized actomyosin contraction couples with actin polymerization and cell-matrix adhesion to regulate cell protrusions and retract trailing edges of migrating cells. While many cells migrate in collective groups during tissue morphogenesis, mechanisms that coordinate actomyosin dynamics in collective cell migration are poorly understood. Migration of Drosophila border cells, a genetically tractable model for collective cell migration, requires non-muscle myosin-II (Myo-II). How Myo-II specifically controls border cell migration and how Myo-II is itself regulated is largely unknown.
Results
We show that Myo-II regulates two essential features of border cell migration: 1) initial detachment of the border cell cluster from the follicular epithelium and 2) the dynamics of cellular protrusions. We further demonstrate that the cell polarity protein Par-1 (MARK), a serine-threonine kinase, regulates the localization and activation of Myo-II in border cells. Par-1 binds to myosin phosphatase and phosphorylates it at a known inactivating site. Par-1 thus promotes phosphorylated Myosin Regulatory Light Chain (MRLC), thereby increasing Myo-II activity. Furthermore, Par-1 localizes to and increases active Myo-II at the cluster rear to promote detachment; in the absence of Par-1, spatially distinct active Myo-II is lost.
Conclusions
We identify a critical new role for Par-1 kinase: spatiotemporal regulation of Myo-II activity within the border cell cluster through localized inhibition of myosin phosphatase. Polarity proteins such as Par-1, which intrinsically localize, can thus directly modulate the actomyosin dynamics required for border cell detachment and migration. Such a link between polarity proteins and cytoskeletal dynamics may also occur in other collective cell migrations.
Introduction
Cells that migrate during embryonic morphogenesis or adult wound healing often move as cohesive groups, in a process termed collective cell migration [1]. Because collective migration occurs in many cancers as part of the tumor invasion process [1, 2], a better understanding of the mechanisms that regulate this mode of migration may provide critical insights into tumor invasion and metastasis. Border cell migration in the Drosophila ovary is a powerful genetic model system to identify and dissect conserved molecular pathways that control directed collective cell migration (reviewed in [3]). During late oogenesis, the 6 to 10 follicle cell-derived border cells form a cohesive group, detach from the follicle cell monolayer epithelium, and migrate ~150 μm between the germline-derived nurse cells to the anterior border of the oocyte (Figures 1A and S1A). Proteins that regulate the actin cytoskeleton, such as cofilin and the small GTPase Rac, are essential for proper border cell migration [4, 5]. Moreover, guidance signaling through the EGF Receptor (EGFR) and the PDGF/VEGF Receptor homolog PVR promotes Rac-dependent formation of actin-rich protrusions at the front of the border cell cluster [5–7]. However, a thorough understanding of how the cytoskeleton is dynamically modulated during border cell migration is still lacking.
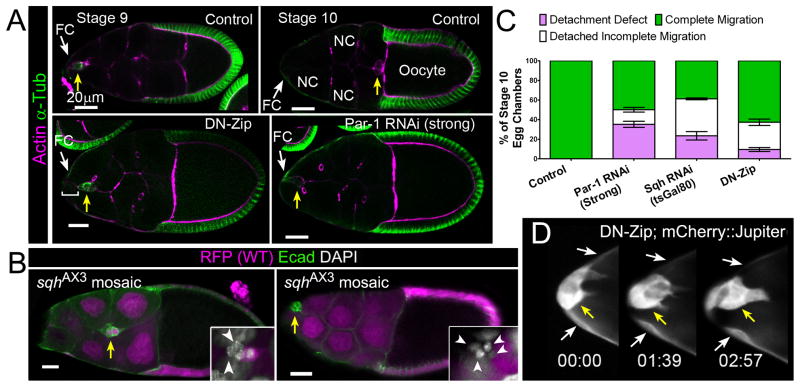
Figure 1. Myo-II Regulates Border Cell Detachment and Migration.
(A) Egg chambers (subunit of the ovary) of the indicated genotypes stained for F-actin (magenta) and α-Tubulin (green). Arrows indicate border cells (yellow) and follicle cells (FC; white). Scale bar, 20 μm. (Top) Normal border cell migration in control (c306-GAL4/+) stage 9 (left) and stage 10 (right) egg chambers. Nurse cells (NC) and oocyte are indicated. (Bottom) Stage 10 egg chambers expressing UAS-DN-Zip (left) or UAS-Par-1 RNAi Strong (right) driven by c306-GAL4; border cells did not migrate and are visibly attached to an anterior FC (bracket). (B) Border cell migration (arrows) in stage 10 sqhAX3 mosaic mutant egg chambers stained for E-cad (green) to mark cell membranes and DAPI to mark nuclei (white, insets); clones are marked by loss of nuclear mRFP (magenta). Partially mutant clusters migrate partway to the oocyte (left), whereas completely mutant clusters do not detach (right). Scale bar, 20 μm. (Insets) Magnified views of border cell clusters; mutant border cells are indicated (arrowheads). (C) Quantification of migration in stage 10 egg chambers of the indicated genotypes, shown as the percentage that did not detach (purple), or detached with incomplete (white) or complete (green) migration. Error bars represent SEM; n ≥ 350 egg chambers in ≥ 3 experiments.(D) Frames from Movie S2 showing a magnified view of the anterior end of an egg chamber expressing UAS-DN-Zip and UAS-mCherry::Jupiter at indicated times (hr:min); border cells (yellow arrow) are attached to anterior FCs (white arrows).Anterior is to the left for this and subsequent figures.
We previously demonstrated that Par-1, a cell polarity protein and serine-threonine kinase, regulates several critical aspects of border cell migration – the proper detachment of the border cell cluster from the follicular epithelium and the directional extension of cell protrusions [8]. Par-1 is known to cooperate with other polarity proteins to establish static apical-basal cell polarity, especially in epithelia. Par-1 has also been implicated in regulation of microtubule stability, Wnt signaling, and neuronal migration [9]. We determined that border cell detachment depends on negative regulation of another polarity protein, Par-3/Bazooka (Baz), by Par-1 [8]. Detachment requires Par-1-dependent restriction of Par-3/Baz to apical domains of detaching border cells. It is not clear, however, whether mutually exclusive partitioning of Par-1 and Par-3/Baz is sufficient for border cell detachment. Moreover, other aspects of border cell migration such as protrusion direction, length, and morphology are independent of Par-3/Baz but dependent on Par-1 [8, 10], suggesting that Par-1 controls these processes through other partners.
