Abstract
Age, family history, and body mass index (BMI) influence the prevalence of hypertension, but very little is known about the interplay of these factors in Chinese populations. The authors examined this issue in Chinese adults (n = 4104) in the People’s Republic of China Study. In young adults (24–39 years), the prevalence of hypertension/1000 persons (95% confidence interval [CI]) at the referent BMI was greater among subjects with a parental history of hypertension (35; 15–54) compared with those without (7; 3–11). Among middle-aged (40–71 years) adults, the prevalence of hypertension was similar regardless of parental history; however, the effect of BMI was modified by parental history status. For example, at BMI = 25 kg/m2, the prevalence difference/1000 persons was 375 (95% CI = 245–506) and 97 (95% CI = 51–144) among subjects with and without a parental history, respectively. These large differences call for further investigation of the genetic and environmental factors that could be driving this interaction.
Keywords: Asian, blood pressure, body mass index, Chinese, family history
Introduction
In China, the prevalence of hypertension among adults aged 18 years and older is substantial (19%) despite low mean levels of body mass index (BMI; 23 kg/m2). 1 Rates of hypertension are expected to rise as levels of obesity increase in China.1 Identifying individuals who present with a greater risk for developing hypertension may help target public health prevention efforts. Risk scores used to predict incident hypertension in Caucasian populations incorporate measures of adiposity, parental history of hypertension, and age.2 Each of these factors contributes to hypertension onset. Older age and higher levels of BMI are well-established risk factors for hypertension in Chinese and other populations3; however, the interaction of parental history of hypertension with obesity has not been well examined and has largely been unexplored in the Chinese.
Parental history of hypertension is often collected in clinical and epidemiological settings, as it captures aspects of shared genetic and environmental factors that are associated with hypertension. Research has shown a parental history of hypertension is associated with higher levels of systolic and diastolic blood pressure among offspring, as well as a greater risk of hypertension.4–6 The correlation of blood pressure levels between parent and offspring has been observed across the life course, as early as at birth, and these associations between parent–child blood pressure levels have been shown to continue through adulthood.7
The aggregation of blood pressure within families is attributed to interactions between shared genes and environments that result in physiologic and biochemical processes that contribute to increased blood pressure.8 It is estimated that 30% to 60% of the interindividual variation in blood pressure is attributed to genetic factors.9,10 In a Chinese population, the correlation of blood pressure between parent and child was 0.16 for both systolic and diastolic pressure.10 Genes that may be passed from parent to child, such as variants in genes encoding angiotensin-converting enzyme, a-adducin, and aldosterone synthase have been associated with higher systolic blood pressure in Chinese populations, and these genes may predispose individuals to hypertension, especially when coupled with a high-sodium diet.11
Epidemiologic studies that examine the association of parental history of hypertension and BMI found that mean BMI levels among offspring with a parental history of hypertension were typically higher, by approximately 0.5 kg/m2 or more in whites and blacks12–15 and by 0.2 kg/m2 in Japanese,5 compared with individuals with no parental history of hypertension. The increased risk of hypertension associated with a positive family history in these ethnicities persists after adjustment for BMI.4,5,12,13 Although individuals may develop hypertension for reasons unrelated to obesity, it is important to note that hypertension and obesity may share some common genetic and environmental risk factors.16 Yet it remains unclear if parental history of hypertension may modify the effect of BMI on hypertension and if the association varies by age. In this article, we address several related research questions. Are individuals with a parental history of hypertension more susceptible to the effects of obesity on blood pressure? Or are individuals with a parental history of hypertension likely to develop hypertension regardless of their weight status, and therefore, more resistant to the effects of obesity? Also, do these relationships vary by age?
