Abstract
1. During nectar and pollen foraging in a temperate climate, honeybees are exposed to a broad range of ambient temperatures, challenging their thermoregulatory ability. The body temperature that the bees exhibit results from endothermic heat production, exogenous heat gain from solar radiation, and heat loss. In addition to profitability of foraging, season was suggested to have a considerable influence on thermoregulation. To assess the relative importance of these factors, the thermoregulatory behaviour of foragers on 33 flowering plants in dependence on season and environmental factors was investigated.
2. The bees (Apis mellifera carnica Pollman) were always endothermic. On average, the thorax surface temperature (Tth) was regulated at a high and rather constant level over a broad range of ambient temperatures (Tth = 33.7–35.7°C, Ta = 10–27°C). However, at a certain Ta, Tth showed a strong variation, depending on the plants from which the bees were foraging. At warmer conditions (Ta = 27–32°C) the Tth increased nearly linearly with Ta to a maximal average level of 42.6 °C. The thorax temperature excess decreased strongly with increasing Ta (Tth−Ta = 21.6 − 3.6°C).
3. The bees used the heat gain from solar radiation to elevate the temperature excess of thorax, head, and abdomen. Seasonal dependance was reflected in a 2.7 °C higher mean Tth in the spring than in the summer. An anova revealed that season had the greatest effect on Tth, followed by Ta and radiation.
4. It was presumed the foragers' motivational status to be the main factor responsible for the variation of Tth between seasons and different plants.
Keywords: Body temperature, flower, foraging, honeybee, season, thermoregulation
Introduction
Honeybees need nectar and pollen to provide for their young bees and brood. Honey supplies energy for heat production to achieve a constant brood temperature and for overwintering in a temperate climate (Stabentheiner et al., 2003a, 2010). During foraging, bees are mostly highly endothermic. They may exhibit thoracic temperatures higher than 40 °C (e.g. Heinrich, 1979a; Cooper et al., 1985; Schmaranzer & Stabentheiner, 1988; Kovac & Schmaranzer, 1996; Schmaranzer, 2000; Kovac et al., 2010). Thermoregulatory investigations of honeybees during foraging on natural sources in their environment are very scarce. Heinrich (1979a) measured thoracic (core) temperatures of Apis mellifera mellifera and Apis mellifera adansonii Linnaeus during foraging on Eucalyptus sp., Bidens pilosa L., and Petrea volubilis L. Thoracic temperatures were regulated between 31 and 32 °C, differing insignificantly between the European honeybee and the African variety. Kovac and Schmaranzer (1996) measured body surface temperatures of honeybees (Apis mellifera carnica Pollman) foraging in the shade in the spring and summer at several different plants. The average thorax temperature varied in a broad range (Tth = 29.3–35.7°C, mean values per flower).
The body temperature of foraging insects is influenced by several environmental factors such as ambient air temperature, solar radiation (Willmer & Unwin, 1981), and convection (for an overview see Heinrich, 1993). The energy gain from solar radiation is of importance for the thermoregulation of foraging bees. An increase of the thorax temperature with increasing insolation was reported in Western honeybees arriving at the nest entrance after their foraging flights (Cena & Clark, 1972; Heinrich, 1979a; Cooper et al., 1985) and during nectar foraging (Heinrich, 1979a). Underwood (1991) reported the same for Indian honeybees collecting sugar syrup under sunny and overcast skies. Kovac et al. (2009a) investigated the influence of solar radiation on the thermoregulation of water-foraging wasps in detail. Vespula and Polistes increased the thorax temperature and reduced the active heat production as solar heat gain increased. In water-foraging honeybees, the relative contribution of endothermic heat production and heat gain from solar radiation on body temperature was observed by Kovac et al. (2010). Up to an ambient temperature of ∼30 °C, bees used solar heat gain for a dual purpose: to reduce energetic expenditure and to increase the thorax temperature by about 1–3 °C, in order to improve force production of flight muscles (Coelho, 1991a) and to speed up suction velocity (Kovac et al., 2010). The aim of the present study was to investigate the contribution of radiative heat gain on the bees' thermoregulation during foraging for nectar and pollen under natural conditions.
Kovac and Schmaranzer (1996), demonstrated in a comparison of honeybees foraging from 13 flowers, considerable variation of the thorax temperature. As a rule, the energy expenditure of individual foragers is balanced with the net energetic gains to the colony (Schmid-Hempel et al., 1985; Seeley et al., 1991; Seeley, 1995). The bees minimise the thermoregulatory costs during foraging by adapting their thorax temperature in response to the profitability of foraging at a food source and the colony's need for nectar and pollen (Stabentheiner & Schmaranzer, 1986, 1987; Dyer & Seeley, 1987; Schmaranzer & Stabentheiner, 1988; Waddington, 1990; Stabentheiner & Hagmüller, 1991; Underwood, 1991; Stabentheiner et al., 1995; Stabentheiner, 2001; Nieh et al., 2006; Sadler & Nieh, 2011). From these investigations, we know the thoracic temperature to vary in a broad range of ∼30–44 °C. As flowers differ considerably in their profitability, i.e. as they vary in the amount of pollen and concentration and flow of nectar, the distance between single blossoms, and because the bees adapt their thorax temperature to profitability, the bees' thorax temperature at a certain flower is not predictable from measurements at other flowers. Therefore, to get a broader overview of the foragers' thermoregulation in their temperate living space, we investigated them on flowers at different locations and environmental conditions.
Under Central European climate conditions, honeybee colonies undergo a typical seasonal population development, influenced by environmental and genetic parameters. The climax of the population strength and brood nest dimension is reached from the middle to the end of June (e.g. Seeley, 1985; Wille, 1985; Winston, 1987; Liebig, 1994; Imdorf et al., 1996). In spring, when the colonies have much brood and low food reserves, the bees should be more motivated to forage. In foraging honeybees, thorax temperature correlates with the insects' motivational state (e.g. Dyer & Seeley, 1987; Stabentheiner & Schmaranzer, 1987; Schmaranzer & Stabentheiner, 1988; Stabentheiner & Hagmüller, 1991; Underwood, 1991; Stabentheiner et al., 1995; Stabentheiner, 2001; Sadler & Nieh, 2011). Kovac and Schmaranzer (1996) presumed that season, beside ambient temperature, has an influence on thermoregulation. However, to test this hypothesis, data from more than 2 years and from multiple flowers were necessary, and measurements in sunshine had to be included (Kovac & Schmaranzer, 1996, had measured in shade). Our investigation covers a complete foraging season under Central European climate conditions. This allowed measurements over the entire range of ambient temperatures and solar radiation to which bees are probably exposed to during their foraging trips. Results should enable assessment of the relative importance of season and environmental factors.
Materials and methods
Animals, field site, and measuring conditions
Measuring locations were the botanical garden in Graz and several orchards and meadows near Graz, Austria, Central Europe. We investigated honeybees (A. mellifera carnica) foraging nectar and pollen on 33 different blossoms of flowers, shrubs and trees, and collecting water from a rainwater barrel. To cover the entire foraging season and range of ambient temperatures honeybees are exposed to under Central European climate conditions, measurements were made on 26 days from March to October in 2006 (Table 1). Measurements were performed in different weather conditions, from overcast sky to bright sunshine. If no flowers were available in shade, a patch of flowers was shaded by a sunshade.
