Abstract
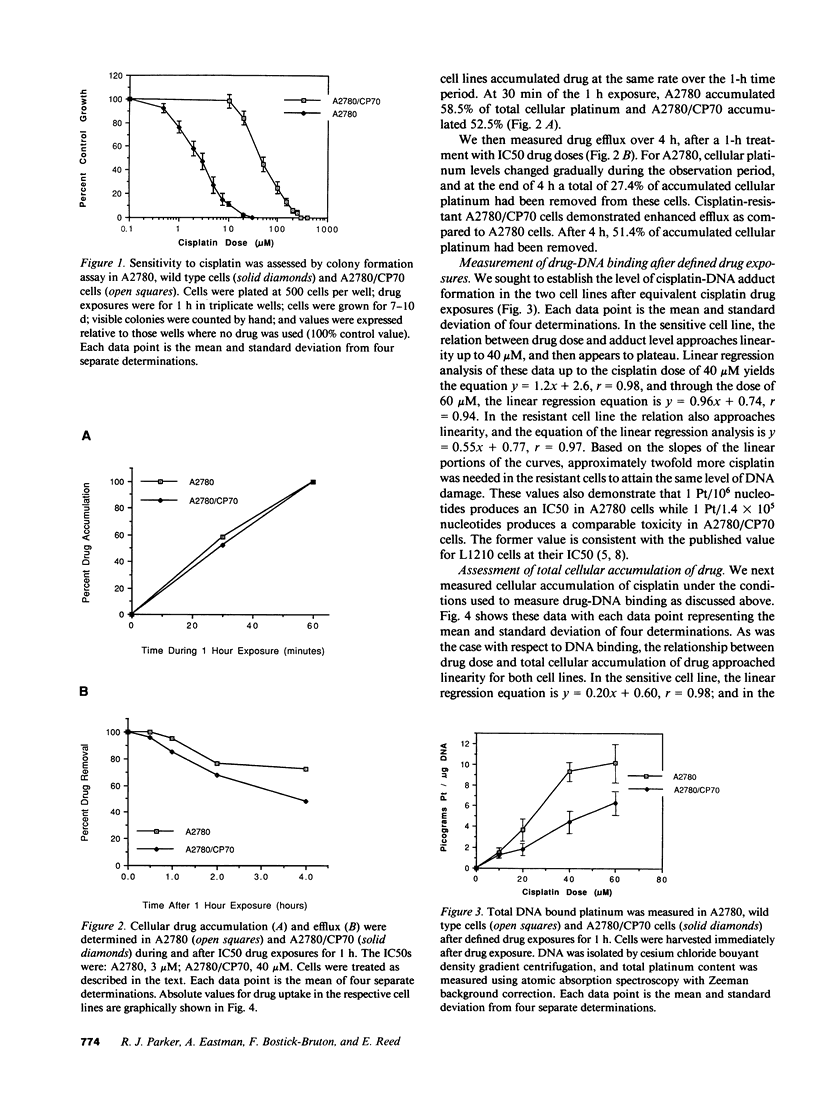
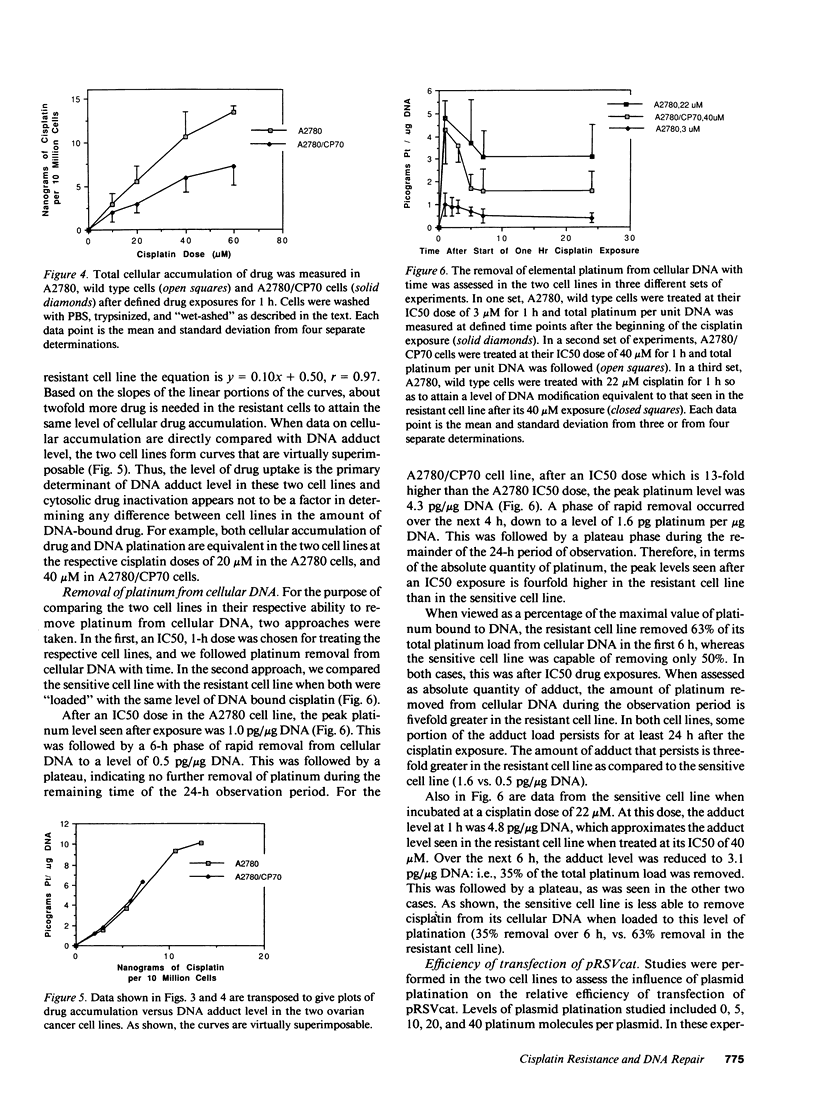
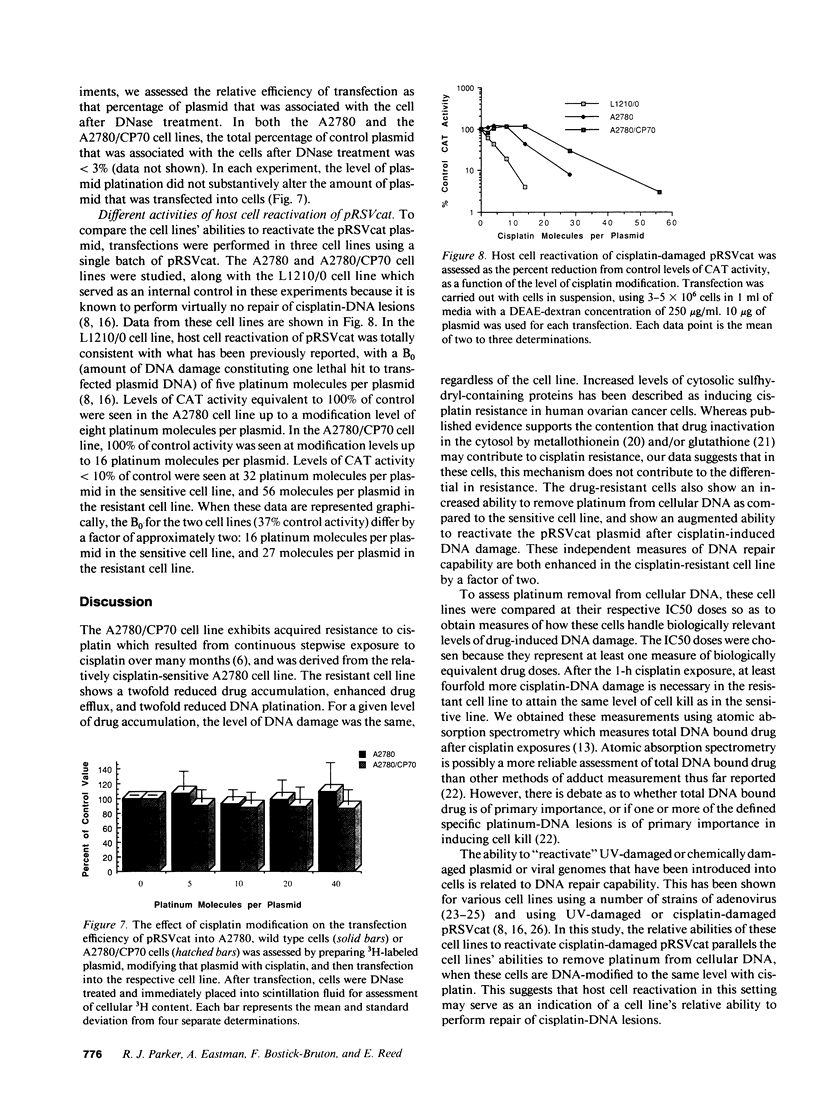
Studies were undertaken to investigate acquired resistance to cisplatin in human ovarian cancer cells. The cell lines A2780 and A2780/CP70 were studied to assess their respective characteristics of drug accumulation and efflux, cytosolic inactivation of drug, and DNA repair. All experiments were performed using 1-h drug exposures. The A2780/CP70 cell line was 13-fold more resistant to cisplatin than A2780 cells. When studied at their respective IC50 doses, drug accumulation rates were similar for the two cell lines. However, the resistant cell line was twofold more efficient at effluxing drug, which was associated with reduced total drug accumulation for equivalent micromolar drug exposures. At equivalent levels of total cellular drug accumulation, the two cell lines formed the same levels of cisplatin-DNA damage, suggesting that cytosolic inactivation of drug does not contribute to the differential in resistance between these cell lines. Resistant cells were also twofold more efficient at repairing cisplatin-DNA lesions in cellular DNA and in transfected plasmid DNA. We conclude that in these paired cell lines, alterations in drug uptake/efflux and in DNA repair are the major contributing factors to acquired resistance to cisplatin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. A., Murphy M. P., Howell S. B. Metallothionein-mediated cisplatin resistance in human ovarian carcinoma cells. Cancer Chemother Pharmacol. 1987;19(2):149–154. doi: 10.1007/BF00254568. [DOI] [PubMed] [Google Scholar]
- Bedford P., Fichtinger-Schepman A. M., Shellard S. A., Walker M. C., Masters J. R., Hill B. T. Differential repair of platinum-DNA adducts in human bladder and testicular tumor continuous cell lines. Cancer Res. 1988 Jun 1;48(11):3019–3024. [PubMed] [Google Scholar]