Materials and Methods
Study Population
Data for this cross-sectional analysis were from the People’s Republic of China (PRC) Study, which examined a community based sample of Chinese men and women from urban and rural areas of Guangzhou and Beijing, China.17 Only data from Guangzhou were included here as young adults were not studied in Beijing. Data were from eligible subjects (n = 4445) collected in 1983 from men and women aged 24 to 71 years at the urban and rural Guangzhou examination centers. Urban participants were currently working or retired employees from 8 workshops of the Guangzhou Shipyard Company and rural participants worked in 14 of the 21 agricultural villages in the Dashi Township of Panyu County at the time of the 1981 census. Participants were excluded if data on BMI (n = 25), parental history of hypertension status (n = 234), smoking (n = 21), or alcohol use (n = 61) were missing. Following these exclusions, 1939 men and 2165 women were included in the analyses. The institutional review board at each field center approved this study, and the analysis was approved by the University of North Carolina at Chapel Hill Institutional Review Board on research involving human subjects.
Measurements
The Collaborative Studies Coordinating Center at the University of North Carolina at Chapel Hill was responsible for protocols and training manuals for all measurements as well as the review, processing, and analyses of all data. Data were collected by trained personnel. Participants wore light clothing without shoes during collection of height, measured to the nearest centimeter (cm), and weight, measured to the nearest kilogram (kg). BMI was calculated as weight (kg)/height (m)2.
Blood pressure was measured 3 times using a random zero mercury sphygmomanometer on the right arm with the participant seated. The average of the last 2 measurements was used in the analyses. Blood pressure thresholds were based on the National High Blood Pressure Education Program Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Participants were classified as hypertensive if (a) systolic blood pressure was ≥140 mm Hg, (b) diastolic blood pressure was ≥90 mm Hg, or (c) self-report of current antihypertensive medication use.
Demographic and lifestyle factors were collected with standardized interviewer administered questionnaires. Cigarettes smoked per day (cig/d) were reported and alcohol consumed per day (g/d) was derived from monthly alcohol consumption levels. Subjects reported highest level of education attained (<senior middle school, senior middle school, and >senior middle school). Parental history of hypertension was determined based on self-report of whether a participant’s mother, father, both, or neither parent had high blood pressure. Subjects had the option to report “unknown,” but this was not selected by any of the study participants. Subjects were classified as having a parental history of hypertension if hypertension was reported by at least one parent.
Statistical Analyses
Our primary objective was to examine the association of BMI with hypertension and determine if this relationship varied by parental history of hypertension status and by age. We found a significant (P = .02) 3-way interaction between BMI, parental history of hypertension status, and age using the Wald test. We also examined effect modification by gender using the Wald test with 1 degree of freedom, but this was not found (P > .1). Therefore, logistic models were stratified into 24- to 39-year and 40- to 71-year age groups and included a BMI–parental history of hypertension interaction, with BMI in the continuous form. We calculated the adjusted prevalence per 1000 persons for BMI values 15 to 30 kg/m2 and the prevalence difference per 1000 persons using a BMI of 18.5 kg/m2, the lowest value within the range of the normal BMI category, as the referent. The “prvalue” command in Stata (Version 10.0) was used to calculate the prevalence and prevalence difference.18 Estimates were adjusted to the mean age, cigarettes, alcohol use, and distribution of gender and field center. The delta method was used to calculate the standard errors and 95% confidence intervals for the prevalence and prevalence difference.
Results
Characteristics of the 4104 participants varied by age and by parental history of hypertension status (Table 1). A parental history of hypertension was reported by 18.6% of the study population. In both age strata, compared with subjects who reported a parental history of hypertension, subjects who reported no parental history of hypertension had slightly lower systolic and diastolic blood pressure, prevalence of hypertension, BMI and education attainment but slightly higher consumption of alcohol and cigarettes. This sample is quite thin as approximately one fifth of the young adults and one fourth of the older adults had a BMI less than 18.5 kg/m2. For both age groups, BMI was positively associated with the prevalence difference for hypertension among both parental history groups (Figures 1 and 2). However, age-related differences in the associations of parental history of hypertension and BMI with hypertension were observed.
Table 1.