Table 1.
Summary statistics for the surface temperature of the head, thorax and abdomen of foraging honeybees on different flowering plants, and blossom surface temperature near the bees' mouthparts (Tblossom), ambient temperature (Ta), relative humidity (rel. hum.), and solar radiation (sol. rad.) for each single measuring day divided in three classes of solar radiation.
| No. | Date | Plant | Rad. classes (W m−2) | Nbees | Nmeans | Thead (°C) | Tthorax (°C) | Tabdomen (°C) | Tblossom (°C) | Ta (°C) | Rel. hum. (%) | Sol. rad. (W m−2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 March 2006 | Crocus vernus | <200 | 1 | 1 | 29.1 ± 0.0 | 37.7 ± 0.0 | 19.4 ± 0.0 | 15.3 ± 0.0 | 17.3 | 33.8 | 193.0 |
| 200–500 | 16 | 250 | 29.4 ± 1.6 | 37.4 ± 1.6 | 19.8 ± 1.2 | 17.9 ± 1.2 | 18.4 | 30.5 | 369.7 | |||
| >500 | 1 | 6 | 27.9 ± 0.8 | 36.8 ± 2.2 | 22.1 ± 0.6 | 18.4 ± 1.2 | 16.8 | 33.6 | 535.7 | |||
| 2 | 4 April 2006 | Salix caprea | <200 | 14 | 137 | 24.2 ± 2.1 | 33.7 ± 2.9 | 14.8 ± 1.0 | 12.8 ± 1.0 | 12.7 | 41.0 | 179.7 |
| 200–500 | 24 | 183 | 25.5 ± 2.9 | 34.3 ± 3.4 | 16.3 ± 1.8 | 14.1 ± 1.8 | 13.1 | 39.5 | 249.6 | |||
| >500 | 24 | 365 | 28.3 ± 3.3 | 35.5 ± 3.4 | 18.9 ± 2.2 | 16.6 ± 3.2 | 14.1 | 38.2 | 903.8 | |||
| 3 | 20 April 2006 | Prunus armeniaca | <200 | 7 | 112 | 30.6 ± 1.6 | 36.6 ± 1.7 | 20.9 ± 1.0 | 18.1 ± 1.3 | 18.2 | 40.7 | 176.7 |
| 200–500 | 11 | 166 | 31.3 ± 1.9 | 36.4 ± 1.9 | 22.1 ± 1.8 | 18.6 ± 1.0 | 18.6 | 40.6 | 301.5 | |||
| >500 | 8 | 115 | 32.9 ± 2.1 | 36.4 ± 2.1 | 24.3 ± 1.6 | 19.5 ± 2.0 | 19.7 | 37.3 | 886.8 | |||
| 4 | 20 April 2006 | Cardamine pratensis | <200 | 14 | 59 | 28.8 ± 1.9 | 34.7 ± 2.9 | 21.3 ± 1.5 | 18.0 ± 1.4 | 18.4 | 35.9 | 186.1 |
| 200–500 | 14 | 69 | 29.0 ± 2.0 | 35.3 ± 2.6 | 21.6 ± 1.3 | 18.2 ± 1.1 | 18.9 | 37.2 | 211.0 | |||
| >500 | 25 | 253 | 30.1 ± 2.5 | 33.8 ± 3.3 | 23.3 ± 1.3 | 19.0 ± 1.2 | 18.7 | 37.1 | 814.0 | |||
| 5 | 20 April 2006 | Water | <200 | 79 | 200 | 29.0 ± 2.0 | 37.1 ± 2.3 | 24.8 ± 1.9 | 21.4 ± 1.5 | 18.0 | 45.4 | 151.1 |
| 200–500 | 18 | 43 | 31.4 ± 1.6 | 37.6 ± 2.5 | 29.6 ± 1.0 | 25.2 ± 1.6 | 19.5 | 37.5 | 317.7 | |||
| >500 | 4 | 7 | 32.6 ± 0.6 | 38.6 ± 1.8 | 30.5 ± 0.5 | 25.8 ± 1.5 | 18.8 | 30.1 | 678.7 | |||
| 6 | 22 April 2006 | Prunus sp. | <200 | 16 | 130 | 30.1 ± 2.1 | 35.5 ± 2.2 | 23.0 ± 1.0 | 20.0 ± 1.5 | 20.7 | 47.0 | 158.1 |
| 200–500 | 9 | 28 | 29.7 ± 1.9 | 35.0 ± 1.8 | 22.5 ± 0.7 | 19.2 ± 1.8 | 19.9 | 45.4 | 286.1 | |||
| >500 | 28 | 155 | 31.2 ± 2.8 | 34.9 ± 3.6 | 24.4 ± 1.4 | 20.9 ± 1.5 | 20.9 | 47.6 | 812.6 | |||
| 7 | 24 April 2006 | Cerasus avium | <200 | 11 | 97 | 28.6 ± 1.6 | 33.4 ± 2.0 | 21.9 ± 1.0 | 19.0 ± 1.3 | 19.4 | 47.8 | 141.3 |
| 200–500 | 4 | 20 | 28.7 ± 1.3 | 33.1 ± 2.0 | 23.2 ± 1.3 | 20.3 ± 1.4 | 18.5 | 51.2 | 355.9 | |||
| >500 | 9 | 29 | 27.5 ± 2.4 | 31.6 ± 2.5 | 22.5 ± 0.8 | 17.5 ± 4.9 | 19.2 | 52.8 | 673.8 | |||
| 8 | 24 April 2006 | Taraxacum officinalis | <200 | 13 | 92 | 32.9 ± 1.8 | 37.7 ± 1.5 | 28.3 ± 1.9 | 23.9 ± 2.2 | 22.7 | 42.8 | 178.1 |
| 200–500 | 9 | 34 | 32.8 ± 2.0 | 37.6 ± 1.2 | 29.8 ± 1.3 | 25.4 ± 2.7 | 22.5 | 39.8 | 212.8 | |||
| >500 | 23 | 212 | 37.2 ± 2.0 | 39.9 ± 1.7 | 34.7 ± 2.5 | 31.4 ± 2.9 | 24.0 | 41.8 | 981.4 | |||
| 9 | 4 May 2006 | Malus domestica | <200 | — | — | — | — | — | — | — | — | — |
| 200–500 | 17 | 71 | 24.7 ± 1.5 | 34.0 ± 1.8 | 19.1 ± 1.4 | 15.5 ± 0.7 | 15.8 | 56.4 | 403.5 | |||
| >500 | 19 | 109 | 27.8 ± 2.0 | 35.8 ± 2.3 | 21.8 ± 1.6 | 17.3 ± 1.2 | 16.8 | 50.4 | 862.2 | |||
| 10 | 4 May 2006 | Taraxacum officinalis | <200 | 9 | 127 | 24.9 ± 1.5 | 36.0 ± 1.8 | 19.8 ± 1.2 | 16.7 ± 1.3 | 16.0 | 57.2 | 153.5 |
| 200–500 | 6 | 78 | 26.2 ± 1.5 | 36.9 ± 1.3 | 20.4 ± 1.5 | 17.8 ± 1.4 | 16.5 | 56.1 | 243.2 | |||
| >500 | 4 | 24 | 29.5 ± 1.1 | 38.0 ± 1.1 | 25.0 ± 1.7 | 22.7 ± 2.7 | 18.4 | 49.7 | 893.8 | |||
