Abstract
Background
Peripheral conversion of androgens to estrogens via aromatase is the primary source of estrogen in postmenopausal women and may play a role in cardiovascular health.
Design
Prospective
Participants, Measurements
The association of an index of aromatase activity (AROM), the serum estrone to androstenedione ratio, with 25 year cardiovascular (CVD) mortality was examined in 819 postmenopausal non-estrogen using women (mean age at baseline=72).
Results
Overall, 247 deaths were attributed to CVD. The median AROM value was 60 (95% range 17–129). AROM was positively correlated with age (r=0.28) and BMI (r=0.22) (P<0.001). The age-adjusted risk for CVD mortality was significantly elevated for women in the lowest (HR=2.01, 95%CI 1.31–3.12) and highest (HR=1.51, 95%CI 1.02–2.22) quintiles of AROM, compared to the middle quintile. This U-shaped association persisted after additional adjustment for BMI, waist-to-hip ratio, exercise, smoking, alcohol use and traditional CVD risk factor covariates. There was a significant interaction of AROM and BMI (P=.001) such that high AROM was associated with a 63% reduction in risk of CVD death for women with low BMI (<22 kg/m2), but with 2.1 to 2.5-fold increased risk in women with mid-range (22–<25 kg/m2) and high (≥25 kg/m2) BMI. Estradiol did not influence AROM associations and was not independently related to CVD death.
Conclusions
These results suggest that aromatase is a novel endocrine factor predictive of CVD mortality among postmenopausal women. If confirmed, additional studies are needed to determine whether extremes of aromatase reflect genetic influences or underlying disease processes.
Keywords: cardiovascular mortality, aromatase, women, epidemiology, aging
INTRODUCTION
Men consistently have 2–4 times higher CVD mortality than women and this is true throughout the world, despite wide regional variations in absolute CVD mortality rates (1). This universal female CVD advantage has long been attributed to the benefits of estrogen. However, several prospective cohort studies have failed to identify a cardioprotective effect of endogenous estrogens (2–4). This may be due in part to the difficulty in measuring the very low levels of estradiol characteristic of postmenopausal women. Until recently, using even the best assay methods, 20 to 25% of postmenopausal women have undetectable estradiol levels.
The main source of estrogens in postmenopausal women is the peripheral conversion of androgens by the aromatase enzyme, testosterone is converted to estradiol, and androstenedione is converted to estrone (5). This occurs primarily in muscle and adipose tissue, accounting for the association of higher estrogen levels with obesity (6). The primary pathway to estrogen after menopause is via conversion of androstenedione to estrone (7), the predominant circulating estrogen in older women. We reasoned that the product-substrate ratio of serum estrone to androstenedione could be used as an index of aromatase activity and that this index might provide a novel means to evaluate the association of estrogen with cardiovascular health in older women.
In addition to adipose tissue, aromatase is found in the gonads, brain, osteoblasts, and of importance to the development of CVD, endothelial and vascular smooth muscle cells (8). Aromatase-deficient mice and humans display a number of atherogenic characteristics including abdominal obesity, elevated lipids and inflammatory markers, insulin resistance and fatty liver disease (6, 9). These observations support the idea that the aromatase enzyme, or its products, may have beneficial biological interactions with the cardiovascular system, and that aromatase deficiency may be associated with increased risk of cardiovascular events.
We report here the prospective association of the aromatase index with 25 year CVD mortality in community-dwelling postmenopausal women who were not using exogenous estrogen. Confounding by traditional CVD risk factors was examined, as well as the influence of other sex hormones, obesity, and obesity-related comorbidities. Based on the existing literature, we hypothesized that lower levels of the aromatase index would predict earlier CVD death in older postmenopausal women.
MATERIALS AND METHODS
Study population
The Rancho Bernardo Study is a population-based study of healthy aging in a predominantly Caucasian Southern California community. Between 1984 and 1987, 82% (n=2480) of surviving community-dwelling participants attended a research clinic visit. During this visit, information regarding medical history, medication use, physical activity, and alcohol consumption was obtained using standard questionnaires. Current medication use was validated by examination of pills and prescriptions brought to the clinic for that purpose. The study protocol was approved by the Institutional Review Board of the University of California, San Diego; all participants gave written informed consent.
Eligibility criteria for the present analysis included 1) age 50 years or older at the 1984–87 visit, 2) availability of stored sera, 3) postmenopausal, and 4) no estrogen or insulin use at the time of the clinic visit. Of the 1351 women who attended the 1984–87 clinic visit, 332 were excluded for current estrogen use; 868 of the remaining women had sufficient stored sera for measurement of sex hormones. Of these, 13 were excluded for age less than 50 years, 8 for pre-menopausal status, 2 because of insulin use, and 10 because they had estradiol levels greater than 550 pmol/L, indicating either premenopausal status or unreported estrogen use. Because aromatase activity has been shown to be elevated during critical illness (10), 20 women with CRP levels >15 mg/L were also excluded. The remaining 819 postmenopausal, non-estrogen using women are the subject of this report.