Demographic Characteristics of Chinese Adults by Age Group and Parental History of Hypertension Status,a the People’s Republic of China Study (1983)
| Parental History of Hypertension Status | Young Adults (24–39 Years)
|
Middle-Aged Adults (40–71 Years)
|
||
|---|---|---|---|---|
| Negative (n = 1866) | Positive (n = 474) | Negative (n = 1473) | Positive (n = 291) | |
| Age in years; mean (SD) | 32.2 (4.0) | 32.6 (3.9) | 49.7 (6.7) | 46.6 (5.4) |
| Gender (% women) | 57.6 | 50.4 | 48.1 | 49.1 |
| Body mass index in kg/m2; mean (SD) | 20.1 (2.0) | 20.4 (2.3) | 20.2 (2.6) | 21.0 (2.9) |
| Body mass index category (%) | ||||
| <18.5 kg/m2 | 20.6 | 18.4 | 27.0 | 23.0 |
| 18.5 to <21.0 kg/m2 | 52.0 | 46.2 | 41.3 | 32.7 |
| 21.0 to <23.0 kg/m2 | 19.8 | 22.8 | 18.9 | 21.3 |
| 23.0 to <25.0 kg/m2 | 5.3 | 9.1 | 8.5 | 13.4 |
| 25.0 to <27.5 kg/m2 | 1.9 | 3.0 | 3.4 | 7.6 |
| ≥27.5 kg/m2 | 0.5 | 0.6 | 0.9 | 2.1 |
| Parental history of hypertension (%) | ||||
| Father | 55.7 | 44.3 | ||
| Mother | 38.0 | 47.8 | ||
| Both parents | 6.3 | 7.9 | ||
| Current cigarette or leaf smokers (%) | 33.9 | 32.5 | 44.7 | 40.2 |
| Cigarettes or leaf smoked in cigarettes/day; mean (SD) | 5.6 (9.4) | 5.3 (8.9) | 8.3 (12.0) | 8.3 (12.0) |
| Current drinkers (%) | 21.9 | 13.9 | 26.1 | 18.9 |
| Alcohol consumed in g/day; mean (SD) | 10.8 (33.9) | 6.7 (26.4) | 17.7 (47.7) | 11.4 (38.1) |
| Educational attainment (%) | ||||
| <Senior middle school | 77.3 | 56.8 | 85.4 | 76.3 |
| Senior middle school | 13.7 | 28.3 | 5.0 | 10.0 |
| >Senior middle school | 1.8 | 4.6 | 1.2 | 4.8 |
| Unknown | 7.1 | 10.3 | 8.4 | 8.9 |
| Field center (% urban) | 43.0 | 75.5 | 44.9 | 69.1 |
| Systolic blood pressure in mm Hg; mean (SD) | 110.3 (11.2) | 113.0 (11.6) | 115.9 (17.9) | 119.7 (20.4) |
| Diastolic blood pressure in mm Hg; mean (SD) | 69.0 (8.8) | 72.0 (9.2) | 74.2 (10.4) | 77.8 (11.4) |
| Hypertension status (%) | ||||
| Prehypertensiveb | 22.8 | 25.3 | 27.7 | 29.2 |
| Hypertensivec | 2.1 | 6.1 | 12.0 | 18.6 |
| Blood pressure medication use (%) | 0.2 | 0.2 | 1.0 | 2.8 |
Subjects were classified as having a positive parental history of hypertension if hypertension was reported by at least one parent and negative if hypertension was not reported in either parent.
Prehypertensive includes subjects who were not hypertensive with a systolic blood pressure of 120 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg.
Hypertensive includes subjects with a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-report of current antihypertensive medication use.
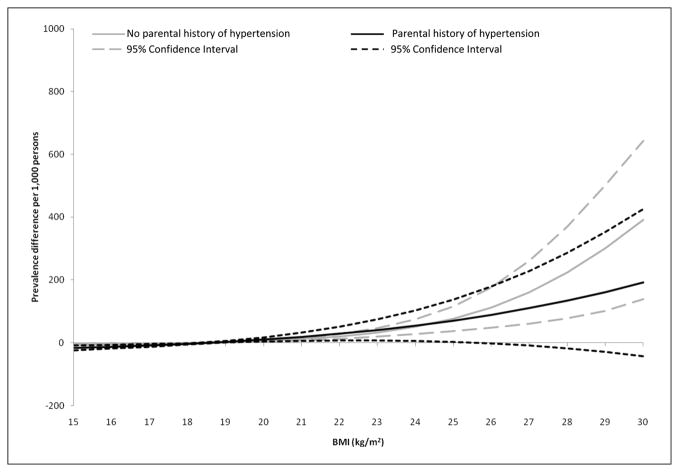
Figure 1.