| 11 | 8 May 2006 | Mahonia aquifolium | <200 | 12 | 166 | 25.5 ± 1.5 | 35.7 ± 1.9 | 18.3 ± 1.1 | 14.6 ± 1.3 | 14.9 | 58.9 | 105.3 |
| 200–500 | 22 | 199 | 26.1 ± 1.9 | 34.9 ± 1.6 | 19.8 ± 2.1 | 16.2 ± 1.7 | 16.1 | 53.5 | 251.0 | |||
| >500 | 7 | 50 | 28.7 ± 1.9 | 35.6 ± 1.1 | 23.3 ± 2.6 | 19.1 ± 2.1 | 18.4 | 54.6 | 793.9 | |||
| 12 | 11 May 2006 | Taraxacum officinalis | <200 | 15 | 211 | 27.2 ± 1.0 | 35.9 ± 1.4 | 21.5 ± 0.8 | 19.0 ± 1.2 | 17.6 | 53.3 | 71.1 |
| 200–500 | 9 | 63 | 27.8 ± 2.0 | 35.0 ± 1.8 | 23.2 ± 3.6 | 20.2 ± 2.8 | 17.9 | 51.0 | 287.9 | |||
| >500 | 30 | 276 | 32.7 ± 2.3 | 36.3 ± 1.9 | 32.4 ± 2.9 | 27.1 ± 3.3 | 20.9 | 76.1 | 1105.0 | |||
| 13 | 11 May 2006 | Mahonia aquifolium | <200 | 16 | 126 | 26.7 ± 1.4 | 34.3 ± 1.7 | 21.6 ± 1.4 | 18.1 ± 1.1 | 18.3 | 39.6 | 179.3 |
| 200–500 | 11 | 97 | 26.5 ± 1.2 | 33.9 ± 1.4 | 21.7 ± 1.6 | 18.7 ± 1.5 | 18.5 | 39.2 | 225.9 | |||
| >500 | 30 | 259 | 30.1 ± 2.1 | 34.4 ± 1.6 | 25.3 ± 2.1 | 21.3 ± 2.6 | 19.1 | 40.4 | 883.2 | |||
| 14 | 18 May 2006 | Brassica napus | <200 | 1 | 3 | 29.3 ± 0.9 | 37.8 ± 2.0 | 25.6 ± 1.3 | 21.4 ± 0.1 | 22.1 | 55.9 | 192.3 |
| 200–500 | 42 | 139 | 29.7 ± 1.6 | 36.0 ± 1.8 | 25.3 ± 1.3 | 22.3 ± 1.2 | 22.7 | 55.1 | 303.0 | |||
| >500 | 38 | 198 | 30.6 ± 1.6 | 35.8 ± 1.8 | 26.5 ± 1.1 | 23.0 ± 1.0 | 22.9 | 55.1 | 818.9 | |||
| 15 | 23 May 2006 | Ranunculus bulbosus | <200 | 9 | 75 | 29.2 ± 1.2 | 35.3 ± 1.3 | 26.5 ± 1.5 | 23.3 ± 1.4 | 22.3 | 64.2 | 128.0 |
| 200–500 | 4 | 21 | 30.6 ± 1.0 | 36.8 ± 1.4 | 25.3 ± 1.8 | 22.5 ± 1.3 | 21.2 | 66.4 | 233.7 | |||
| >500 | 14 | 84 | 33.3 ± 1.4 | 37.2 ± 1.3 | 30.7 ± 1.3 | 26.7 ± 1.6 | 23.6 | 63.7 | 966.4 | |||
| 16 | 23 May 2006 | Crepis sp. | <200 | — | — | — | — | — | — | — | — | — |
| 200–500 | 15 | 94 | 28.3 ± 1.0 | 32.5 ± 1.4 | 25.6 ± 0.8 | 23.4 ± 1.0 | 22.1 | 61.8 | 404.1 | |||
| >500 | 9 | 58 | 30.0 ± 1.3 | 33.2 ± 1.1 | 27.6 ± 1.4 | 25.1 ± 1.2 | 23.2 | 61.9 | 721.5 | |||
| 17 | 8 June 2006 | Angelica archangelica | <200 | 11 | 85 | 26.2 ± 1.3 | 33.0 ± 1.8 | 19.0 ± 1.0 | 15.0 ± 1.0 | 16.3 | 54.4 | 154.8 |
| 200–500 | 26 | 146 | 26.3 ± 1.7 | 31.6 ± 1.4 | 19.8 ± 2.3 | 15.7 ± 2.0 | 16.5 | 56.4 | 302.7 | |||
| >500 | 30 | 142 | 29.9 ± 2.1 | 32.7 ± 1.5 | 25.6 ± 2.4 | 19.1 ± 1.6 | 18.4 | 50.6 | 1027.9 | |||
| 18 | 8 June 2006 | Water | <200 | 12 | 122 | 25.6 ± 1.9 | 35.8 ± 2.8 | 23.7 ± 1.0 | 19.3 ± 0.7 | 16.6 | 57.4 | 155.6 |
| 200–500 | 5 | 17 | 26.7 ± 1.2 | 35.7 ± 1.7 | 24.7 ± 1.8 | 20.4 ± 1.3 | 16.3 | 57.1 | 307.6 | |||
| >500 | — | — | — | — | — | — | — | — | — | |||
| 19 | 8 June 2006 | Rubus idaeus | <200 | 8 | 80 | 24.3 ± 1.4 | 30.9 ± 1.6 | 18.9 ± 0.8 | 16.5 ± 0.8 | 17.2 | 50.3 | 157.3 |
| 200–500 | 17 | 136 | 24.7 ± 1.2 | 31.1 ± 1.4 | 19.2 ± 1.0 | 16.8 ± 1.0 | 17.2 | 48.5 | 254.3 | |||
| >500 | 1 | 7 | 27.4 ± 0.6 | 32.5 ± 1.0 | 24.8 ± 1.3 | 20.9 ± 1.5 | 19.0 | 51.1 | 969.4 | |||
| 20 | 12 June 2006 | Cornus sanguinea | <200 | 27 | 300 | 30.3 ± 1.2 | 35.5 ± 2.0 | 26.5 ± 0.9 | 22.6 ± 1.0 | 22.8 | 41.1 | 84.6 |
| 200–500 | 1 | 1 | 35.2 ± 0.0 | 38.2 ± 0.0 | 32.2 ± 0.0 | 27.0 ± 0.0 | 26.3 | 36.2 | 465.0 | |||
| >500 | 34 | 297 | 33.2 ± 2.1 | 36.0 ± 2.0 | 30.9 ± 2.0 | 24.8 ± 2.2 | 23.8 | 41.7 | 854.2 | |||
| 21 | 20 June 2006 | Rubus sp. | <200 | 37 | 212 | 32.7 ± 2.1 | 35.7 ± 2.3 | 31.6 ± 1.7 | 28.2 ± 1.5 | 28.2 | 42.5 | 98.7 |
| 200–500 | 1 | 3 | 37.5 ± 0.8 | 40.2 ± 0.8 | 35.8 ± 0.1 | 32.3 ± 1.3 | 29.2 | 45.5 | 479.0 | |||
| >500 | 39 | 200 | 36.6 ± 1.4 | 39.5 ± 1.5 | 35.2 ± 2.0 | 31.4 ± 1.8 | 28.9 | 43.1 | 983.5 | |||
| 22 | 20 June 2006 | Aegopodium podagraria | <200 | 20 | 227 | 32.7 ± 1.2 | 36.0 ± 2.1 | 30.2 ± 0.8 | 26.9 ± 0.7 | 29.6 | 73.1 | 101.5 |
| 200–500 | 1 | 1 | 37.1 ± 0.0 | 38.1 ± 0.0 | 35.7 ± 0.0 | 27.6 ± 0.0 | 29.8 | 47.5 | 440.0 | |||
| >500 | 25 | 285 | 37.6 ± 2.1 | 40.1 ± 1.9 | 35.3 ± 2.1 | 28.6 ± 0.9 | 30.5 | 54.8 | 1048.2 | |||