Non-muscle myosin II (Myo-II) regulates cell migration [11] by inducing localized contraction of the actin cytoskeleton, establishing migrating cell polarity, modulating cell adhesions, and retracting trailing edges. In Drosophila, Myo-II is required for epithelial remodeling and movement during tissue and organ formation, such as occurs during dorsal closure, gastrulation and border cell migration [12, 13]. Myo-II contains two copies of each of three subunits: the Drosophila heavy chain (MHC) is Zipper (Zip), the essential light chain is Myosin light chain-cytoplasmic (Mlc-c), and the regulatory light chain (MRLC) is Spaghetti squash (Sqh). The latter is targeted by Rho-associated Kinase (Rok; also known as ROCK) and Myosin Light Chain Kinase (MLCK) [11, 14], which phosphorylate MRLC/Sqh at Thr-18/Ser-19 (Thr-19/Ser-20 in Drosophila). Phosphorylation of MRLC increases the ATPase activity of the MHC and stabilizes myosin-containing filaments [11]. Activation by Rok further helps localize Myo-II to cortical cell membranes and is required for Myo-II function in vivo [15–18]. Conversely, myosin phosphatase dephosphorylates MRLC/Sqh to inactivate Myo-II [19].
In this study, we show that active Myo-II modulates both border cell cluster detachment and the refinement of cellular protrusions important for cell motility. In turn, Par-1 promotes the localized phosphorylation and activation of Myo-II. We show that Par-1 activates Myo-II by phosphorylating and inhibiting the myosin phosphatase complex. While myosin phosphatase is uniformly distributed, Par-1 is localized to the rear of the detaching cluster, resulting in phosphatase inhibition and Myo-II activation at the cluster rear. Our results thus show for the first time that a Par-1/Myo-II regulatory pathway connects dynamic cell polarity with cytoskeletal dynamics to regulate collective cell migration.
Results
Myo-II is Required for Border Cell Detachment and Protrusion Dynamics
Previous studies found that global loss of sqh (Drosophila MRLC) throughout the ovary strongly inhibited border cell migration [13]. In addition, single border cells mutant for sqh extended long, abnormal protrusions [20]. These studies, however, did not specifically address which cells require Myo-II nor whether Myo-II regulates other aspects of border cell migration. To further investigate Myo-II function in border cell migration, we performed mosaic clonal analysis using a sqh null allele, sqhAX3 [17]. Border cell clusters containing at least one mutant border cell exhibited migration defects and were found anywhere from 25% to 70% of the normal migration distance (Figure 1B and not shown). These mutant border cells were usually located at the back of the cluster (68%, n = 28), similar to other mutants that disrupt border cell motility [6]. In most of these egg chambers, anterior epithelial follicle cells were also mutant (Figure 1B and not shown). Only a few clusters were observed in which all border cells were mutant (13%, n = 32); in each case, the cluster failed to detach from the epithelium and did not move away from the anterior end of the egg chamber (Figure 1B). We then confirmed that activation of Myo-II is important. We performed mosaic clonal analysis with a strongmutant allele of rok, rok2 [15], which is a known Myo-II activator. We observed comparable migration defects in egg chambers with rok mosaic mutant clones (n = 15; not shown). These results are consistent with a role for active Myo-II in border cells and possibly anterior follicle cells.
To better understand why border cells defective for Myo-II do not migrate, we performed live time-lapse imaging of egg chambers in culture [7]. Since completely mutant sqh or rok border cell clusters were difficult to obtain (not shown), we knocked down the function of Myo-II in all border cells using the GAL4/UAS system. A dominant-negative form of Zip (DN-Zip), which lacks the MHC catalytic motor domain, disrupts the formation of functional Myo-II [21]. Expression of DN-Zip in border cells and follicle cells using the follicle cell specific driver c306-GAL4 (Figure S1A) significantly inhibited border cell detachment and migration in fixed egg chambers (Figures 1A and 1C). Thus, loss of the MHC Zip is similar to loss of MRLC/Sqh in sqh loss-of-function mosaic clones or Sqh RNAi (Figure 1). Using an alternative border cell driver, slbo-GAL4, we further confirmed that Myo-II was required in border cells (Fig. S1B). The central polar cells trigger JAK/STAT signaling to recruit surrounding cells to form the border cell cluster and stimulate their motility [3]. Specific knock down of Myo-II in polar cells by upd-GAL4 did not disrupt border cell migration (Fig. S1C).
In live egg chambers, control (wild-type) border cells extend protrusions in the direction of migration, detach from the epithelium, and migrate to the oocyte over the course of 4–6 hr (Movie S1) [7]. Control protrusions before detachment are longer in length and lifetime than those that extend after border cells detach and begin to migrate (Figures S1E and S1F). Expression of DN-Zip disrupted the ability of some border cells to properly detach from the follicle cell epithelium and move forward (Figure 1D; Movie S2). DN-Zip did not affect the direction of protrusion extension (not shown). However, DN-Zip border cell clusters that did not detach initially had normal protrusions, but these protrusions failed to resolve into protrusions of shorter length and duration like in control (Figures S1E and S1F). Notably, DN-Zip border cell clusters that detached initially extended protrusions with normal characteristics, but after detachment protrusions were slightly longer in length (P = 0.04; unpaired two-tailed t-test) and had significantly longer lifetime (P = 0.001) than the control (Figures S1E and S1F).
Activated Myo-II is Required Downstream of Par-1 in Border Cell Migration
Defects in border cell detachment were also found in border cells in which Par-1 function was disrupted, either by c306-GAL4-driven RNAi knock down (Figures 1A and 1C) or loss-of-function mosaic clones [8]. Moreover, previously published studies of border cells mutant for sqh [20] and par-1 [8] reported examples of similar, misshapen long protrusions. We hypothesized that Par-1 and Myo-II may act in the same pathway to regulate border cell migration. In support of this, we identified a genetic interaction between par-1 and sqh (Figure 2A). Weak expression of RNAi transgenes for Par-1 or Sqh (UAS-RNAi driven by c306-GAL4) each mildly disrupted border cell migration. Co-expression of weak RNAi for both Par-1 and Sqh dramatically increased the migration defects by 2.5-fold over the expected additive phenotype. par-1 and rok also exhibited a genetic interaction (Figure 2B). Stronger RNAi knock down of Par-1 (UAS-Par-1 RNAi driven by c306-GAL4 in a heterozygous par-1 mutant background) significantly disrupted border cell detachment, whereas Rok RNAi disrupted detachment less frequently. Co-expression of RNAi for both Par-1 and Rok enhanced the detachment defect to almost twice the expected additive phenotype. These results indicate that Rok and Par-1 both regulate border cell detachment, potentially through a common substrate such as Myo-II.