Adjusted prevalence difference and 95% confidence interval for hypertension by body mass index (BMI) and parental history of hypertension status in Chinese young adults (24–39 years)
Models adjusted to the mean age, cigarettes (smoked), alcohol consumed (g/d), and distribution of gender and field center. Prevalence differences were computed using a BMI of 18.5 kg/m2 as the referent.
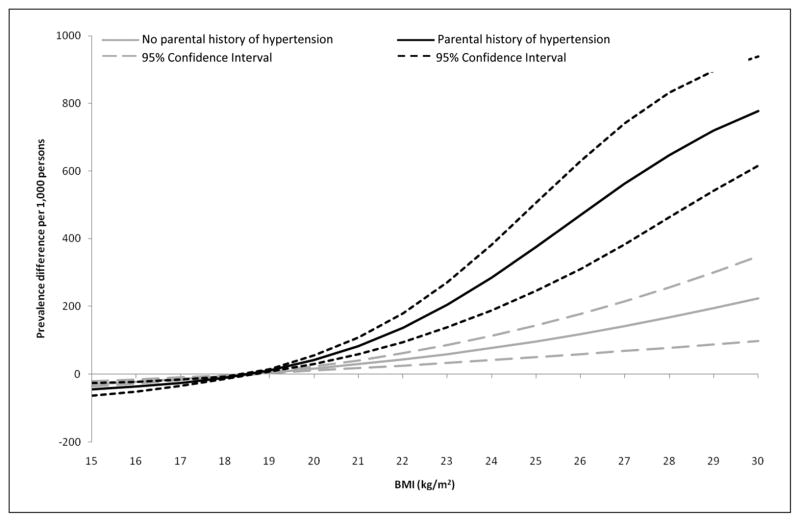
Figure 2.
Adjusted prevalence difference and 95% confidence interval for hypertension by body mass index (BMI) and parental history of hypertension status in Chinese middle-aged adults (40–71 years)
Models adjusted to the mean age, cigarettes (smoked), alcohol consumed (g/d), and distribution of gender and field center. Prevalence differences were computed using a BMI of 18.5 kg/m2 as the referent.
Among young adults (24–39 years), the adjusted prevalence (95% confidence interval [CI]) of hypertension was significantly associated with hypertension prevalence. The prevalence of hypertension at a BMI of 18.5 kg/m2 was approximately 4-fold greater among subjects who reported a parental history of hypertension (35; 95% CI = 15–54) per 1000 persons) compared with subjects who reported no parental history (7; 95% CI = 3–11) per 1000 persons; Table 2. The adjusted prevalence difference associated with an increase in BMI >18.5 kg/m2 was similar between subjects regardless of parental history and almost identical within the underweight (<18.5 kg/m2) and normal weight (18.5–24.9 kg/m2) categories (Figure 1). This suggests that among young Chinese adults parental history of hypertension is associated with hypertension among offspring, but parental history status does not modify the prevalence difference of BMI with hypertension.
Table 2.
Adjusted Prevalencea of Hypertension per 1000 Persons (95% CI) at a BMI of 18.5 kg/m2 by Age Group and Parental History of Hypertensionb in Chinese Adults
| Young Adults (24–39 Years)
|
Middle-Aged Adults (40–71 Years)
|
|||
|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |
| Hypertensionc | 7 (3, 11) | 35 (15, 54) | 77 (61, 94) | 61 (27, 96) |
Abbreviations: CI, confidence interval; BMI, body mass index.
Model includes age, alcohol, smoking, gender, and field center.