| 23 | 27 June 2006 | Castanea sativa | <200 | 12 | 89 | 32.7 ± 1.5 | 35.1 ± 1.7 | 30.2 ± 1.4 | 28.1 ± 0.9 | 28.3 | 57.1 | 145.2 |
| 200–500 | — | — | — | — | — | — | — | — | — | |||
| >500 | 13 | 70 | 36.6 ± 2.0 | 38.5 ± 1.6 | 33.8 ± 1.8 | 30.8 ± 2.1 | 29.0 | 58.0 | 884.3 | |||
| 24 | 27 June 2006 | Trifolium repens | <200 | 5 | 23 | 34.9 ± 0.9 | 38.4 ± 1.3 | 31.4 ± 1.0 | 29.5 ± 0.8 | 29.5 | 74.1 | 190.2 |
| 200–500 | 9 | 118 | 35.2 ± 0.9 | 38.9 ± 1.2 | 31.6 ± 0.8 | 29.7 ± 0.7 | 29.4 | 72.6 | 244.4 | |||
| >500 | 11 | 150 | 40.0 ± 1.7 | 41.9 ± 1.4 | 35.9 ± 1.6 | 32.5 ± 1.4 | 31.5 | 66.2 | 999.6 | |||
| 25 | 30 June 2006 | Tilia cordata | <200 | — | — | — | — | — | — | — | — | — |
| 200–500 | 29 | 247 | 27.8 ± 1.9 | 30.8 ± 2.6 | 24.2 ± 1.3 | 21.7 ± 0.8 | 22.5 | 58.2 | 369.9 | |||
| >500 | 10 | 114 | 33.2 ± 2.7 | 34.8 ± 2.4 | 29.8 ± 2.5 | 25.6 ± 2.0 | 25.1 | 55.5 | 1031.5 | |||
| 26 | 6 July 2006 | Lavendula sp. | <200 | 21 | 226 | 28.3 ± 2.0 | 32.2 ± 2.0 | 24.5 ± 1.8 | 23.5 ± 1.7 | 24.9 | 51.4 | 80.1 |
| 200–500 | — | — | — | — | — | — | — | — | — | |||
| >500 | 26 | 194 | 31.1 ± 2.1 | 33.4 ± 1.9 | 27.8 ± 2.1 | 25.8 ± 2.0 | 25.6 | 52.0 | 926.1 | |||
| 27 | 12 July 2006 | Cirsium arvense | <200 | 14 | 218 | 29.3 ± 1.3 | 30.8 ± 1.7 | 28.4 ± 1.0 | 26.4 ± 0.6 | 27.3 | 60.5 | 122.1 |
| 200–500 | 1 | 1 | 30.2 ± 0.0 | 32.2 ± 0.0 | 28.9 ± 0.0 | 27.1 ± 0.0 | 29.2 | 51.7 | 212.0 | |||
| >500 | 18 | 137 | 35.4 ± 1.6 | 36.8 ± 1.6 | 33.7 ± 1.8 | 31.8 ± 2.0 | 28.7 | 60.1 | 892.6 | |||
| 28 | 19 July 2006 | Begonia semperflorens | <200 | 14 | 98 | 32.1 ± 3.4 | 34.6 ± 3.2 | 30.6 ± 3.4 | 27.2 ± 2.5 | 28.5 | 40.9 | 115.9 |
| 200–500 | 11 | 39 | 35.5 ± 3.5 | 37.7 ± 3.4 | 33.8 ± 3.1 | 30.1 ± 2.2 | 30.5 | 43.0 | 256.4 | |||
| >500 | 20 | 104 | 37.2 ± 2.9 | 39.1 ± 2.5 | 35.6 ± 2.9 | 32.0 ± 2.3 | 29.9 | 41.9 | 1027.0 | |||
| 29 | 19 July 2006 | Mentha longifolia | <200 | 9 | 104 | 30.6 ± 2.8 | 32.2 ± 3.1 | 30.0 ± 2.5 | 27.4 ± 2.1 | 27.8 | 40.8 | 109.3 |
| 200–500 | — | — | — | — | — | — | — | — | — | |||
| >500 | 12 | 110 | 37.8 ± 0.9 | 39.5 ± 0.8 | 36.2 ± 1.0 | 31.9 ± 1.4 | 29.5 | 40.9 | 1073.1 | |||
| 30 | 23 August 2006 | Cirsium oleraceum | <200 | — | — | — | — | — | — | — | — | — |
| 200–500 | 15 | 75 | 29.7 ± 1.5 | 37.4 ± 2.9 | 26.9 ± 1.2 | 24.1 ± 1.3 | 21.4 | 59.4 | 323.4 | |||
| >500 | 4 | 13 | 31.0 ± 1.3 | 34.7 ± 1.9 | 28.1 ± 1.5 | 24.5 ± 1.2 | 23.0 | 55.4 | 1075.8 | |||
| 31 | 23 August 2006 | Water | <200 | — | — | — | — | — | — | — | — | — |
| 200–500 | 15 | 75 | 29.7 ± 1.5 | 37.4 ± 2.9 | 26.9 ± 1.2 | 24.1 ± 1.3 | 21.4 | 59.4 | 323.4 | |||
| >500 | 4 | 13 | 31.0 ± 1.3 | 34.7 ± 1.9 | 28.1 ± 1.5 | 24.5 ± 1.2 | 23.0 | 55.4 | 1075.8 | |||
| 32 | 28 August 2006 | Helianthus annuus | <200 | 9 | 130 | 27.4 ± 0.8 | 29.8 ± 1.6 | 24.7 ± 0.7 | 25.8 ± 1.2 | 22.0 | 47.4 | 119.4 |
| 200–500 | 5 | 58 | 29.3 ± 0.8 | 30.0 ± 0.9 | 26.6 ± 1.1 | 29.6 ± 1.3 | 21.9 | 44.2 | 370.3 | |||
| >500 | 14 | 167 | 34.0 ± 2.5 | 34.9 ± 2.8 | 31.5 ± 2.9 | 34.1 ± 2.6 | 23.4 | 44.7 | 768.9 | |||
| 33 | 28 August 2006 | Zinnia sp. | <200 | 10 | 98 | 26.2 ± 1.5 | 28.7 ± 1.6 | 24.9 ± 1.4 | 23.1 ± 1.2 | 21.9 | 46.9 | 97.6 |
| 200–500 | 2 | 6 | 28.9 ± 0.8 | 31.2 ± 1.3 | 27.5 ± 1.8 | 25.2 ± 1.6 | 22.0 | 46.8 | 466.0 | |||
| >500 | 8 | 37 | 33.0 ± 2.3 | 34.8 ± 2.3 | 31.2 ± 2.5 | 30.4 ± 3.0 | 23.4 | 45.4 | 923.0 | |||
| 34 | 6 September 2006 | Solidago gigantea | <200 | — | — | — | — | — | — | — | — | — |
| 200–500 | 3 | 20 | 33.4 ± 1.0 | 35.1 ± 0.6 | 31.2 ± 1.3 | 28.1 ± 1.3 | 26.1 | 59.1 | 458.0 | |||
| >500 | 19 | 161 | 32.7 ± 1.5 | 34.5 ± 1.5 | 29.7 ± 1.6 | 26.5 ± 1.5 | 25.3 | 56.2 | 608.9 | |||
| 35 | 10 September 2006 | Solidago gigantea | <200 | 14 | 172 | 27.1 ± 1.1 | 31.5 ± 1.6 | 22.5 ± 0.9 | 20.6 ± 0.8 | 21.2 | 50.4 | 73.4 |