Measurements
Height, weight, and waist and hip girth were measured in the clinic with participants wearing light clothing and no shoes. Body mass index (BMI, kg/m2) and waist to hip ratio (WHR) were used as estimates of overall and central adiposity, respectively. Blood pressures were measured twice in seated resting subjects using the Hypertension Detection and Follow–Up Program protocol (11).
Blood samples were obtained by venipuncturebetween 0730 h and 1100 h after a requested 12-h fast; serum and plasma were separated and frozen at −70° C. Sex hormone levels weremeasured on first-thawed serum samples between1992 and 1994 in the UCSD Reproductive Endocrinology research laboratory. Androstenedione, estrone, testosterone and estradiol levels were measured by radioimmunoassay after solvent extraction and celite column chromatography. The sensitivity and the intra- and inter- assay coefficients ofvariation (CV), respectively, were 11 pmol/L, 6.0%, and 7.7% for estrone; 11 pmol/L, 5.9%, and 7.1% for estradiol; 0.06 nmol/L, 4.3%, 4.3% for androstenedione; and 0.07 nmol/L, 4.0%, and 4.9% for testosterone. Androstenedione and testosterone levels for all participants were abovethe assay sensitivity. Estrone and estradiol levels were set to the assay sensitivities for women with estrone (n=22) or estradiol (n=176) levels below the assay sensitivity.
Fasting plasma total, HDL, and LDL cholesterol and triglyceride levels were measured in a Center for Disease Control Certified Lipid Research Clinic Laboratory using established methods (9). Plasma IL-6 and CRP levels were measured on a subset of 596 women in 2000 using previously described methods (9).
Prevalent conditions and mortality assessment
Medical history was obtained at baseline using standardized questionnaires. Prevalent CVD was defined as physician-diagnosed MI, coronary artery revascularization, congestive heart failure, stroke or transient ischemic attack, carotid surgery, peripheral arterial surgery, or physician-diagnosed intermittent claudication. Validation of self-reported heart attack (by chest pain, enzyme elevation, and ECG) was achieved for 72% of a subset for whom hospital records could be obtained. Diabetes was defined by physician diagnosis, fasting plasma glucose ≥ 7.0 mmol/L, 2 hr post-challenge glucose ≥ 11.1 mmol/L or use of diabetes medications. The metabolic syndrome was defined according to the 2002 Adult Treatment Panel III criteria (12). Hypertension was defined as blood pressure ≥140/90 mmHg or use of antihypertensive medication. Estimated glomerular filtration rate (eGFR, ml/min/1.73m2) was calculated by the MDRD equation (13) and classified as normal (eGFR ≥90), mild CKD (chronic kidney disease) (eGFR 60–89), and moderate CKD (eGFR<60). Comorbidities recorded included thyroid, liver, kidney and heart disease, diabetes, cancer (non-skin), emphysema, arthritis, hip fracture and hypertension.
Participants were followed through April 2009, with 98% ascertainment of vital status. Death certificateswere classified for underlying cause of death by a certifiednosologist using the International Classification of Diseases, Ninth Revision. CVD deaths included codes 401–448.
Statistical analysis
Estrogen, IL-6, CRP and triglyceride levels were not normally distributed and were log-transformed for analyses; reported values are geometric means or medians and 95% ranges. The aromatase index (AROM) was defined as the molar ratio of estrone to androstenedione.
The association of AROM with baseline characteristics was assessed by linear regressions with AROM as the dependent variable. The association between baseline AROM and CVD mortality was determined using Cox proportional hazards regressions; model assumptions were tested by applying the time-dependent covariate test (14), by Schoenfeld residual visualizations (15), and by visualization of log-log survival plots and Kaplan-Meier versus Cox estimated survivor functions (16); all models presented met the proportional hazards assumption. Based on an a priori hypothesis of a low (enzyme deficiency) threshold association, time-to-event analyses were performed using quintiles of AROM for the entire population to evaluate non-linear associations. Three separate regression models were assessed: the first adjusted for age, the second added adjustment for adiposity (BMI, WHR) and the third added adjustment for lifestyle characteristics including physical activity (3+ times per week, yes/no), alcohol use (1+ drinks/day vs. less or none), and current smoking habit (yes/no). Additional multivariate models added adjustment for AROM covariates. Sensitivity analyses tested the influence of individual sex hormones. Interaction terms were used to test for effect modification. There was no significant multicollinearity (variance inflation factor >2) between the independent variables.
The association of AROM with time to CVD mortality was also modeled using accelerated failure time (AFT) regressions to facilitate display of the continuous AROM-CVD mortality association. In AFT regressions the outcome is age at death, rather than time to death as in Cox regressions. AROM was modeled as a third order continuous variable in this fully parametric survival model.
All p-values presented are 2-tailed; p<0.05 was considered statistically significant. Data were analyzed using STATA (v11.1; Stata Corp., College Station, TX)) and SPSS (version v15; SPSS Inc., Chicago, IL).
RESULTS
Baseline characteristics (Table 1)
Table 1.