Subjects were classified as having a positive parental history of hypertension if hypertension was reported by at least one parent and negative if hypertension was not reported in either parent.
Hypertension was defined as a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-report of current antihypertensive medication use.
In middle-aged (40–71 years) adults, the adjusted prevalence of hypertension per 1000 persons at the reference level of BMI (18.5 kg/m2) was similar by parental history status: 77 (95% CI = 61–94) for subjects with no parental history and 61 (95% CI = 27–96) for subjects with parental history (Table 2). Although the baseline prevalence at the referent value was similar between groups, the prevalence difference for hypertension associated with BMI varied by parental history (Figure 2). Across BMI, even at values within the normal BMI range (18.5–24.9 kg/m2), the risk associated with BMI was greater among subjects with a parental history of hypertension. This suggests that among middle-aged adults, parental history of hypertension alone is not associated with a greater risk of hypertension, but subjects with a parental history of hypertension are more sensitive to the effects of obesity as observed by the greater prevalence difference for hypertension associated with BMI.
Discussion
Our goal was to determine the association of BMI with prevalent hypertension in Chinese adults and to examine if this relationship differed by parental history of hypertension and by age. In both age groups, BMI was positively associated with hypertension prevalence. However, the association of parental history with hypertension prevalence was not consistent across age stratum. In the young adults (24–39 years), parental history was associated with a 4-fold greater risk of hypertension but the effects of obesity on prevalence differences were similar across parental history groups. In the middle-aged (40–71 years) group, the prevalence of hypertension was similar by parental history status, but subjects with a parental history of hypertension were much more susceptible to the effects of BMI as observed by the associations with the prevalence difference.
When familial associations of blood pressure thresholds, were examined by age, studies in Caucasians have shown parental hypertension that develops before age 60 is associated with a greater risk of hypertension in offspring, whereas onset of parental hypertension at 70 years or older, was associated with no greater risk of hypertension in offspring than that of the general population.4,19 It is thought that hypertension that occurs much later in life is associated with weaker genetic and environmental factors that are less likely to be shared between parent and child. One explanation as to why parental hypertension status was not related to hypertension onset in the middle-aged adults in our sample may be related to differences in age of hypertension onset among their parents. Data from the Johns Hopkins Precursors Study, which included 1160 White medical students with more than 54 years of follow-up, found that among subjects with parental history of hypertension in at least one parent, hypertension risk among offspring was highest in early adulthood (before age 35 years) and progressively declined with age.20 This was similar to our findings whereby parental history was associated with hypertension in young adults but not middle-aged adults. However, we cannot fully examine these associations as data related to the age of hypertension onset among subjects or their parents were not collected in this study.
Few studies have explored the interaction of parental history of hypertension status and BMI with hypertension risk. Examination of this relationship, which included Caucasians and African Americans in the Atherosclerosis Risk in Communities Study (ARIC), indicated no effect modification of parental history status by BMI.21 A cross-sectional study of 503 Swedish middle aged adults examined the independent and combined effects of parental history and obesity (a relative BMI percentage of ≥120%) with hypertension.22 Compared with non-obese subjects with no parental history of hypertension, the odds ratios, adjusted for age, gender, and physical activity, were 3.0 (95% CI = 1.1–8.0) for a parental history of hypertension, 4.8 (95% CI = 1.8–13.0) for obesity, and 9.1 (95% CI = 4.1–20.1) for the joint effects of obesity and a parental history of hypertension. Another cross-sectional study that examined the independent and joint effects of obesity (BMI > 26 kg/m2) and family history of hypertension (in siblings and parents) with hypertension included a large Japanese sample (n = 9914).23 Odds ratios, adjusted for age, gender, smoking, alcohol use, and exercise, were graphed without confidence intervals. The approximate values were 2.4 for obesity, 2.8 for a family history of hypertension, and 7.0 for their joint effects. In both the Swedish and Japanese cohorts, the independent and joint effects of obesity and family history of hypertension were associated with a greater risk of hypertension compared with non-obese subjects with no parental history of hypertension. Although the risks of their joint effects were larger than the sum of their independent effects, interactions between these factors did not appear statistically significant.