- Day R. S., 3rd Cellular reactivation of ultraviolet-irradiated human adenovirus 2 in normal and xeroderma pigmentosum fibroblasts. Photochem Photobiol. 1974 Jan;19(1):9–13. doi: 10.1111/j.1751-1097.1974.tb06467.x. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Giuffrida A. S., Dingman C. W. Repair by human cells of adenovirus-2 damaged by psoralen plus near ultraviolet light treatment. Mutat Res. 1975 Dec;33(2-3):311–320. doi: 10.1016/0027-5107(75)90206-7. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Scudiero D., Dimattina M. Excision repair by human fibroblasts of DNA damaged by r-7, t-8-dihyroxy-t-9,10-oxy-7,8,9,10- tetrahydrobenzo(a)pyrene. Mutat Res. 1978 Jun;50(3):383–394. doi: 10.1016/0027-5107(78)90043-x. [DOI] [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Eastman A., Schulte N. Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry. 1988 Jun 28;27(13):4730–4734. doi: 10.1021/bi00413a022. [DOI] [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Knox R. J., Friedlos F., Lydall D. A., Roberts J. J. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986 Apr;46(4 Pt 2):1972–1979. [PubMed] [Google Scholar]
- Lai G. M., Ozols R. F., Smyth J. F., Young R. C., Hamilton T. C. Enhanced DNA repair and resistance to cisplatin in human ovarian cancer. Biochem Pharmacol. 1988 Dec 15;37(24):4597–4600. doi: 10.1016/0006-2952(88)90325-5. [DOI] [PubMed] [Google Scholar]
- Lai G. M., Ozols R. F., Young R. C., Hamilton T. C. Effect of glutathione on DNA repair in cisplatin-resistant human ovarian cancer cell lines. J Natl Cancer Inst. 1989 Apr 5;81(7):535–539. doi: 10.1093/jnci/81.7.535. [DOI] [PubMed] [Google Scholar]
- Masuda H., Tanaka T., Matsuda H., Kusaba I. Increased removal of DNA-bound platinum in a human ovarian cancer cell line resistant to cis-diamminedichloroplatinum(II). Cancer Res. 1990 Mar 15;50(6):1863–1866. [PubMed] [Google Scholar]