Population characteristics and linear associations with AROM
| Means | β with AROMf | |
|---|---|---|
| Age and body size, mean (SD) | ||
| Age (yr) | 72.2 (9.0) | 0.28c |
| BMI (kg/m2) | 24.4 (3.7) | 0.22c |
| WHR (waist to hip ratio) | 0.80 (0.07) | 0.09a |
| Lifestyle Parameters (%) | ||
| Alcohol (1+drinks/day) | 38.0 | −0.19b |
| Current smoker | 13.7 | −0.35c |
| Exercise (3+ times/week) | 77.9 | −0.13a |
| Prevalent Conditions (%) | ||
| Cardiovascular disease | 12.1 | 0.26a |
| Diabetes | 14.1 | 0.24a |
| Metabolic syndrome | 16.4 | 0.19a |
| Hypertension | 71.8 | 0.18a |
| Health status indicators | ||
| eGFRd 90+, 60–80, <60 | 65.8, 31.6, 2.6 | 0.19b |
| Number of medications | 0.941 (1.05) | 0.09a |
| Number of comorbidities | 0.918 (0.96) | 0.10b |
| Weight loss >10 lbs (%) | 18.9 | 0.09 |
| Metabolic factors, mean (SD) | ||
| Total cholesterol (mmol/L) | 6.02 (1.03) | −0.08a |
| HDL cholesterol (mmol/L)e | 1.66 (0.51) | −0.08a |
| LDL cholesterol (mmol/L) | 3.71 (1.00) | −0.06 |
| Triglycerides (mmol/L)e | 1.13 0.87) | 0.09b |
| Fasting plasma glucose (mg/dl) | 5.52 (0.85) | 0.07a |
| Systolic blood pressure (mmHg) | 142.1 (22.1) | 0.11b |
| Diastolic blood pressure (mmHg) | 75.7 (9.5) | −0.03 |
| CRP (mg/L)e | 1.66 (1.69) | 0.24c |
| IL-6 (pg/ml)e | 2.36 (1.22) | 0.16c |
P <0.05,
P <0.01,
P <0.001
eGFR units = ml/min/1.73m2
Log transformed for analysis, values represent geometric mean (SD), available on a subset of 596
Standardized coefficients for continuous variables; binary variables were left untransformed.
The mean age of the 819 women was 72.2 years (range 51– 90); 29% were classified as overweight (BMI 25–30 kg/m2), and 8% as obese (BMI>30 kg/m2); 16% had undergone bilateral oophorectomy. Only 14% reported current smoking, 38% consumed at least one alcohol drink daily, and 78% reported moderate physical exercise 3 or more times per week.
The median value for the aromatase index (AROM) was 60.0 (95% range 16.6 to 129.1) (Table 2). The correlation of AROM values with estrone and androstenedione concentrations was 0.46 and −0.36, respectively (both P <0.001), indicating that the value of AROM was dependent on both hormone concentrations, but contained information distinct from the two hormones. AROM correlated significantly (P’s <0.001) with estradiol (r = 0.30) and with the estradiol/testosterone ratio (r = 0.36), but not with testosterone alone (r<0.01, P = 0.84).
Table 2.
Sex hormones, sex hormone ratios and correlation with AROM
| Hormones, hormone ratios | Median | 95% Range | R with AROMa |
|---|---|---|---|
| Estrone/Androstenedioneb | 60.0 | 16.6– 129.1 | -- |
| Androstenedione (nmol/L) | 1.07 | 0.35 – 2.55 | −0.36 |
| Estrone (pmol/L) | 62.9 | 7.4 – 155.4 | 0.46 |
| Testosterone (nmol/L) | 0.50 | 0.10–1.61 | −0.01 |
| Estradiol (pmol/L) | 18.4 | 7.3–47.7 | 0.30 |
| Estradiol/Testosteroneb | 40.0 | 10.9 – 151.1 | 0.28 |
P <0.001 for all except testosterone (P = 0.84)
Hormone ratios are pmol/L divided by nmol/L
AROM covariates
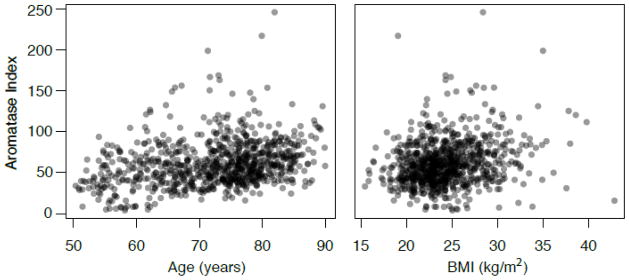
The associations of AROM values with baseline characteristics are presented in Table 1. AROM values were positively correlated with age and BMI (Figure 1). Together age and BMI explained 15% of the variability of AROM. Higher AROM was also related to higher levels of several other CVD risk factors including WHR, triglycerides, diastolic blood pressure, fasting plasma glucose, and IL-6 and CRP levels, and with lower levels of HDL cholesterol (all P <0.001). Prevalent CVD, diabetes, metabolic syndrome, hypertension, and mild CKD also associated with higher AROM (all P<0.001), whereas current smoking, daily alcohol use and exercising 3 or more times per week associated with lower AROM (all P<0.05). Although statistically significant, most of these associations were relatively weak and only the CRP association was independent of age and BMI (data not shown). Based on testing quadratic terms, the only variable with a significant non-linear association with AROM was SBP (P=.041).