A very limited number of studies have examined the association of parental hypertension with offspring hypertension in the context of both age and obesity. In 1979, a nationwide screening program of 720 000 U.S. whites and blacks found family history was independently associated with hypertension prevalence in both 20- to 39-year-old and 40- to 64-year-old adults.24 When self-report weight class was also considered, the prevalence of hypertension was 3 to 4 times greater among overweight individuals with a parental history status compared with normal weight individuals with no parental history of hypertension. Excess cases of hypertension attributed to overweight among individuals with a parental history was 136 per 1000 among 20- to 39-year-olds and 262 per 1000 among the 40- to 64-year-olds. To our knowledge, no other studies on hypertension have reported results by weight and parental history of hypertension.
Parental hypertension status was not validated in our sample and therefore, recall and information bias may be a problem. Reports of parental history of disease have been linked to disease prevalence, access to care, family communication behaviors, and health-seeking behaviors. These factors differ within and between populations. Among Chinese adults, rates of hypertension awareness (44.7%), treatment (28.2%), and control (8.1%)25 are low compared with rates in the United States where rates of awareness (75.7%), treatment (65.1%), and control (36.8%) are much higher.26 If only about half of Chinese mothers and fathers who were hypertensive were aware of their hypertension, offspring reports of parental history status were most likely underestimated in our sample. Results from U.S. studies suggest that the sensitivity and specificity of self-report parental history of hypertension are fairly high, at 76% and 84%, respectively.27 These rates may not be applicable to our population. A study of West Africans in Gambia28 found that subjects who reported a family history of noncommunicable disease were younger, were more likely to live in a city, have higher education status, and were more likely to be female. This was similar to our findings in which subjects reporting a parental history tended to be younger, female, have higher education, and be from the urban center. Most likely, when examining parental history status, this would bias our estimates toward the null. As hypertension awareness changes across time, older individuals may be even less aware of parental history of hypertension compared with younger generations.
Although our sample was population based, subjects were from one region in southern China, Guangzhou. Regional differences in the prevalence of hypertension within China have been observed.29 Hypertension is less common in southern China, where diet and exercise patterns are substantially different from the north. In addition, the prevalence of hypertension is lower in rural than in urban areas, which has also been suggested to be related to higher levels of physical activity and lower levels of overweight. Diet and physical activity patterns have evolved over time and dietary and physical activity patterns of previous generations may have protected individuals from developing hypertension. The lack of association between parental history of hypertension and hypertension prevalence in the 40- to 71-year-olds may in part be because of shifts in dietary and physical activity patterns or to other factors described above such as age-related differences in parental hypertension onset and hypertension awareness.
In summary, interactions between BMI, parental history of hypertension, and age were found to influence hypertension risk in Chinese. Strengths of this work include the large population-based sample of Chinese, a group in which parental history of hypertension has largely been unexplored, use of measured anthropometrics and blood pressure, and estimation using difference rather than ratio measures.30 The present study may be limited in that our analysis was cross-sectional, limited to one region of China, and information regarding the age of hypertension onset among subjects were not collected. It is possible that subjects in the 40- to 71-year-old age stratum may have developed hypertension during young adulthood. Therefore, we cannot clearly separate the different findings between age stratums. Additional limitations include a lack of dietary, physical activity, or parental BMI data and use of self-report parental history of hypertension. The relationship between parental history status, BMI, and age may be clarified with a prospective cohort begun in early adulthood and collected validated measures of parental history status. Hypertension and obesity are preventable risk factors for cardiovascular disease, which is a leading cause of death in China. The interaction of BMI and parental history of hypertension on hypertension prevalence has not been well studied and deserves further attention as this information could help inform public health efforts designed to identify individuals at risk of hypertension for prevention and early treatment.
Acknowledgments
The authors thank the staff and participants of the People’s Republic of China Study for their important contributions.