- Milman G., Herzberg M. Efficient DNA transfection and rapid assay for thymidine kinase activity and viral antigenic determinants. Somatic Cell Genet. 1981 Mar;7(2):161–170. doi: 10.1007/BF01567655. [DOI] [PubMed] [Google Scholar]
- Poirier M. C., Egorin M. J., Fichtinger-Schepman A. M., Yuspa S. H., Reed E. DNA adducts of cisplatin and carboplatin in tissues of cancer patients. IARC Sci Publ. 1988;(89):313–320. [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed E., Ostchega Y., Steinberg S. M., Yuspa S. H., Young R. C., Ozols R. F., Poirier M. C. Evaluation of platinum-DNA adduct levels relative to known prognostic variables in a cohort of ovarian cancer patients. Cancer Res. 1990 Apr 15;50(8):2256–2260. [PubMed] [Google Scholar]
- Reed E., Ozols R. F., Tarone R., Yuspa S. H., Poirier M. C. Platinum-DNA adducts in leukocyte DNA correlate with disease response in ovarian cancer patients receiving platinum-based chemotherapy. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5024–5028. doi: 10.1073/pnas.84.14.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed E., Yuspa S. H., Zwelling L. A., Ozols R. F., Poirier M. C. Quantitation of cis-diamminedichloroplatinum II (cisplatin)-DNA-intrastrand adducts in testicular and ovarian cancer patients receiving cisplatin chemotherapy. J Clin Invest. 1986 Feb;77(2):545–550. doi: 10.1172/JCI112335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani N., Jennerwein M. M., Eastman A. DNA repair in cells sensitive and resistant to cis-diamminedichloroplatinum(II): host cell reactivation of damaged plasmid DNA. Biochemistry. 1989 Apr 4;28(7):3120–3124. doi: 10.1021/bi00433a055. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Anderson T., Kohn K. W. DNA-protein and DNA interstrand cross-linking by cis- and trans-platinum(II) diamminedichloride in L1210 mouse leukemia cells and relation to cytotoxicity. Cancer Res. 1979 Feb;39(2 Pt 1):365–369. [PubMed] [Google Scholar]