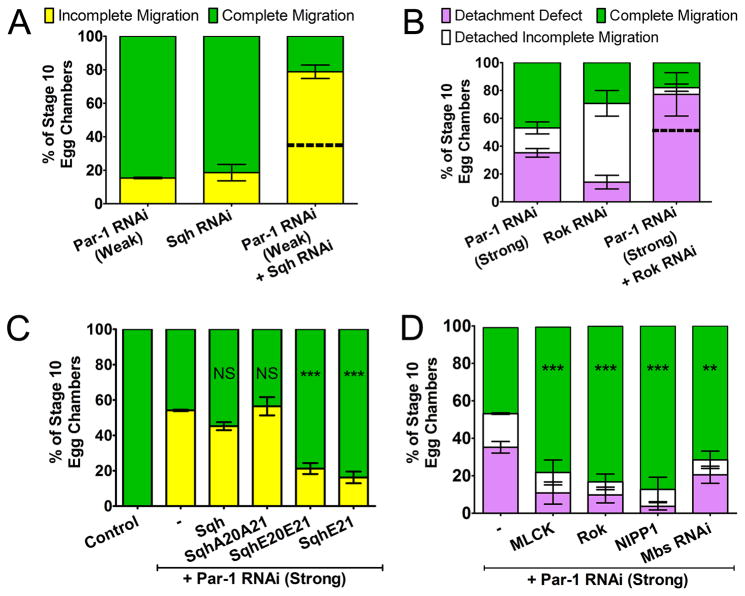
Figure 2. Activated Myo-II is Required Downstream of Par-1.
Quantification of migration in stage 10 egg chambers of the indicated genotypes is shown as the percentage of incomplete (yellow) versus complete (green) migration (A, C) or the percentage with detachment defects (purple) versus detached incomplete (white) or complete (green) migration (B, D). (NS) P ≥ 0.05; (**) P ≤ 0.01; (***) P ≤ 0.001 (unpaired two-tailed t test); error bars represent SEM. (A, B) Genetic interaction between sqh and par-1 (A) and between rok and par-1 (B); predicted additive effects (dotted line) are shown. (A) N ≥ 199 egg chambers in ≥ 3 experiments (P< 0.001). (B) N ≥ 81 egg chambers in ≥ 3 experiments (P < 0.01 for detachment defect). (C) Single copies of Sqh wild-type or mutant transgenes in the Par-1 RNAi (strong) background; n ≥ 153 egg chambers in ≥ 3 experiments. (D) Overexpression of MLCK, Rok or the PP1c inhibitor NIPP1, or knock down of Mbs by RNAi in the Par-1 RNAi (Strong) background; n ≥ 160 egg chambers in ≥ 3 experiments. Par-1 RNAi (Strong) migration defects (Figure 1C) are shown for comparison (B–D).
The interaction between par-1 and sqh led us to investigate whether Myo-II could suppress the migration defects caused by loss of par-1. We used a series of transgenes expressing MRLC/Sqh under the control of the endogenous sqh promoter [15, 17, 22]. These transgenes are either unmutated (wild-type), singly mutated at the primary phosphorylation site (Ser-21), or doubly mutated at the primary and secondary (Thr-20) phosphorylation sites. Phosphomimetic, active forms of Sqh (mutation to glutamic acid; E20E21 or E21) and non-phosphorylatable, inactive Sqh (mutation to alanine; A20A21) have been shown to respectively rescue or enhance phenotypes caused by loss of the Myo-II activator rok in Drosophila [15].
Expression of a single copy of any of the sqh transgenes in a wild-type background did not disrupt border cell migration (Figure S2A). Neither non-phosphorylatable sqhA20A21 nor wild-type sqh transgenes suppressed the Par-1 RNAi phenotype (Figure 2C). Conversely, a single copy of either of the phosphomimetic mutant transgenes (sqhE21 or sqhE20E21)strongly suppressed the Par-1 RNAi migration defects (Figure 2C). Importantly, none of the transgenes suppressed the migration defects caused by disruption of Par-3/Baz (Figure S2B), a known target of Par-1 in polarized cells [23] and border cells [8]. Furthermore, sqh-E21 did not rescue the border cell motility or detachment defects found in mutants of two other genes known to regulate border cell migration (Figures S2C and S2D). These results suggest that activated Myo-II is specifically required downstream of Par-1. In agreement with this, overexpression of Par-1 did not rescue the migration defects caused by Sqh RNAi (Figure S2E).
We next determined whether activation of endogenous Myo-II could suppress the phenotypes caused by disruption of Par-1 function. Activation of Myo-II can occur either by increasing Myo-II kinase levels or by inactivating myosin phosphatase [11]. Ectopic expression of the Myo-II kinases MLCK or Rok in vivo leads to greater phosphorylation of MRLC/Sqh and activation of endogenous Myo-II [11, 24] (see below). Conversely, myosin phosphatase inactivates Myo-II by dephosphorylating MRLC/Sqh and consists of a type 1 protein phosphatase catalytic subunit (PP1c), a subunit M20 of unknown function, and the substrate-specific myosin binding subunit Mbs (Mbs; also known as MYPT). Therefore, inactivation of myosin phosphatase increases the activity of Myo-II in cells [19, 25] (see below). To inhibit myosin phosphatase function in vivo, we expressed an inhibitor of PP1c, Nuclear Inhibitor of PP1 (NIPP1) [26], or Mbs RNAi. Increasing the levels of activated Myo-II in a wild-type background, either by overexpressing Myo-II kinases or inhibiting myosin phosphatase, disrupted both border cell migration and detachment from the epithelium (Figure S2F). Thus, border cells, like many migrating cells [11], require precise levels and/or distribution of active Myo-II for normal movement. Nonetheless, overexpression of Myo-II kinases or inhibition of myosin phosphatase efficiently suppressed the Par-1 RNAi-induced border cell detachment and migration defects (Figure 2D). These results confirm that activation of Myo-II rescues the detachment and migration defects caused by loss of par-1.
Par-1 Regulates the Distribution and Dynamics of Myo-II in Border Cells
It has been noted that Myo-II accumulates at cortical membranes of migrating border cells [13]. Using time-lapse imaging, we examined the subcellular distribution of a functional GFP-tagged Sqh transgene driven by its own promoter (Sqh:GFP). This transgene rescues sqh mutant flies to viability and accurately reports the localization of Myo-II in vivo [22]. Sqh:GFP is highly expressed in live border cells throughout their migration (Movie S3) and accumulates in transient, intense fluorescent cortical puncta or “foci” (Figure 3A and Movie S4); these foci resembled those described in a variety of epithelia [12, 27–29]. Sqh:GFP foci were particularly evident where the cluster displayed an obvious change in morphology, such as at the cluster rear during detachment (Movie S3; see below). Control border cell clusters had an average of 6 foci per cluster with a median lifetime of 92 seconds (Figures 3A, 3C–D; Movie S4).