Figure 1.
Plots of AROM values versus age (R=0.28) and BMI (R=0.22) (both P<0.001).
AROM and CVD mortality
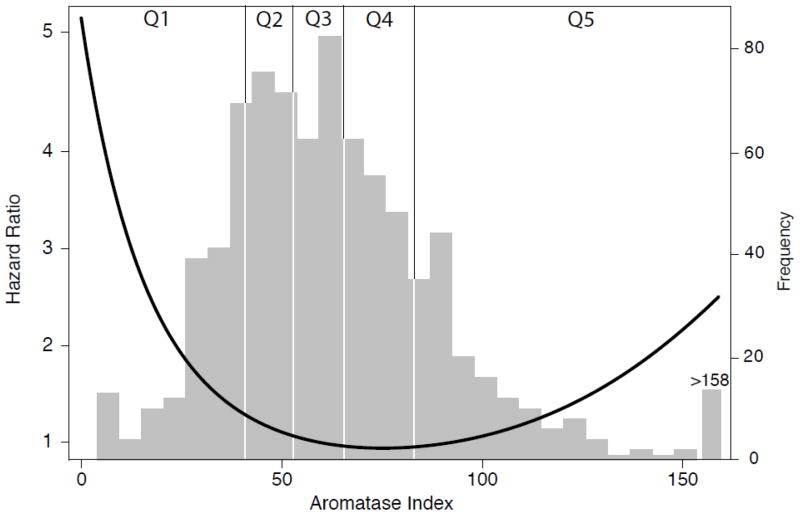
During a median follow-up of 14.7 years, 507 (63%) women died; 49% (n=247) of deaths were attributed to CVD. Age-adjusted quintile analysis suggested a U-shaped association of AROM with CVD mortality (P<0.001 for quadratic trend). Accordingly, the AROM-CVD mortality association was tested using the middle quintile (Q3) as the reference level (Table 3). In age-adjusted analyses, the risk of death was significantly elevated for women in both the lowest (low AROM) and the highest (high AROM) quintiles, compared with those in Q3, but did not differ significantly for women in quintile 2 or 4. Compared to Q3, CVD mortality risk was increased 101% (P=0.002) for women with low AROM and 51% (P= 0.043) for women with high AROM (Model 1). This U-shaped association persisted after additional adjustment for adiposity (Model 2) and lifestyle (Model 3). The age, adiposity and lifestyle adjusted association of AROM with age at CVD death is depicted in Figure 2, with the distribution of AROM values displayed in the background. As shown, the hazard is highest at very low values of AROM, is low through the mid-range, and rises again across the top 20% of AROM values (P=0.004).
Table 3.
Hazards ratios for CVD mortality by quintile of Aromatase Index (AROM)
| AROM quintile (range) | CVD Mortality Rate a | Model 1b HR (95% CI) |
Model 2c HR (95% CI) |
Model 3d HR (95% CI) |
Model 4e HR (95% CI) |
Model 5f HR (95% CI) |
|---|---|---|---|---|---|---|
| Q1 (0.37–3.88) | 33 | 2.01 (1.31–3.12) | 1.89 (1.22–2.92) | 1.99 (1.27–3.09) | 1.88 (1.20–2.94) | 2.23 (1.41–3.53) |
| Q2 (3.89–5.01) | 25 | 1.26 (0.94–1.91) | 1.24 (0.83–1.89) | 1.27 (0.84–1.93) | 1.20 (0.79–1.83) | 1.21 (0.79–1.85) |
| Q3 (5.02–6.21) | 19 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q4 (6.22–7.87) | 19 | 0.97 (0.64–1.48) | 0.97 (0.64–1.48) | 0.95 (0.63–1.45) | 0.91 (0.60–1.40) | 1.01 (0.66–1.54) |
| Q5 (7.88–119.3) | 32 | 1.51 (1.02–2.22) | 1.51 (1.03–2.23) | 1.49 (1.01–2.20) | 1.44 (0.98–2.13) | 1.41 (0.94–2.10) |
Ref, Reference category.
Bold indicates P<0.05
Adjusted for age, deaths per 100.
Model 1, adjusted for age.
Model 2, adjusted for age, BMI, and WHR.
Model 3, model 2 + alcohol use, current smoking, and exercise.
Model 4, model 3 + triglycerides, HDL, hypertension, fasting blood glucose
Model 5, model 3 + eGFR category, weight loss >10 lbs, number of medications, number of comorbidities
Figure 2.
CVD mortality hazard function for AROM overlying the relative distribution of AROM. The hazards function is based on an accelerated failure time model adjusted for age, BMI, WHR, alcohol intake, smoking and exercise with AROM modeled as a third order continuous variable, P=0.004 overall for AROM.