| 200–500 | — | — | — | — | — | — | — | — | — | |||
| >500 | 24 | 166 | 31.9 ± 1.9 | 33.8 ± 1.9 | 28.6 ± 2.2 | 24.4 ± 1.9 | 23.8 | 50.7 | 679.7 | |||
| 36 | 11 September 2006 | Sedum spectabile | <200 | 25 | 132 | 27.9 ± 1.8 | 32.5 ± 2.4 | 24.5 ± 1.4 | 21.9 ± 1.1 | 21.6 | 44.5 | 108.9 |
| 200–500 | — | — | — | — | — | — | — | — | — | |||
| >500 | 29 | 177 | 34.9 ± 1.9 | 37.6 ± 1.7 | 34.2 ± 2.7 | 28.8 ± 2.8 | 24.4 | 46.3 | 793.6 | |||
| 37 | 11 September 2006 | Echinacea purpurea | <200 | 15 | 83 | 25.8 ± 1.6 | 29.0 ± 2.4 | 23.7 ± 1.7 | 23.5 ± 2.2 | 21.3 | 43.3 | 106.7 |
| 200–500 | — | — | — | — | — | — | — | — | — | |||
| >500 | 18 | 111 | 30.5 ± 1.4 | 33.0 ± 1.2 | 28.5 ± 1.8 | 29.4 ± 2.4 | 22.2 | 46.0 | 817.6 | |||
| 38 | 2 October 2006 | Aster sp. | <200 | 68 | 149 | 26.9 ± 1.0 | 31.0 ± 1.5 | 22.7 ± 1.1 | 20.7 ± 1.1 | 20.7 | 76.5 | 161.0 |
| 200–500 | 105 | 211 | 28.5 ± 1.8 | 31.1 ± 1.7 | 25.7 ± 2.3 | 23.6 ± 2.0 | 22.5 | 69.9 | 327.3 | |||
| >500 | 23 | 40 | 30.3 ± 2.2 | 32.6 ± 1.8 | 28.0 ± 2.2 | 25.4 ± 2.2 | 23.6 | 68.6 | 553.7 | |||
| 39 | 9 October 2006 | Aster sp. | <200 | 61 | 168 | 27.1 ± 1.6 | 33.4 ± 2.3 | 22.4 ± 1.0 | 19.6 ± 0.8 | 19.5 | 56.3 | 64.5 |
| 200–500 | — | — | — | — | — | — | — | — | — | |||
| >500 | 81 | 224 | 30.1 ± 1.6 | 32.9 ± 1.5 | 26.8 ± 1.9 | 22.8 ± 2.0 | 20.3 | 56.5 | 593.6 | |||
| 40 | 23 October 2006 | Sinapis arvensis | <200 | 6 | 21 | 26.8 ± 2.1 | 33.1 ± 3.4 | 21.4 ± 1.7 | 18.8 ± 1.0 | 19.9 | 63.9 | 100.5 |
| 200–500 | 15 | 66 | 26.7 ± 1.5 | 33.4 ± 1.6 | 19.8 ± 1.3 | 17.2 ± 1.4 | 17.2 | 70.1 | 359.3 | |||
| >500 | 19 | 82 | 28.6 ± 1.7 | 33.8 ± 2.0 | 22.7 ± 1.6 | 19.5 ± 1.7 | 18.6 | 65.6 | 670.3 | |||
| 41 | 23 October 2006 | Helianthus annuus | <200 | 11 | 103 | 27.1 ± 1.6 | 29.3 ± 1.7 | 25.2 ± 2.0 | 25.0 ± 2.6 | 20.8 | 62.0 | 108.8 |
| 200–500 | 7 | 38 | 28.3 ± 2.9 | 30.6 ± 2.7 | 24.1 ± 3.2 | 27.4 ± 3.2 | 18.3 | 67.8 | 352.5 | |||
| >500 | 17 | 133 | 34.5 ± 2.8 | 35.7 ± 2.8 | 31.6 ± 2.9 | 33.3 ± 3.1 | 21.2 | 63.2 | 638.6 |
Data presented as means ± SD or only means. Nbees = number of measured bees, Nmeans = number of measurements.
Measurements
The bees were filmed during the foraging stays at the blossoms (if possible from landing until takeoff) with an infrared camera (ThermaCam SC2000 NTS, FLIR, Stockholm, Sweden). We used infrared thermography because it allows temperature measurements without contact and behavioural impairment (e.g. Stabentheiner & Schmaranzer, 1987; Schmaranzer & Stabentheiner, 1988; Kleinhenz et al., 2003; Kovac et al., 2009a,b; Stabentheiner et al., 2010). In addition, it allows simultaneous temperature monitoring of all body parts during the entire foraging stay at one blossom. This is especially important in insects with a variable body temperature like honeybees. The behaviour of the insects was not impaired, which would not have been possible with ‘grab and stab’ methods with thermocouples or thermoneedles (Stone & Willmer, 1989). This outweighs the disadvantage of the method, which measures surface and not core temperatures. The surface temperature of a thorax heated to 40 °C at an ambient temperature of 21.5 °C is ∼1 °C below the subcuticular temperature (Stabentheiner & Schmaranzer, 1987; B. Heinrich, pers. comm.). The infrared camera was calibrated periodically by slotting in a self-constructed peltier-driven reference source of known temperature and emissivity (for details of calibration see Stabentheiner & Schmaranzer, 1987; Schmaranzer & Stabentheiner, 1988). Thermographic data were stored digitally with a 14-bit resolution on a portable computer (DOLCH Flexpac-400-XG, Munich, Germany) at a rate of 3–5 frames s−1. On 3 days, in addition to the nectar-gathering bees, water-collecting bees foraging at a rainwater barrel a few metres away from the nectar-foragers were also measured.