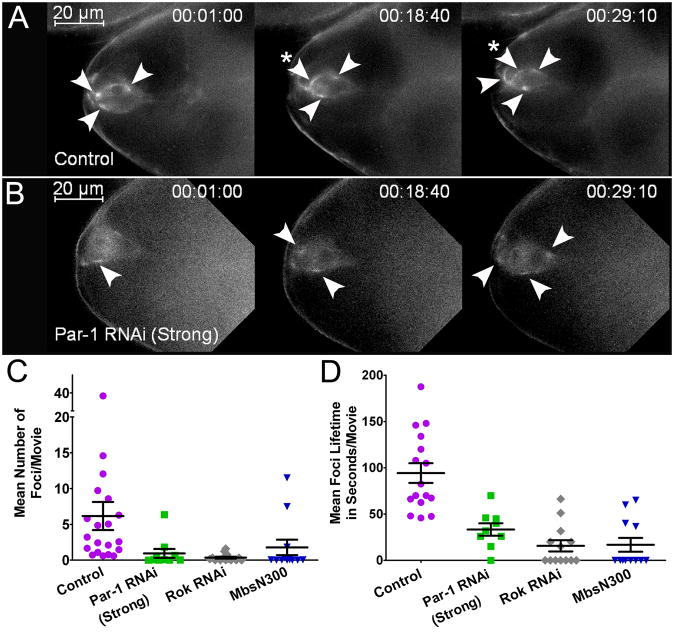
Figure 3. Myo-II Forms Localized Foci in a Par-1-dependent Manner.
(A–B) Frames from time-lapse control (A) and Par-1 RNAi Strong (B) movies (Movie S4) showing Sqh:GFP localization in border cells before detachment at the indicated times (hr:min:s). Transiently enriched foci (arrowheads) were observed throughout the movies. A stable focus is indicated with an asterisk in (A). Par-1 RNAi border cells have fewer foci and Sqh:GFP is generally more diffuse than control. Scale bar, 20 μm. (C–D) Quantification of mean Sqh:GFP foci number (C) and lifetime (D) in movies of the indicated genotypes. Each point represents the average of individual foci measured within a single movie. N ≥ 9 movies for each genotype; P ≤ 0.0001 (unpaired two-tailed t test). Error bars represent SEM. See Supplemental Experimental Procedures for details on analyses.
We asked whether Par-1 influences Sqh:GFP foci in border cells (Figures 3B–D; Movie S4). Sqh:GFP was more diffuse and fewer foci formed in Par-1 RNAi border cells. The foci that formed had a shortened median lifetime (Figure 3D). To determine whether Sqh:GFP foci formed in response to activated Myo-II, we knocked down Rok or expressed a constitutively active form of myosin phosphatase (MbsN300) [30]. In both genotypes, fewer Sqh:GFP foci formed and they were less persistent (Figures 3C and 3D; Movie S5 and not shown), suggesting that Sqh:GFP foci dynamics depend on Myo-II activation. Thus, Par-1 regulates the localized formation and/or stability of Myo-II foci in border cells.
Par-1 Regulates the Levels of Activated Myo-II in Border Cells
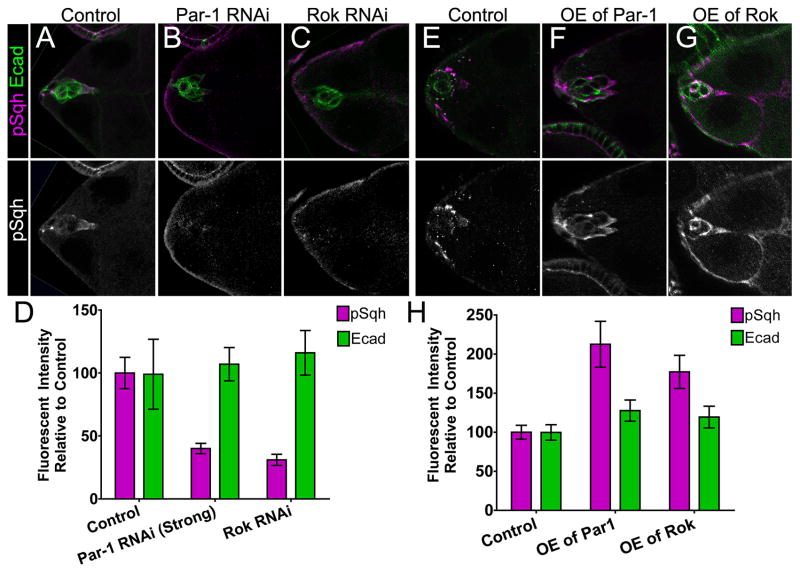
To determine whether Par-1 influences Myo-II activity in vivo, we examined the levels of activated MRLC/Sqh in border cells. We used an antibody that specifically recognizes MRLC/Sqh when it is phosphorylated at the activating Ser-21 (p-MRLC/Sqh) [11, 17, 18]. Control border cells expressed high levels of p-MRLC/Sqh at the cell cortex (Figure 4A). Border cells in which Par-1 (Figure 4B) or the known activator Rok (Figure 4C) were knocked down by RNAi had reduced levels of p-MRLC/Sqh compared to control (Figure 4D). In contrast, levels of the cell-junction protein E-cadherin were unaffected (Figures 4A–D). Importantly, the overall levels of endogenous MRLC/Sqh were unchanged when Par-1 was ubiquitously knocked down (Figure S3A). Conversely, Par-1- or Rok-overexpressing border cells had quantifiably higher levels of p-MRLC/Sqh compared to control, but E-cadherin levels were unchanged (Figures 4E–H). Furthermore, Mbs RNAi also significantly increased p-MRLC/Sqh levels (Figures S3B and S3C), confirming that myosin phosphatase inhibits Myo-II activation in border cells. Together, these results show that Par-1 promotes phosphorylation and activation of MRLC/Sqh in vivo.
Figure 4. Par-1 Regulates Levels of Phosphorylated MRLC/Sqh in vivo.
(A–C, E–G) Representative egg chambers stained for p-MRLC/Sqh (magenta, top panels; white, bottom panels) and E-cadherin (E-cad, green in top panels) in the indicated genotypes. (D, H) Quantification of fluorescence intensity in border cells relative to control. Samples were prepared at the same time and images captured using the same camera exposure. Brightness or contrast of images were adjusted for better visualization, but were not used for quantification. See Supplemental Experimental Procedures for detailed description of the analyses. (D) Loss of Par-1 or Rok reduced p-MRLC/Sqh; n ≥ 14 egg chambers in ≥ 2 experiments (P ≤ 0.0001; unpaired two-tailed t test). E-cadherin levels were not altered (P ≥ 0.69). (H) Overexpression of Par-1 or Rok increased p-MRLC/Sqh levels; n ≥ 10 egg chambers in 2 experiments (P = 0.0001; unpaired two-tailed t test). E-cadherin levels were not altered (P ≥ 0.09).