Potential confounding or mediation was examined by adding additional variables to the age, adiposity and lifestyle adjusted model (Model 3). Risk estimates for low and high AROM were only modestly attenuated by further adjustment for CVD risk factor covariates (Model 4) or by adjusting for markers of poor health status (Model 5). Sequential adjustment for the most commonly used medications (anti-hypertensives, aspirin, and thyroid medications used by 33%, 19% and 13% of participants, respectively) also failed to influence results (data not shown).
Other secondary analyses tested the influence of comorbid conditions (Table 4). Individually or jointly adjusting for prevalent CVD, diabetes or metabolic syndrome had minimal influence on results, although excluding women with these conditions moderately attenuated the association for high AROM, with exclusion of diabetics having the largest effect. We also tested the effect of comorbidity and occult disease in separate analyses excluding women whose fatal CVD event occurred during the first 2 (n=19) or first 5 (n=76) years of follow-up, with only minor changes in risk estimates for low and high AROM. Exclusion of 132 women with bilateral oophorectomy also had minimal influence on results. Adjusting for IL-6 and CRP levels had minimal effect on AROM associations in the subset with these cytokine measures (Table 4).
Table 4.
Hazards ratios of low and high AROM for CVD mortality adjusting for, or excluding, potential covariates
| Q1 vs. Q3 HR (95 % CI) |
Q5 vs. Q3 HR (95 % CI) |
|
|---|---|---|
| Base modela | 1.99 (1.27, 3.09) | 1.49 (1.01, 2.20) |
| Base modela plus: | ||
| Diabetes | 1.97 (1.27, 3.08) | 1.50 (1.01, 2.21) |
| Prevalent CVD | 1.98 (1.28, 3.10) | 1.50 (1.28, 3.10) |
| Metabolic syndrome | 1.94 (1.24, 3.03) | 1.48 (1.00, 2.18) |
| All of the above conditions | 1.83 (1.17, 2.87) | 1.45 (0.98, 2.14) |
| CRP, IL-6b | 2.23 (1.31, 3.40) | 1.51 (0.95, 2.40) |
| Estrone, androstenedione | 1.94 (1.10, 3.42) | 1.44 (0.89, 2.33) |
| Base modela excluding: | ||
| Current smokers (n=115) | 2.13 (1.30, 3.49) | 1.64 (1.09, 2.49) |
| Oophorectomy (n=132) | 1.85 (1.13, 3.00) | 1.44 (0.95, 1.03) |
| Diabetes (n=118) | 1.99 (1.23, 3.25) | 1.29 (0.84, 1.99) |
| Prevalent CVD (n=103) | 1.98 (1.23, 3.18) | 1.40 (0.91, 2.15) |
| Metabolic syndrome (n =137) | 1.93 (1.18, 3.16) | 1.39 (0.91, 2.14) |
| CVD death within 2 years (n=19) | 2.00 (1.27, 3.15) | 1.53 (1.03, 2.29) |
| CVD death within 5 years (n=76) | 1.89 (1.19, 3.04) | 1.49 (0.99, 2.25) |
| Stratified by BMI (kg/m2) groupc: | ||
| <22 (n=212) | 1.50 (0.70–3.22) | 0.37 (0.16–0.88) |
| 22–<25 (n=298) | 1.74 (0.83–3.67) | 2.18 (1.07–4.45) |
| 25+ (n=306) | 3.84 (1.63–9.06) | 2.48 (1.23–4.98) |
Bold indicates P<0.05
Base model is adjusted for age, BMI, WHR, smoking, exercise, and alcohol use.
CRP, IL-6 available on a subset of 596 women: HR for Q1 vs Q3 = 2.23 (1.31, 3.80), HR for Q5 vs Q3 = 1.50 (0.95, 2.39) for this subset for the base model.
P=.011 for interaction of BMI subgroup and AROM
The association of AROM with CVD mortality was not modified by age, WHR, alcohol use, current smoking, or prevalent CVD (P’s for interaction >0.18); however, there was a significant interaction of AROM and BMI (P=.011). In BMI-stratified analyses (Table 4), low AROM tended to be associated with increased risk of CVD mortality in all BMI strata, although the hazards ratio only reached significance in women with BMI ≥25 kg/m2. In contrast, high AROM was associated with a 63% reduction in risk of CVD death for women with low BMI (<22 kg/m2), but with 2.1 and 2.5-fold increased risk in women with mid-range (22–<25 kg/m2) and high (≥25 kg/m2) BMI, respectively. Age-adjusted CVD mortality rates per 100 persons were 33, 26, and 32, respectively, for the low, mid, and high BMI strata.