The ambient air temperature (Ta) was measured near the foraging bees (∼1–5 cm) with thermocouples. In the near vicinity of the insects (<1 m), we also measured the relative humidity with NTC-sensors (in shade) and the solar radiation with a miniature global radiation sensor (FLA613-GS mini spezial, Ahlborn, Holzkirchen, Germany). Care was taken so that the radiation sensor was exposed to the same ambient conditions as the foraging bees. The temperature and radiation data were stored every 2 s with ALMEMO data loggers (Ahlborn, Holzkirchen, Germany).
Data evaluation and statistics
The temperature of the three bee body parts and of the blossoms' surfaces (in close vicinity to the bees' mouthparts) was calculated from the infrared thermograms (Fig. 1) by means of the AGEMA Research software (FLIR, Stockholm, Sweden) controlled by a self-written Excel VBA-macro (Microsoft Corporation, Santa Rosa, California). The environmental data were automatically extracted from the datalogger files. Values of the body temperature during foraging were taken in regular intervals of about 3–5 s immediately after the insects' landing until their takeoff. This interval was chosen, because bees are able to increase or decrease body temperature within this time and temperature could vary considerably during foraging on one blossom (Fig. 2). The surface temperatures of the head (Thd), thorax (Tth) and abdomen (Tab) were calculated with an infrared emissivity of 0.97, determined for the honeybee cuticle (Stabentheiner & Schmaranzer, 1987; Schmaranzer & Stabentheiner, 1988). Because the ThermaCam works in the long-wave infrared range (7.5–13 µm), the reflected radiation from the bees' cuticle produced only a small measurement error (0.2 °C for 1000 W m−2), which was compensated for. In this way we reached an accuracy of 0.7 °C for the body surface temperature of the bees at a sensitivity of <0.1 °C. The blossom surface temperature was calculated with an infrared emissivity of 0.95, representing a typical value for plants (Lamprecht et al., 2006).
Fig. 1.
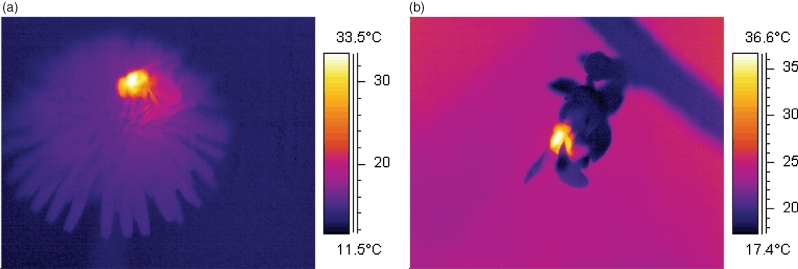
Thermograms of foraging honeybees on dandelion (Taraxacum officinalis, a) and apricot (Prunus armeniaca, b). (a) Tthorax = 34.6, Thead = 26.0, Tabdomen = 21.7°C, Tblossom = 19.3°C, Ta = 17.3°C, radiation = 66 W m−2. (b) Tthorax = 39.8, Thead = 33.6, Tabdomen = 22.7°C, Tblossom = 21.0°C, Ta = 18.7°C, radiation = 199 W m−2.
Fig. 2.
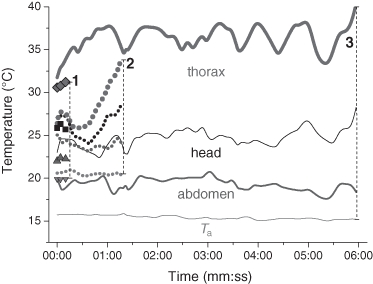
Body and ambient temperatures (Ta) of a bee's short foraging stay on Aster sp. (1, symbols), a stay of medium duration on Cirsium oleraceum (2, dotted lines) and a long lasting stay on Taraxacum officinalis (3, continous lines). From the top to the bottom: thorax (red), head (black), abdomen (blue), and Ta (green).
The temperature gradient between the thorax and the ambient air (thorax temperature excess = Tthorax−Ta) was used as a measure to assess the bees' endothermic capability. To evaluate the influence of the radiative heat gain on the body temperature, three classes of solar radiation were established: shade, <200 W m−2, overcast sky, 200–500 W m−2, and sunshine, >500 W m−2. The mean of all foraging bees on one blossom type was calculated and values were divided into the three radiation classes. The values for the temperature excess of the head and abdomen were calculated in the same way.
The relationship between body temperature, temperature excess, and Ta was described by linear, exponential or polynomial regression functions and tested with anova. Data analysis and statistics were performed using the Statgraphics package (Statistical Graphics Corporation, Warrenton, Virginia) and ORIGIN software (OriginLab Corporation, Northampton, Massachusetts).
Results
In 2006, we measured honeybees (A. mellifera carnica) foraging on 33 different flowering plants. Figure 1 shows thermograms of foraging bees on dandelion and apricot blossoms. From 1666 single forging stays we got 12 685 thermograms and evaluated the body surface temperatures of the head (Thd), thorax (Tth), and abdomen (Tab) as well as the blossom surface temperature (Tblossom) where the bees were sucking. We covered the complete foraging season (March–October) and the entire range of ambient temperatures (Ta =∼ 10–33°C) and solar radiation (50–1400 W m−2) to which they are likely to be exposed in their natural environment during a foraging trip in Central Europe. It must be noted that the investigated flowers often deliver both nectar and pollen (see Droege, 1989). We were unable to determine the relation of nectar and pollen load in the free-ranging individuals.
Body temperature and blossom surface temperature
The body surface temperatures during nectar and pollen collection on one blossom were not constant but fluctuated, especially during longer-lasting stays (Fig. 2). The continuous measurement with infrared thermography enabled the registration of this variability within the foraging stay. The mean body surface temperatures per plant and date varied in a wide range, Tth from 23.2 to 44.2 °C, Thd from 18.6 to 43.2 °C, and Tab from 13.0 to 41.3 °C at ambient temperatures from 10.8 to 32.9 °C. A plot of all measurement data (Fig. 3) shows that at ambient temperatures of about 10–27 °C, Tth was regulated rather independent of Ta on average. At Ta > ∼27°C, however, it increased nearly linearly with Ta (Fig. 3). The head and abdomen exhibited a stronger dependence on Ta but both of them were regulated well above Ta. The head was warmer and better regulated than the abdomen (Fig. 3). The abdominal temperature increased nearly linearly with Ta. The relation of body temperature and ambient air temperature could be described best with an exponential function for the thorax (radiation: 0–1400 W m−2, R2 = 0.16185, Fig. 3, Table 2; A – E are the fit parameters):
Fig. 3.