Par-1 Interacts with and Phosphorylates the Myosin Phosphatase Complex
We next investigated the mechanistic basis for how Par-1 controls the levels of activated MRLC/Sqh. Kinases such as Rok activate Myo-II directly by phosphorylating MRLC/Sqh and indirectly by inactivating myosin phosphatase [19, 24, 25], whereas other kinases, such as NUAK1 and MRCK, primarily inactivate myosin phosphatase [31, 32]. Myosin phosphatase is inhibited by phosphorylation of the myosin binding subunit, Mbs, at several sites including Thr-594 (Thr-696 in mammalian MYPT1) [19, 25]. We did not detect Par-1 and Sqh in a protein complex; GFP-tagged Par-1 or Sqh:GFP did not co-immunoprecipitate the respective endogenous proteins from fly extracts (not shown). Notably, Par-1 was present in an immunocomplex with myosin phosphatase (Figures 5A, 5B, S4A and S4B). Endogenous Mbs was immunoprecipitated from S2 cell (Figure 5A) and ovarian (Figure S4A) lysates with GFP-tagged Par-1. Conversely, endogenous Par-1 was immunoprecipitated with HA-tagged Mbs from S2 cell lysates (Figure 5B). Flapwing (Flw/PP1β9C; PP1c homolog), a PP1 catalytic subunit that specifically regulates Myo-II [33], also immunoprecipitated endogenous Par-1 and Mbs from ovarian and whole fly extracts (Figure S4B, S4C, and not shown).
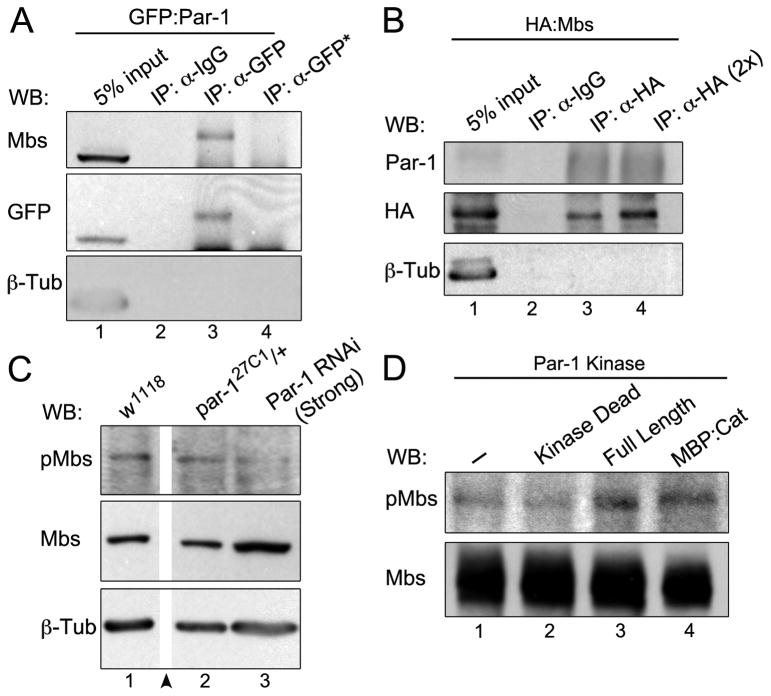
Figure 5. Par-1 is Present in a Complex with Myosin Phosphatase and Regulates Mbs Phosphorylation.
(A) Extracts from S2 cells expressing GFP:Par-1 were immunoprecipitated with anti-GFP antiserum (IP: α-GFP) or non-specific antibody as control (IP: α-IgG). Blots were probed for Mbs and stripped and reprobed for GFP and β-Tub. Extracts from untransfected S2 cells were used as a specificity control (lane 4; IP α-GFP*). (B) Extracts from S2 cells expressing HA:Mbs were immunoprecipitated with anti-HA antiserum (IP: α-HA) or non-specific antibody as control (IP: α-IgG). Twice the amount of extract was used in lane 4 (IP: α-HA 2x). Blots were probed for Par-1 and HA and stripped and reprobed for β-Tub. (C) Ovarian lysates from the indicated genotypes were subjected to Western blots with anti-p-Mbs (p-T594) and Mbs antibodies. Par-1 RNAi (Strong) reduced p-Mbs levels compared to controls (w1118 or par-127C1/+). Intervening lanes on the blot were removed for clarity (arrowhead). (D) In vitro kinase assays using either GFP:Par-1 (Kinase Dead or Full Length) immunopurified from whole fly extracts or bacterially expressed and purified MBP:Par-1 catalytic (kinase) domain as sources of kinase. HA:Mbs was purified from S2 cells and used as the substrate. Blots were probed for p-T594 Mbs and Mbs. Active Par-1 kinase increased the levels of p-T594 Mbs (lanes 3 and 4).
To test whether Par-1 regulates myosin phosphatase activity, we used an antibody that recognizes phosphorylated Drosophila Mbs at a known inhibitory site (p-T594 Mbs; Figure S4D) [19]. We observed a decrease in p-T594 Mbs levels in ovarian lysates when Par-1 was ubiquitously knocked down (Figure 5C). To test whether Par-1 regulates phosphorylation of Mbs, we performed an in vitro kinase assay using immunopurified HA:Mbs as the substrate (Figure 5D). Addition of GFP-Par-1 purified from fly lysates or bacterially purified Par-1 catalytic domain [23] increased the levels of p-T594 Mbs in vitro, whereas kinase-dead Par-1 did not (Figure 5D). Thus, Par-1 phosphorylates Mbs at the known Thr-594 inhibitory site.
Par-1 Promotes Polarized Distribution of Activated Myo-II in the Border Cell Cluster
Border cells retain epithelial polarity, especially before they detach from the epithelium [8, 10], with clear basolateral (back) and apical (front) sides. Par-1 localizes to the basolateral side of the cluster (Figure 6A) [8]. While Mbs has a fairly uniform cytoplasmic and cortical localization within the cluster, Mbs overlaps with Par-1 specifically at the basolateral side (Figure 6A). In addition, we observed increased Sqh:GFP signal at the basolateral side of the cluster as it detached (Figure 6B). Fixed wild-type border cell clusters also exhibited polarized enrichment of p-MRLC/Sqh (Figures 4A, 6C and 6D). In some cases this biased enrichment was along the axis of migration at both the apical and basolateral sides of the cluster (front/back bias), whereas in others it occurred at the sides, perpendicular to the axis of migration (side bias) (Figure 6C).