Specificity of AROM-CVD mortality
The specificity of the AROM-CVD mortality association was examined by testing other sex hormone-CVD mortality associations using the age, adiposity and lifestyle adjusted model (data not shown). Estrone and androstenedione levels did not significantly predict CVD mortality when considered alone or jointly and did not significantly alter the AROM-CVD association when entered in the AROM model (Table 4). Adjusting for estradiol levels also failed to influence the AROM–CVD associations and estradiol was not independently related to CVD death. The ratio of estradiol to testosterone was also unrelated to CVD death. Finally, we examined the association of AROM with death from non-CVD causes (n=260) and found no significant association [HR=1.28 (0.87–1.91) and 0.84 (0.57–1.25), for low and high AROM, respectively]. No significant interactions with age, BMI or WHR were detected for any of these analyses.
DISCUSSION
To our knowledge, this is the first population-based study to show that an index of aromatase activity, the ratio of serum estrone to androstenedione concentrations, independently predicts cardiovascular death over a 25 year follow-up in community-dwelling postmenopausal women who were not using estrogen therapy. The association of AROM with CVD mortality was U-shaped with 2-fold higher risk of CVD death for women in the lowest 20% of AROM and 50% elevated risk for women in the highest 20% of AROM, compared to their counterparts without extreme levels. These results were not explained by age and adiposity or other traditional CVD risk factors.
Why use the ratio of estrone to androstenedione to estimate aromatase activity since the aromatase enzyme also catalyzes the conversion of testosterone to estradiol? Aromatase has 10 times higher affinity for androstenedione than testosterone, thus the primary pathway to estrogen after menopause is via peripheral conversion of androstenedione to estrone. Most estradiol is derived from the conversion of estrone to estradiol via 17-β hydroxysteroid dehydrogenase (17). Aromatase activity increases as a function of adiposity (18) and aromatase gene expression per volume of adipose tissue is 2–4 fold higher in older, compared to younger, women (19, 20). Thus, the association of higher AROM values with increasing age and BMI observed in the present study provides an indirect validation of this index of aromatase activity.
Aromatase activity has been shown to increase during periods of critical illness and traumatic injury, resulting in higher levels of circulating estrogens and lower androgens (10). In 301 critically ill and injured surgical patients, the degree of hyperestrogenemia predicted 28-day all-cause mortality (21). Hence, the unexpected association of high AROM values with CVD death observed in the present study could simply be because elevated AROM is a biomarker of pre-existing illness. We addressed this possibility in several ways. First, women with CRP >15 mg/dl (n=20) were excluded to avoid any confounding by acute illness. Next, the influence of known disease was tested by excluding women with pre-existing CVD, diabetes or metabolic syndrome, and then by adjusting for a series of markers of poor health status; associations with high AROM were reduced, but risk estimates remained above one. The influence of unknown or occult disease was tested by excluding women whose deaths occurred within either the first 2 or 5 years of follow-up, with negligible effect. Taken together, this evidence argues against reverse causality as the basis for the association of high AROM values with increased risk of CVD mortality, although this possibility cannot be ruled out.
Several lines of evidence point to a role for endogenous estrogens in cardiometabolic disease in postmenopausal women. Women with higher estradiol levels have greater risk of insulin resistance and glucose intolerance and are more likely to develop type 2 diabetes (22–27). Although most prospective studies, including this one, have failed to demonstrate an association of endogenous estrogens with CVD events or death (2–4), a recent InCHIANTI study used an ultrasensitive assay and showed that higher estradiol concentrations predicted increased risk of 9-year all cause mortality in late postmenopausal women (information on cause of death was not available) (28). In the present study, the association of high AROM with CVD mortality appears to be due to factors other than obesity since hazards ratios were similarly elevated for normal weight (BMI 22 to <25 kg/m2) and overweight/obese (BMI>25 kg/m2) women. Interestingly, high values of AROM actually had a protective association in the thinnest women (BMI <22 kg/m2), reducing the risk of CVD death by 67% and suggesting that higher levels of intracellular aromatase activity may be beneficial in the setting of low body fat and hypoestrogenemia.
As we hypothesized, women with low values of AROM also experienced earlier CVD death, and the low AROM association was stronger and more robust to adjustment for covariates than that for high AROM. Animal models of aromatase deficiency display an obesity phenotype characterized by central adiposity, hypercholesterolemia and insulin resistance (29, 30) that is reversed by estrogen treatment (31). To date, about 20 humans have been identified with natural mutations of the aromatase gene, more than half of whom are women (32). Most of these women were diagnosed around the time of puberty and placed on estrogen therapy, thus it has not been possible to evaluate their CVD risk profile. Men with aromatase deficiency display a number of metabolic abnormalities including in one case elevated LDL cholesterol, low HDL cholesterol, hypertriglyceridemia, hyperglycemia and carotid plaques, all of which improved with estradiol replacement (33). There is also some evidence to suggest that cardiovascular risk in breast cancer patients is increased with aromatase inhibitor (AI) therapy, however most AI trials have used tamoxifen treatment as a comparator, and whether risk differences are due to putative cardioprotective effects of tamoxifen, or adverse effects of aromatase inhibitors, is not clear (34).