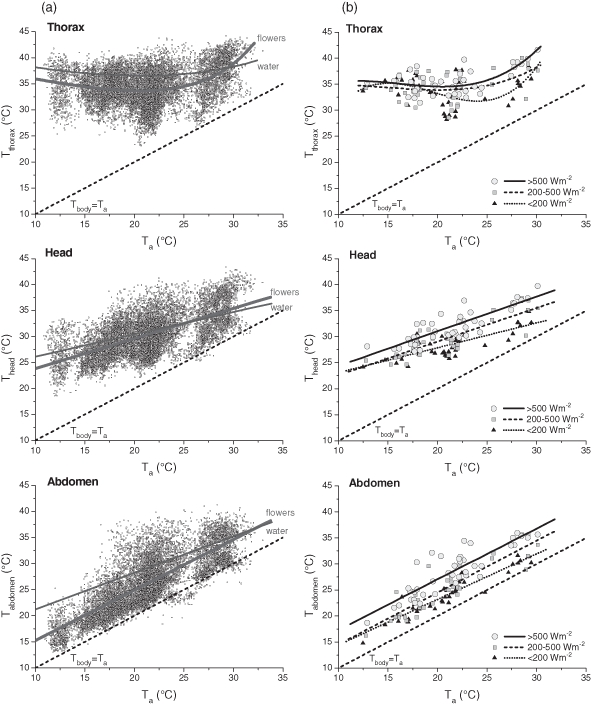
Surface temperature of the thorax, head and abdomen of foraging honeybees in dependence on ambient temperature (Ta). (a) Foraging on flowering plants (dots are single values; bold red lines are regressions), and foraging water (thin blue lines; from Kovac et al., 2010). (b) Means per flowering plant and day at three different classes of solar radiation. Equations for linear and non-linear regressions, number of observations, and regression statistics in Table 2.
Table 2.
Equations of linear and non-linear regressions for the temperature of the thorax (Tth), head (Thd), and abdomen (Tab) of honeybees foraging on flowers or foraging for water (*, Kovac et al., 2010), and of the blossom temperature, in dependence on ambient temperature (Ta) and solar radiation (Fig. 3).
| Body part | Radiation (W m−2) | Equations | R2 | P | N |
|---|---|---|---|---|---|
| Bees on flowers | |||||
| Thorax | 0–1400 | Tth = 10.15733 + 29.83926 × 0.98433Ta + 0.05332 × 1.19123Ta | 0.16185 | — | 12685 |
| <200 | 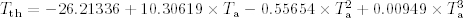 |
0.20651 | — | 38 | |
| — | Tth = 33.67525 + 0.00041 ×Ta | 0.00000 | >0.05 | 38 | |
| 200–500 | 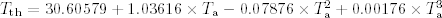 |
0.18030 | — | 34 | |
| — | Tth = 30.22421 + 0.21173 ×Ta | 0.10899 | >0.05 | 34 | |
| >500 | 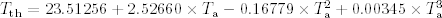 |
0.48709 | — | 40 | |
| — | Tth = 28.90354 + 0.30973 ×Ta | 0.26565 | <0.001 | 40 | |
| Head | 0–1400 | Thd = 18.00959 + 0.58012 ×Ta | 0.41795 | <0.0001 | 12503 |
| <200 | Thd = 18.32716 + 0.47753 ×Ta | 0.59942 | <0.0001 | 38 | |
| 200–500 | Thd = 16.13789 + 0.64590 ×Ta | 0.72383 | <0.0001 | 34 | |
| >500 | Thd = 17.69125 + 0.66659 ×Ta | 0.72718 | <0.0001 | 40 | |
| Abdomen | 0–1400 | Tab = 5.56068 + 0.96864 ×Ta | 0.64091 | <0.0001 | 12645 |
| <200 | Tab = 5.71561 + 0.87379 ×Ta | 0.87821 | <0.0001 | 38 | |
| 200–500 | Tab = 4.54687 + 0.99761 ×Ta | 0.82406 | <0.0001 | 34 | |
| >500 | Tab = 7.57345 + 0.97364 ×Ta | 0.76073 | <0.0001 | 40 | |
| Bees foraging water* | |||||
| Thorax | 0–1200 | Tth = −10.25678 + 1.68281 × 1.06859Ta + 50.40771 × 0.98906Ta | 0.18480 | — | 11340 |
| Head | 0–1200 | Thd = 21.86636 + 0.42776 ×Ta | 0.59319 | <0.0001 | 11290 |
| Abdomen | 0–1200 | Tab = 14.22541 + 0.70079 ×Ta | 0.81478 | <0.0001 | 11334 |
| Blossom surface temperature | |||||
| 0–1200 | Tbl = 3.39642 + 0.94273 ×Ta | 0.60852 | <0.0001 | 11340 | |
| <200 | Tbl = 2.19283 + 0.91810 ×Ta | 0.87219 | <0.0001 | 38 | |
| 200–500 | Tbl = 3.30773 + 0.92522 ×Ta | 0.73901 | <0.0001 | 34 | |
| >500 | Tbl = 4.05296 + 0.98745 ×Ta | 0.64128 | <0.0001 | 40 |
R2 = squared correlation coefficient, P = probability, N = means per flower and day (<50) or number of measurements.
| (1) |
and with a simple linear regression for the head and the abdomen (radiation: 0–1400 W m−2, head: R2 = 0.41795, abdomen: R2 = 0.64091, Fig. 3, Table 2):
| (2) |
At a low Ta of 10 °C, the average values of Tth, Thd, and Tab derived from the regression lines were 35.6, 24.3, and 16.0 °C, respectively. In the medium range of Ta, at about 20 °C, the Tth decreased to 33.7 °C, the Thd increased to 29.6, and the Tab increased to 24.9 °C. At the highest Ta measured (∼33 °C), Tth, Thd, and Tab increased to 44.4, 37.2, and 37.5 °C, respectively. In order to allow a comparison of the results of flower-visiting bees with water-foraging honeybees (from the paper of Kovac et al., 2010), the regression lines for the three body parts of the water foraging bees are also displayed in Fig. 3 (for statistical details see Table 2).
Plotting the body temperature in dependence on three levels of solar radiation (<200, 200–500, >500 W m−2; Fig. 3) revealed that bees foraging in sunshine were mostly warmer than bees foraging in shade. The relation of thorax temperature and ambient air temperature could be described best with a polynomial function (radiation: <200 W m−2: R2 = 0.20651, 200–500 W m−2: R2 = 0.18030, >500 W m−2: R2 = 0.48709, Fig. 3, Table 2; A – D are the fit parameters):
| (3) |
and with a simple linear regression [eqn (2)] for the head (radiation <200 W m−2: R2 = 0.59942, 200–500 W m−2: R2 = 0.72383, >500 W m−2: R2 = 0.72718) and the abdomen (radiation <200 W m−2: R2 = 0.87821, 200–500 W m−2: R2 = 0.82406, >500 W m−2: R2 = 0.76073). For further statistical and graphical details see Table 2 and Fig. 3. The temperature difference between >500 and <200 W m−2 as estimated from the regression lines of Fig. 3 was smaller at low and greater at high Ta (Ta = 12°C: difference Tth = 2.0, Thd = 1.7, Tab = 3.0°C; Ta = 30°C: difference Tth = 3.3, Thd = 5.0, Tab = 4.8°C).