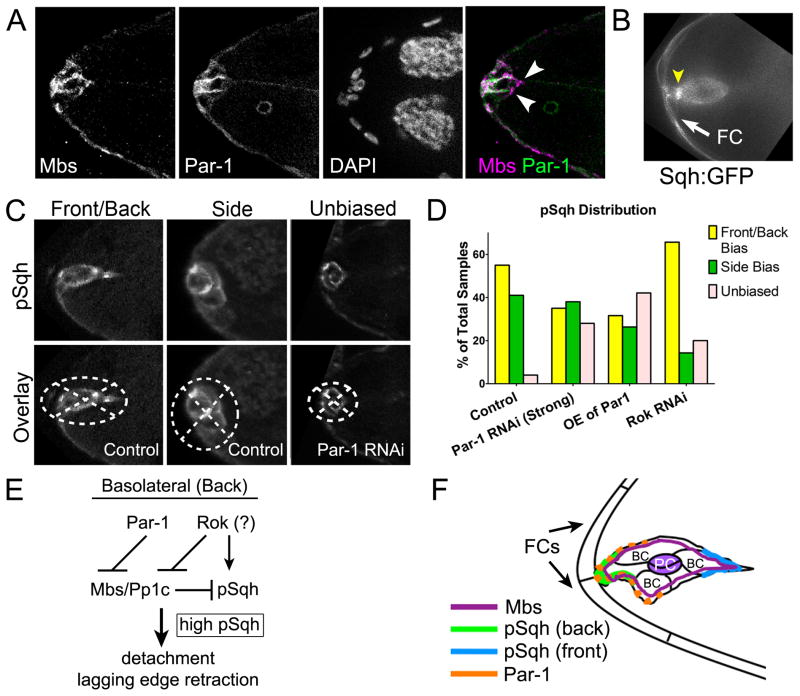
Figure 6. Active Myo-II is Polarized Within the Border Cell Cluster.
(A) Stage 9 wild-type egg chamber stained for Mbs (magenta), Par-1 (green), and DAPI to label nuclei. Mbs is uniform in the border cell cluster and overlaps with Par-1 (white) at the basolateral/back but not at the apical/front (arrowheads). (B) Frame from a time-lapse movie showing an intense Sqh:GFP foci (arrowhead) at the basolateral/back of the cluster during detachment from an anterior follicle cell (white arrow; observed in 7/9 movies). (C, D) Polarized distribution of p-MRLC/Sqh in border cells. (C) Border cell clusters are overlaid with the quadrants (dotted lines) used to measure fluorescence distribution (see Supplemental Experimental Procedures). Brightness/contrast of images (from experiments shown in Figure 4) were adjusted for better visualization, but not used for quantification. (D) Quantification of p-MRLC/Sqh distribution in the indicated genotypes. (E, F) Molecular pathway (E) and model (F) for Myo-II activation at the basolateral/back of border cells. See text for details.
The polarized localization of Par-1 led us to ask whether Par-1 contributes to polarized accumulation of p-MRLC/Sqh in border cells before they detach. Although the overall levels of p-MRLC/Sqh were significantly reduced when Par-1 was knocked down (Figures 4B and 4D), some cortical p-MRLC/Sqh was still detected (Figures 6C and 6D); however, p-MRLC/Sqh signal was less polarized (unbiased) than control (Figure 6D). Par-1 overexpression caused a similar unbiased distribution, indicating that overall levels of Par-1 activity are important. In contrast, border cell clusters depleted of Rok by RNAi retained polarized p-MRLC/Sqh, raising the possibility that Rok regulates p-MRLC/Sqh levels but not its distribution. These results indicate that Par-1 not only regulates the levels of activated MRLC/Sqh, but also is important for the polarized enrichment of p-MRLC/Sqh in detaching border cells.
Discussion
Myo-II plays a fundamental role in establishing the front-rear axis of migrating cells to promote directional migration [11]. Myo-II localizes to the cell rear and stimulates motility at the front [34, 35], likely by local stabilization of adhesions and actomyosin bundles at the cell rear but not at the front [36]. In contrast to single cells, the mechanisms that set up or maintain polarized actomyosin contraction during collective migration are still poorly understood. We identified a new role for Par-1 kinase, namely that Par-1 regulates myosin phosphatase to control Myo-II activation. We propose a model in which Myo-II is activated in a polarized manner (Figures 6E and 6F). Myosin phosphatase, which is distributed uniformly in the cluster, is locally inactivated by Par-1 at the basolateral/back side of the cluster. The consequent polarization of active Myo-II induces contraction and cell morphological changes critical for detachment and motility. The question of how Par-1 becomes localized to the basolateral side of border cells is largely unknown. Phosphorylation by the apical polarity protein aPKC restricts Par-1 to basolateral membranes in epithelial cells [37] and is also critical for Par-1 function in border cells [8]. This mechanism may thus restrict basolateral localization of Par-1 in border cells, although a role for border cell-specific factors cannot be ruled out.
Our observation that there is an increase in Sqh:GFP at the rear of the border cell cluster during detachment is consistent with a specific role for Myo-II in promoting epithelial detachment of border cells. Loss-of-function sqh mosaic clone experiments demonstrated a requirement for Myo-II in border cells and possibly adjacent epithelial follicle cells. Indeed, live imaging analyses revealed that disruption of Myo-II function inhibited the ability of border cells to detach. This raises the question of how Myo-II contributes to detachment. Activation of Rok by Rho GTPase can destabilize cell-cell junctions by inducing actomyosin contraction in normal and tumor-derived epithelial cells [38]. In other contexts, however, Rho-dependent Myo-II stabilizes cell junctions through regulation of the junctional protein E-cadherin [39]. The overall levels of E-cadherin were unchanged when Rok was knocked down in border cells, suggesting that activated Myo-II more likely contributes directly to detachment. We suggest that the localized increase in active Myo-II at the rear specifically contracts the border cell cluster and helps it pull away from the epithelium. In the absence of Par-1, overall levels of activated Myo-II were decreased and Sqh:GFP foci, which correlate with active Myo-II, exhibited altered dynamics; this potentially leads to uncoordinated or decreased contractile forces and thus to defects in detachment.
Par-1 promotes increased p-MRLC/Sqh levels and higher levels of activated myosin by phosphorylation of myosin phosphatase at a known inactivating threonine. Regulation of myosin phosphatase activity is essential for many cellular processes, including cell motility and epithelial morphogenesis [19]. Despite identification of kinases that inactivate myosin phosphatase in vitro, few have been shown to do so in vivo during migration. Notably, vertebrate MARK2 (Par-1) phosphorylates the Mbs homolog MYPT1 in vitro at several sites, including the conserved threonine examined here [31], although this has not been confirmed in vivo. This raises the intriguing possibility that vertebrate Par-1 homologs regulate myosin phosphatase, and thus Myo-II, during cell migration. However, Par-1-mediated regulation of Myo-II phosphorylation via myosin phosphatase may be cell- or context-specific. In contrast to the situation in border cells, Par-1 does not colocalize with myosin phosphatase in Drosophila ovarian epithelial follicle cells; active p-MRLC/Sqh and Mbs localized to apical domains in follicle cells whereas Par-1 localizes to basolateral membranes [18, 23] (not shown).
Active Myo-II accumulates at the apical side/front of the border cell cluster in addition to its localization at the rear. Myo-II that is localized near the leading edge of single cells has been proposed to promote retraction by coordinating cell-substrate adhesions with the actin cytoskeleton [40, 41]. Likely roles for Myo-II at apical/front side of the border cell cluster include retraction of protrusions [20], as well as resolving protrusion dynamics from the pre- to post-detachment phases of migration (this study). Our data do not explicitly support a role for Par-1 at the apical side of the cluster. Moreover, in the absence of Par-1 we detected a low level of phosphorylated MRLC/Sqh that was still partially localized. Thus, Myo-II is activated by at least one other kinase in addition to Par-1.