Our results are consistent with the idea that aromatase deficiency also has a deleterious effect on the vascular system in postmenopausal women, although causality cannot be assumed. This seems to be especially true for overweight/obese women, who would be expected to have high, not low, levels of aromatase. It is not clear whether low AROM in these women in the face of apparently ample adipose tissue reflects some genetic abnormality (35) or disrupted steroid metabolism. Estrogens have well-documented effects on body weight regulation, opposing adipose tissue accumulation by increasing lipolysis and inhibiting lipogenesis (36). Thus, aromatase deficiency and lower estrogen activity may contribute to increased adipose tissue mass in this group, particularly atherogenic visceral adiposity (36).
Simpson and colleagues have emphasized the importance of local tissue-specific estrogen synthesis and action in postmenopausal women (6). They propose that critical paracrine and intracrine actions of estrogens may not be discernible by measurement of peripheral hormone concentrations. Estrone and estradiol levels did not predict CVD death in this study, even in models stratified by BMI. On the other hand, we observed moderately strong signals for CVD mortality, especially at the low end of the AROM index, and this association persisted after adjustment for multiple CVD risk factors and comorbidities. If confirmed, our findings suggest that the estrone to androstenedione estimate of aromatase activity may be a better indicator of CVD-related estrogen action than circulating estrogen concentrations. Additional studies with other endpoints such as bone mineral density and fractures are needed to validate the usefulness of the AROM index in elucidating the link between endogenous estrogens and postmenopausal health.
This study has some limitations. Results were based on a largely Caucasian middle- to upper-middle-class community and may not apply to other ethnic and socioeconomic groups. Like most epidemiological studies, hormone levels were based on a single assay. However, single measurements of androgens in postmenopausal women have been shown to reliably characterize average levels over a 2- to 3-year period, and estrone levels are more reproducible than estradiol (37). In addition, blood for hormone measurements was obtained in the morning from fasting women (minimizing any diurnal variation), the laboratory methods used were the gold standard at the time the assays were performed, and all androstenedione levels and all but 22 estrone values were within the assay sensitivity. Changes in hormone levels during long term storage were unlikely to explain observed associations; hormone levels were measured in never previously thawed plasma, and levels did not vary by season of sampling or duration of storage. In addition, others have shown that levels of steroid hormones are relatively stable in frozen plasma stored for 3–10 years (38, 39). It is possible that CVD death was misclassified in this elderly population; however, 85% of fatal CVD events were confirmed by medical record review in a subset with available information. Of importance, all of these sources of misclassification, if present, would be likely to diminish associations, not cause them. We did not have direct measurements of fat mass and residual confounding may exist.
Conclusions
These findings suggest that aromatase is a novel endocrine factor predictive of CVD mortality among postmenopausal women. Additional studies are needed to determine whether the aromatase –CVD association reflects genetic influences or underlying disease processes, to confirm whether associations of aromatase activity with CVD events differs by level of adiposity in other settings, and to evaluate whether use of the aromatase index may provide novel insights into other sex hormone-related postmenopausal diseases such as breast cancer and osteoporosis.
Acknowledgments
Funding Sources: The Rancho Bernardo Study was funded by research grants AG028507 and AG018339 from the National Institute on Aging and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases. This work was supported by grants from the American Heart Association (GAL, LBD) and by a grant from the Sandra Daugherty Foundation (GAL).
ACKNOWLEDGEMENTS: None
Footnotes
Disclosures: The authors have nothing to disclose
References
- 1.Kalin MF, Zumoff B. Sex hormones and coronary disease: a review of the clinical studies. Steroids. 1990 Aug;55(8):330–52. doi: 10.1016/0039-128x(90)90058-j. [DOI] [PubMed] [Google Scholar]
- 2.Barrett-Connor E, Goodman-Gruen D. Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. Bmj. 1995 Nov 4;311(7014):1193–6. doi: 10.1136/bmj.311.7014.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips GB, Yano K, Stemmermann GN. Serum sex hormone levels and myocardial infarction in the Honolulu Heart Program. Pitfalls in prospective studies on sex hormones. J Clin Epidemiol. 1988;41(12):1151–6. doi: 10.1016/0895-4356(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 4.Rexrode KM, Manson JE, Lee IM, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003 Oct 7;108(14):1688–93. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 5.Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973 Feb;36(2):207–14. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- 6.Simpson ER, Misso M, Hewitt KN, et al. Estrogen--the good, the bad, and the unexpected. Endocr Rev. 2005 May;26(3):322–30. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 7.Longcope C, Baker S. Androgen and estrogen dynamics: relationships with age, weight, and menopausal status. J Clin Endocrinol Metab. 1993 Mar;76(3):601–4. doi: 10.1210/jcem.76.3.8445016. [DOI] [PubMed] [Google Scholar]