The People’s Republic of China Study was carried out by the National Heart, Lung, and Blood Institute under contracts N01-HV-12243, N01-HV-08112, and N01-HV-59224 with the University of North Carolina at Chapel Hill and the People’s Republic of China Ministry of Public Health, the Cardiovascular Institute, and Fu Wai Hospital, Chinese Academy of Medical Sciences, Beijing, and the Guangdong Provincial Cardiovascular Institute, Guangzhou.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:
This study was supported by the Carolina Program for Health and Aging Research (CPHAR) of the University of North Carolina Institute on Aging (2T32AG000272-06A2); by a grant (RR00046) from the General Clinical Research Centers program of the Division of Research Resources, National Institutes of Health; by a grant (R01 DK069678) from the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK056350-05S2); and by a grant from the National Center for Research Resources (ULIRR025747).
Footnotes
Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the conse quences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007;31:177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- 2.Parikh NI, Pencina MJ, Wang TJ, et al. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Intern Med. 2008;148:102–110. doi: 10.7326/0003-4819-148-2-200801150-00005. [DOI] [PubMed] [Google Scholar]
- 3.Stevens J, Truesdale KP, Katz EG, Cai J. Impact of body mass index on incident hypertension and diabetes in Chinese Asians, American Whites, and American Blacks: the People’s Republic of China Study and the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2008;167:1365–1374. doi: 10.1093/aje/kwn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lascaux-Lefebvre V, Ruidavets J, Arveiler D, et al. Influence of parental history of hypertension on blood pressure. J Hum Hypertens. 1999;13:631–636. doi: 10.1038/sj.jhh.1000884. [DOI] [PubMed] [Google Scholar]
- 5.Tozawa M, Oshiro S, Iseki C, et al. Family history of hypertension and blood pressure in a screened cohort. Hypertens Res. 2001;24:93–98. [Google Scholar]
- 6.Guanglin W, Huimin Y, Xiuying Q, Zhenlin J. A case-control for the association between change in weight, family history and hypertension at different ages. Asia Pac J Public Health. 2001;13:96–99. doi: 10.1177/101053950101300207. [DOI] [PubMed] [Google Scholar]
- 7.Mongeau JG. Heredity and blood pressure in humans: an overview. Pediatr Nephrol. 1987;1:69–75. doi: 10.1007/BF00866887. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Kan D, Province M, et al. An updated meta-analysis of genome scans for hypertension and blood pressure in the NHLBI Family Blood Pressure Program (FBPP) Am J Hypertens. 2006;19:122–127. doi: 10.1016/j.amjhyper.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Hottenga JJ, Boomsma DI, Kupper N, et al. Heritability and stability of resting blood pressure. Twin Res Hum Genet. 2005;8:499–508. doi: 10.1375/183242705774310123. [DOI] [PubMed] [Google Scholar]
- 10.Chien KL, Hsu HC, Chen WJ, Chen MF, Su TC, Lee YT. Familial aggregation of metabolic syndrome among the Chinese: report from the Chin-Shan community family study. Diabetes Res Clin Pract. 2007;76:418–424. doi: 10.1016/j.diabres.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Wang JG, Liu L, Zagato L, et al. Blood pressure in relation to three candidate genes in a Chinese population. J Hypertens. 2004;22:937–944. doi: 10.1097/00004872-200405000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Burke GL, Savage PJ, Sprafka JM, et al. Relation of risk factor levels in young adulthood to parental history of disease. The CARDIA study. Circulation. 1991;84:1176–1187. doi: 10.1161/01.cir.84.3.1176. [DOI] [PubMed] [Google Scholar]
- 13.Grotto I, Huerta M, Kark JD, Shpilberg O, Meyerovitch J. Relation of parental history of coronary heart disease to obesity in young adults. Int J Obes Relat Metab Disord. 2003;27:362–368. doi: 10.1038/sj.ijo.0802242. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Alpert BS, Walker SS, Somes GW. Longitudinal relationship of parental hypertension with body mass index, blood pressure, and cardiovascular reactivity in children. J Pediatr. 2007;150:498–502. doi: 10.1016/j.jpeds.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 15.van den Elzen AP, de Ridder MA, Grobbee DE, Hofman A, Witteman JC, Uiterwaal CS. Families and the natural history of blood pressure. A 27-year follow-up study. Am J Hypertens. 2004;17:936–940. doi: 10.1016/j.amjhyper.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Simsolo RB, Romo MM, Rabinovich L, Bonanno M, Grunfeld B. Family history of essential hypertension versus obesity as risk factors for hypertension in adolescents. Am J Hypertens. 1999;12:260–263. doi: 10.1016/s0895-7061(98)00253-2. [DOI] [PubMed] [Google Scholar]
- 17.An epidemiological study of cardiovascular and cardiopulmonary disease risk factors in four populations in the People’s Republic of China. Baseline report from the P.R.C.-U.S.A. Collaborative Study. People’s Republic of China–United States Cardiovascular and Cardiopulmonary Epidemiology Research Group. Circulation. 1992;85:1083–1096. doi: 10.1161/01.cir.85.3.1083. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Long JS. [Accessed April 24, 2011];Using the delta method to construct confidence intervals for predicted probabilities, rates, and discrete changes. http://www.indiana.edu/~jslsoc/stata/ci_computations/spost_deltaci.pdf.