We hypothesize that Par-1 promotes higher levels of Myo-II activity at the basolateral/back, whereas another kinase activates Myo-II (and/or inactivates the phosphatase) specifically at the front. Rok can phosphorylate both MRLC and myosin phosphatase [24, 25]. Knock down of Rok by RNAi significantly reduced p-MRLC/Sqh levels and disrupted border migration. The combined depletion of both Par-1 and Rok almost completely abolished detachment, suggesting that the two kinases converge (directly or indirectly) on the same target (Myo-II). Epithelial morphogenesis during C. elegans embryonic elongation and Drosophila larval tissue development require multiple kinases to optimally activate Myo-II [42, 43]. Furthermore, different kinases have been shown to regulate MRLC activation at discrete locations within single migrating cells. For example, MLCK phosphorylates MRLC at the front/leading edge, while Rok targets MRLC in the cell body and at the trailing edge of fibroblasts [14]. However, it remains to be determined whether Rok and/or additional kinases have a polarized or more general role in Myo-II activation in border cells.
Our study demonstrates that maintaining localized Myo-II activity is a critical feature of collective cell detachment and motility and identifies the conserved polarity kinase Par-1 as a key new regulator of this pathway. Active Myo-II is polarized within the border cell cluster, rather than in individual border cells, emphasizing that asymmetrically activated Myo-II contributes to collective behavior. Notably, in a model of collective cancer cell invasion, high actomyosin activity at cell-matrix contacts combined with low activity at contacts between cells within the group, produced optimal contractile force around the outside and thus promoted collective cell movement [44]. It will be important to determine whether vertebrate Par-1 homologs also regulate actomyosin contraction during processes that depend on collective cell motility, such as wound healing or tumor invasion and metastasis. Given that many metastasizing tumors detach from epithelia both as single cells and collective groups, it will be important to further probe the mechanisms of myosin-mediated contraction in this process.
Experimental Procedures
Drosophila Genetics
Crosses were performed at 25°C, except tsGAL80 crosses were at 18 °C. Flies were incubated at 29°C for ≥ 14 hr before dissection for optimal GAL4/UAS transgene expression. Strain information is in the Supplemental Experimental Procedures. GAL4 lines outcrossed to w1118 were used as controls. The genotype for Par-1 RNAi (Strong) is c306-GAL4/+; +/par-127C1; +/UAS-Par-1 RNAi and for Par-1 RNAi (Weak) is c306-GAL4/+; +/UAS-Par-1 RNAi. Sqh RNAi (Strong) genotype is sqhAX3/+; UAS-Sqh RNAi/+; equivalent phenotypes were obtained with UAS-Sqh RNAi (tsGAL80). Mosaic mutant clones of sqhAX3 [17]and rok2 [15] were generated with the FLP/FRT system using ubi-mRFP.nls, hsFLP, FRT 19A.
Immunofluorescence and Live Imaging
Ovary dissection and staining methods were performed as described [8]. Fixation was in 4% formaldehyde in phosphate buffer (pH 7.4) for 10 min; for p-MRLC/Sqh staining, ovaries were fixed in 8% formaldehyde in PBS for 5 min. The following primary antibodies were used: rat anti-DE-cadherin 1:25 (DCAD2; Developmental Studies Hybridoma Bank, DSHB); mouse anti-α-Tubulin 1:400 (DM1A, Sigma); guinea pig anti-Par-1 1:10 [8]; rabbit anti-Mbs 1:1000 (from C. Tan); rabbit anti-pSer19 MRLC 1:10 (p-MRLC/Sqh; Cell Signaling Technologies). Secondary antibodies or phalloidin (to label F-actin) conjugated to Alexa Fluor 488 or Alexa Fluor 568 (1:400; Invitrogen) were used. DAPI (0.05 μg/mL; Sigma) was used to stain nuclei.
Ovary dissection, live culturing, and time-lapse imaging were performed as described [7]. Imaging was performed on a Zeiss AxioImager Z1 motorized upright microscope with a Zeiss Colibri LED light source (live imaging), or HXP 120 light source and the ApoTome system (fixed samples), and a Zeiss AxioCam MRm CCD camera controlled with AxioVision 4.8.2 software. Movies S1 and S2 were acquired using 75–100% LED power (LED module 540–580 nm) at 3-min intervals for 4–5 hr, either using a Plan-Apochromat 20× 0.75 NA or a Plan-Apochromat 10× 0.45 NA objective. For Movies S4 and S5, time-lapse images were captured using 25% LED power (LED module 455) at 10-sec intervals for 30 min, and 10 z-stacks were acquired at 0.5 μm intervals using a Plan-Neofluar 40× 1.3 NA oil objective. For Movie S3, images were acquired on a Nikon TE-2000 microscope using a 20× 0.75 NA objective and the PerkinElmer UltraVIEW VoX spinning disk confocal with a Hamamatsu cooled 14-bit EMCCD C9100-50 camera controlled by PerkinElmer Volocity software. Descriptions of the image analyses are in the Supplemental Experimental Procedures.
Immunoprecipitation, Western Blot Analysis and In Vitro Kinase Assays
For immunoprecipitations, samples were lysed in homogenization buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2.5 mM EDTA, 0.1% Triton X-100) supplemented with Halt protease and phosphatase inhibitor cocktail (Thermo Scientific). 5 μg of antibody IgG (rabbit anti-GFP or anti-HA; Invitrogen) per milligram of magnetic Dynabeads Protein A (Invitrogen) were preincubated before adding lysate. Lysate was added to antibody-bound beads and incubated at 4°C overnight, washed in homogenization buffer and prepared for SDS-PAGE followed by Western blot analysis using standard protocols. In vitro kinase assays were performed as described [45] on isolated HA:Mbs protein using purified Par-1 kinase. Details on sample preparation, antibodies and Western blotting are in the Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Active Myo-II promotes border cell epithelial detachment and protrusion dynamics
Par-1 regulates Myo-II dynamics and activity levels in collective cell movement
Par-1 kinase phosphorylates inactivating site of myosin phosphatase
Localized Par-1 establishes spatially defined active Myo-II to promote migration
Acknowledgments
We thank Drs. R. Karess, D. St Johnston, D. Kiehart, M. VanBerkum, J. Treisman, L. Luo, V. Riechmann, C. Tan, A. Wodarz, B. Lu, G. Longmore, and S. Horne-Badinovac, the Bloomington Stock Center, the Vienna Drosophila RNAi Center, the NIG-Fly Stock Center, the Drosophila Genomics Resource Center and the Developmental Studies Hybridoma Bank at the University of Iowa for fly stocks, antibodies, and plasmid DNA. Special thanks to Drs. M. Starz-Gaiano, S. Misra and A. Zhu for helpful advice and discussions; and to A. Vasanji of ImageIQ for help with custom image analyses. This work was supported by NIH/NIGMS R01GM078526 and by American Recovery and Reinvestment Act (ARRA) funds through this grant (J.A.M.); and NIH/NICHD training grant T32 HD007104 (G.A.).