- 8.Simpson ER. Aromatase: biologic relevance of tissue-specific expression. Semin Reprod Med. 2004 Feb;22(1):11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 9.McCullough LD, Blizzard K, Simpson ER, et al. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003 Sep 24;23(25):8701–5. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spratt DI, Morton JR, Kramer RS, et al. Increases in serum estrogen levels during major illness are caused by increased peripheral aromatization. Am J Physiol Endocrinol Metab. 2006 Sep;291(3):E631–8. doi: 10.1152/ajpendo.00467.2005. [DOI] [PubMed] [Google Scholar]
- 11.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976 Jun;5(2):207–15. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 12.National Cholesterol Education Program (NCEP) Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(23):3143–421. [PubMed] [Google Scholar]
- 13.Levey AS, Greene T, Kusek JW, et al. A simplified equation to predict glomerular filtration rate from serum creatinine {Abstract} J Am Soc Nephrol. 2001;11:A0828. [Google Scholar]
- 14.Bradburn MJ, Clark TG, Love SB, et al. Survival analysis Part III: multivariate data analysis -- choosing a model and assessing its adequacy and fit. Br J Cancer. 2003 Aug 18;89(4):605–11. doi: 10.1038/sj.bjc.6601120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collett D. Modelling Survival Data in Medical Research. London: Chapman & Hall; 1994. [Google Scholar]
- 16.Cleves MW. An Introduction to Survival Analysis Using Stata: Revised Edition. College Station, TX: Stata Press; 2004. [Google Scholar]
- 17.Longcope C. Hormone dynamics at the menopause. Ann N Y Acad Sci. 1990;592:21–30. doi: 10.1111/j.1749-6632.1990.tb30313.x. discussion 44–51. [DOI] [PubMed] [Google Scholar]
- 18.Bulun SE, Zeitoun K, Sasano H, et al. Aromatase in aging women. Semin Reprod Endocrinol. 1999;17(4):349–58. doi: 10.1055/s-2007-1016244. [DOI] [PubMed] [Google Scholar]
- 19.Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994 Feb;78(2):428–32. doi: 10.1210/jcem.78.2.8106632. [DOI] [PubMed] [Google Scholar]
- 20.Misso ML, Jang C, Adams J, et al. Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause. 2005 Mar;12(2):210–5. doi: 10.1097/00042192-200512020-00016. [DOI] [PubMed] [Google Scholar]
- 21.May AK, Dossett LA, Norris PR, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med. 2008 Jan;36(1):62–8. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding EL, Song Y, Manson JE, et al. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007 Oct;50(10):2076–84. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- 23.Oh JY, Barrett-Connor E, Wedick NM, et al. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002 Jan;25(1):55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Kalish GM, Barrett-Connor E, Laughlin GA, et al. Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. J Clin Endocrinol Metab. 2003 Apr;88(4):1646–52. doi: 10.1210/jc.2002-021375. [DOI] [PubMed] [Google Scholar]
- 25.Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2006 Mar 15;295(11):1288–99. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 26.Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000 Jul;23(7):912–8. doi: 10.2337/diacare.23.7.912. [DOI] [PubMed] [Google Scholar]
- 27.Golden SH, Dobs AS, Vaidya D, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007 Apr;92(4):1289–95. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- 28.Maggio M, Ceda GP, Lauretani F, et al. Relationship between higher estradiol levels and 9-year mortality in older women: the Invecchiare in Chianti study. J Am Geriatr Soc. 2009 Oct;57(10):1810–5. doi: 10.1111/j.1532-5415.2009.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher CR, Graves KH, Parlow AF, et al. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6965–70. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12735–40. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misso ML, Murata Y, Boon WC, et al. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003 Apr;144(4):1474–80. doi: 10.1210/en.2002-221123. [DOI] [PubMed] [Google Scholar]
- 32.Jones ME, Boon WC, McInnes K, et al. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab. 2007 May;3(5):414–21. doi: 10.1038/ncpendmet0477. [DOI] [PubMed] [Google Scholar]
- 33.Maffei L, Murata Y, Rochira V, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004 Jan;89(1):61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- 34.Janni W, Hepp P. Adjuvant aromatase inhibitor therapy: outcomes and safety. Cancer Treat Rev. 2010 May;36(3):249–61. doi: 10.1016/j.ctrv.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Tworoger SS, Chubak J, Aiello EJ, et al. Association of CYP17, CYP19, CYP1B1, and COMT polymorphisms with serum and urinary sex hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2004 Jan;13(1):94–101. doi: 10.1158/1055-9965.epi-03-0026. [DOI] [PubMed] [Google Scholar]
- 36.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood) 2004 Dec;229(11):1127–35. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 37.Hankinson SE, Manson JE, Spiegelman D, et al. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiol Biomarkers Prev. 1995 Sep;4(6):649–54. [PubMed] [Google Scholar]
- 38.Bolelli G, Muti P, Micheli A, et al. Validity for epidemiological studies of long-term cryoconservation of steroid and protein hormones in serum and plasma. Cancer Epidemiol Biomarkers Prev. 1995 Jul-Aug;4(5):509–13. [PubMed] [Google Scholar]
- 39.Kley HK, Schlaghecke R, Kruskemper HL. Stability of steroids in plasma over a 10-year period. J Clin Chem Clin Biochem. 1985 Dec;23(12):875–8. [PubMed] [Google Scholar]