- 19.Goldstein IB, Shapiro D, Guthrie D. Ambulatory blood pressure and family history of hypertension in healthy men and women. Am J Hypertens. 2006;19:486–491. doi: 10.1016/j.amjhyper.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Wang NY, Young JH, Meoni LA, Ford DE, Erlinger TP, Klag MJ. Blood pressure change and risk of hypertension associated with parental hypertension: the Johns Hopkins Precursors Study. Arch Intern Med. 2008;168:643–648. doi: 10.1001/archinte.168.6.643. [DOI] [PubMed] [Google Scholar]
- 21.Liese AD, Mayer-Davis EJ, Tyroler HA, et al. Familial components of the multiple metabolic syndrome: the ARIC study. Diabetologia. 1997;40:963–970. doi: 10.1007/s001250050775. [DOI] [PubMed] [Google Scholar]
- 22.Cederholm J, Wibell L. Influences of familial and environmental factors on hypertension. Ups J Med Sci. 1991;96:239–246. doi: 10.3109/03009739109179276. [DOI] [PubMed] [Google Scholar]
- 23.Tozawa M, Oshiro S, Iseki C, et al. Multiple risk factor clustering of hypertension in a screened cohort. J Hypertens. 2000;18:1379–1385. doi: 10.1097/00004872-200018100-00004. [DOI] [PubMed] [Google Scholar]
- 24.Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Family (parental) history and prevalence of hypertension. Results of a nationwide screening program. JAMA. 1979;241:43–46. [PubMed] [Google Scholar]
- 25.Gu D, Reynolds K, Wu X, et al. Prevalence, awareness, treatment, and control of hypertension in china. Hypertension. 2002;40:920–927. doi: 10.1161/01.hyp.0000040263.94619.d5. [DOI] [PubMed] [Google Scholar]
- 26.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 27.Bensen JT, Liese AD, Rushing JT, et al. Accuracy of proband reported family history: the NHLBI Family Heart Study (FHS) Genet Epidemiol. 1999;17:141–150. doi: 10.1002/(SICI)1098-2272(1999)17:2<141::AID-GEPI4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.van der Sande MA, Walraven GE, Milligan PJ, et al. Family history: an opportunity for early interventions and improved control of hypertension, obesity and diabetes. Bull World Health Organ. 2001;79:321–328. [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Z, Wu X, Stamler J, et al. A north-south comparison of blood pressure and factors related to blood pressure in the People’s Republic of China: a report from the PRC-USA Collaborative Study of Cardiovascular Epidemiology. J Hypertens. 1994;12:1103–1112. [PubMed] [Google Scholar]
- 30.Stevens J. Ethnic-specific revisions of body mass index cutoffs to define overweight and obesity in Asians are not warranted. Int J Obes Relat Metab Disord. 2003;27:1297–1299. doi: 10.1038/sj.ijo.0802417. [DOI] [PubMed] [Google Scholar]