Footnotes
Supplemental Data include Supplemental Experimental Procedures, four figures, and five movies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res. 2010;8:629–642. doi: 10.1158/1541-7786.MCR-10-0139. [DOI] [PubMed] [Google Scholar]
- 3.Montell DJ. Border-cell migration: the race is on. Nat Rev Mol Cell Biol. 2003;4:13–24. doi: 10.1038/nrm1006. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Luo J, Wan P, Wu J, Laski F, Chen J. Regulation of cofilin phosphorylation and asymmetry in collective cell migration during morphogenesis. Development. 2011;138:455–464. doi: 10.1242/dev.046870. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, He L, Wu YI, Hahn KM, Montell DJ. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol. 2010;12:591–597. doi: 10.1038/ncb2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, Rorth P. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 2007;448:362–365. doi: 10.1038/nature05965. [DOI] [PubMed] [Google Scholar]
- 7.Prasad M, Montell DJ. Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev Cell. 2007;12:997–1005. doi: 10.1016/j.devcel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 8.McDonald JA, Khodyakova A, Aranjuez G, Dudley C, Montell DJ. PAR-1 kinase regulates epithelial detachment and directional protrusion of migrating border cells. Curr Biol. 2008;18:1659–1667. doi: 10.1016/j.cub.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matenia D, Mandelkow EM. The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem Sci. 2009;34:332–342. doi: 10.1016/j.tibs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Pinheiro EM, Montell DJ. Requirement for Par-6 and Bazooka in Drosophila border cell migration. Development. 2004;131:5243–5251. doi: 10.1242/dev.01412. [DOI] [PubMed] [Google Scholar]
- 11.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorfinkiel N, Blanchard GB. Dynamics of actomyosin contractile activity during epithelial morphogenesis. Curr Opin Cell Biol. 2011 doi: 10.1016/j.ceb.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Edwards KA, Kiehart DP. Drosophila nonmuscle myosin II has multiple roles in imaginal disc and egg chamber morphogenesis. Development. 1996;122:1499–1511. doi: 10.1242/dev.122.5.1499. [DOI] [PubMed] [Google Scholar]
- 14.Tan I, Leung T. Myosin light chain kinases: division of work in cell migration. Cell Adh Migr. 2009;3:256–258. doi: 10.4161/cam.3.3.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 16.Dean SO, Spudich JA. Rho kinase’s role in myosin recruitment to the equatorial cortex of mitotic Drosophila S2 cells is for myosin regulatory light chain phosphorylation. PLoS One. 2006;1:e131. doi: 10.1371/journal.pone.0000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan P, Karess R. Myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J Cell Biol. 1997;139:1805–1819. doi: 10.1083/jcb.139.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Riechmann V. The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr Biol. 2007;17:1349–1355. doi: 10.1016/j.cub.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 19.Grassie ME, Moffat LD, Walsh MP, Macdonald JA. The myosin phosphatase targeting protein (MYPT) family: A regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1delta. Arch Biochem Biophys. 2011 doi: 10.1016/j.abb.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Fulga TA, Rorth P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nat Cell Biol. 2002;4:715–719. doi: 10.1038/ncb848. [DOI] [PubMed] [Google Scholar]
- 21.Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II is required for cell proliferation, cell sheet adhesion and wing hair morphology during wing morphogenesis. Dev Biol. 2010;345:117–132. doi: 10.1016/j.ydbio.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royou A, Field C, Sisson JC, Sullivan W, Karess R. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol Biol Cell. 2004;15:838–850. doi: 10.1091/mbc.E03-06-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- 24.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 25.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 26.Bennett D, Szoor B, Gross S, Vereshchagina N, Alphey L. Ectopic expression of inhibitors of protein phosphatase type 1 (PP1) can be used to analyze roles of PP1 in Drosophila development. Genetics. 2003;164:235–245. doi: 10.1093/genetics/164.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L, Wang X, Tang HL, Montell DJ. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat Cell Biol. 2010;12:1133–1142. doi: 10.1038/ncb2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauzi M, Lenne PF, Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468:1110–1114. doi: 10.1038/nature09566. [DOI] [PubMed] [Google Scholar]
- 29.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee A, Treisman JE. Excessive Myosin activity in mbs mutants causes photoreceptor movement out of the Drosophila eye disc epithelium. Mol Biol Cell. 2004;15:3285–3295. doi: 10.1091/mbc.E04-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zagorska A, Deak M, Campbell DG, Banerjee S, Hirano M, Aizawa S, Prescott AR, Alessi DR. New roles for the LKB1-NUAK pathway in controlling myosin phosphatase complexes and cell adhesion. Sci Signal. 2010;3:ra25. doi: 10.1126/scisignal.2000616. [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 33.Vereshchagina N, Bennett D, Szoor B, Kirchner J, Gross S, Vissi E, White-Cooper H, Alphey L. The essential role of PP1beta in Drosophila is to regulate nonmuscle myosin. Mol Biol Cell. 2004;15:4395–4405. doi: 10.1091/mbc.E04-02-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yam PT, Wilson CA, Ji L, Hebert B, Barnhart EL, Dye NA, Wiseman PW, Danuser G, Theriot JA. Actin-myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. J Cell Biol. 2007;178:1207–1221. doi: 10.1083/jcb.200706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verkhovsky AB, Svitkina TM, Borisy GG. Self-polarization and directional motility of cytoplasm. Curr Biol. 1999;9:11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- 36.Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, Horwitz AR. Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells. J Cell Biol. 2011;193:381–396. doi: 10.1083/jcb.201012159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol. 2004;14:736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002;4:408–415. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- 39.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Dobereiner HG, Freund Y, Borisy G, et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neubueser D, Hipfner DR. Overlapping roles of Drosophila Drak and Rok kinases in epithelial tissue morphogenesis. Mol Biol Cell. 2010;21:2869–2879. doi: 10.1091/mbc.E10-04-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gally C, Wissler F, Zahreddine H, Quintin S, Landmann F, Labouesse M. Myosin II regulation during C. elegans embryonic elongation: LET-502/ROCK, MRCK-1 and PAK-1, three kinases with different roles. Development. 2009;136:3109–3119. doi: 10.1242/dev.039412. [DOI] [PubMed] [Google Scholar]
- 44.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benton R, Palacios IM, St Johnston D. Drosophila 14-3-3/PAR-5 is an essential mediator of PAR-1 function in axis formation. Dev Cell. 2002;3:659–671. doi: 10.1016/s1534-5807(02)00320-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.