The blossom surface temperature (range Tbl = 9.5–42.2°C) measured closely beside the bees' mouthparts increased linearly in dependence on Ta at all three categories of radiation (Fig. 4, Table 1, statistical details in Table 2). In sunshine the blossoms' temperature was about 4 °C elevated above the ambient air temperature. Under (partly) overcast skies (200–500 W m−2) the Tbl was also always higher than the ambient air temperature. However, the blossoms' temperature in shade was similar to the ambient air. The three regression lines differed significantly (anova, P < 0.0001, F-Ratio = 68.35, d.f. = 5), and the intercepts of values in sunshine versus the two other categories of radiation were also significantly different (P < 0.01; F-Ratio = 6.83, 9.53, 36.32; d.f. = 1).
Fig. 4.
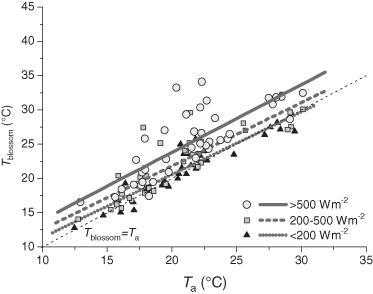
Temperature of the blossom surface near the honeybee mouthparts (means per flowering plant and day) in dependence on ambient temperature (Ta) at three different classes of solar radiation. Equations of linear regressions, number of observations, and regression statistics in Table 2.
Temperature excess and solar radiation
The bees were always endothermic as the thorax (the centre of heat production) was clearly more elevated above the ambient air than were the other body parts. The thorax temperature excess (Tth−Ta) depended strongly on Ta. It decreased significantly with Ta in the sunshine and in the shade (values calculated from linear regressions in Table 3; Tth−Ta = 20.6 − 8.2°C at Ta = 12–30°C and radiation >500 W m−2; Tth−Ta = 21.6 − 3.6°C at Ta = 12–30°C and radiation <200 W m−2; P < 0.0001). The temperature excess of the intermediate radiation range (overcast sky, 200–500 W m−2) showed a similar course. An anova confirmed the difference in thorax temperature excess between sunshine and shade (P < 0.01, F-ratio = 11.01, d.f. = 1 for intercepts, and P < 0.05, F-ratio = 4.61, d.f. = 1 for slopes).
3.
Equations of linear regressions for the temperature excess (Tbody−Ta) of honeybees foraging on flowers in dependence on ambient temperature (Ta), for three classes of solar radiation.
| Body part | Radiation (W m−2) | Equations | R2 | P | N |
|---|---|---|---|---|---|
| Thorax | <200 | Tth = 33.67525 − 0.99959 ×Ta | 0.68966 | <0.0001 | 38 |
| 200–500 | Tth = 30.22421 − 0.78827 ×Ta | 0.62899 | <0.0001 | 34 | |
| >500 | Tth = 28.90354 − 0.69027 ×Ta | 0.64243 | <0.0001 | 40 | |
| Head | <200 | Thd = 18.32716 − 0.52247 ×Ta | 0.64175 | <0.0001 | 38 |
| 200–500 | Thd = 16.13789 − 0.35410 ×Ta | 0.44063 | <0.0001 | 34 | |
| >500 | Thd = 17.69125 − 0.33341 ×Ta | 0.40007 | <0.0001 | 40 | |
| Abdomen | <200 | Tab = 5.71561 − 0.12621 ×Ta | 0.13078 | 0.02569 | 38 |
| 200–500 | Tab = 4.54687 − 0.00239 ×Ta | 0.00003 | 0.97678 | 34 | |
| >500 | Tab = 7.57345 − 0.02636 ×Ta | 0.00232 | 0.76767 | 40 |
R2 = squared correlation coefficient, P = probability, N = number of means per flower and day.
The excess temperature of the head decreased with Ta as well, but the slopes were somewhat flatter than for the thorax. The decrease was still significant (P < 0.0001, Table 3). However, the temperature excess of the abdomen decreased with Ta only in the shade (P < 0.05) and remained constant between 12 and 33 °C in the sunshine and overcast sky (Table 3). An anova confirmed the difference in temperature excess between the sunshine and the shade (head: P < 0.0001, F-ratio = 75.98, d.f. = 1 for intercepts, and P < 0.05, F-ratio = 4.50, d.f. = 1 for slopes; abdomen: P < 0.0001, F-ratio = 102.15, d.f. = 1 for intercepts, and P > 0.05, F-ratio = 0.77, d.f. = 1 for slopes).
Temperature and season
In Fig. 5, the mean temperatures of the three body parts during foraging in the shade are plotted against the date of observation (a) and ambient temperature (b) for each flowering plant. The Tth revealed a clear dependence on the season. The average value in the spring (March–June) as calculated from the means per stay was 35.2 ± 2.3°C, (N = 218). In the summer (July–September), it was only 31.4 ± 2.4°C (N = 127) in spite of the higher Ta in the summer. The difference could be statistically confirmed (Mann–Whitney/Wilcoxon's test, P < 0.0001; W = 3259.0). Testing the effect of season on average Tth per foraging stay and bee with anova (removing the effect of Ta and radiation) showed the same result (main factor season: P ≪ 0.0001, F-ratio = 247.07, d.f. = 1; covariate Ta: P ≪ 0.0001, F-ratio = 40.62, d.f. = 1; covariate radiation: P = 0.4224, F-ratio = 0.65, d.f. = 1; N = 345). F-ratios indicate that season had the greatest effect followed by Ta, and radiation had no effect. Plotting the average values of Tth against the ambient temperature (Fig. 5b) and calculating means for ranges of Ta according to Kovac and Schmaranzer (1996) for 12–20 °C and 20–30 °C revealed only a weak statistical difference [Ta = 12–20°C: Tth = 34.4 ± 2.3°C, (N = 86); Ta = 20–30°C: Tth = 33.6 ± 3.1°C, (N = 259); Mann–Whitney/Wilcoxon's test, P < 0.04; W = 9492.0].
Fig. 5.
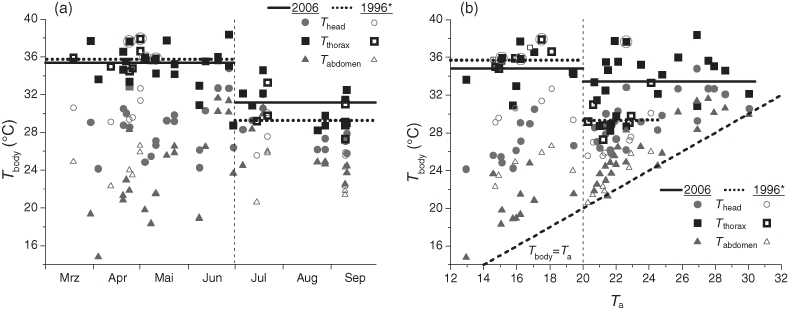
Temperature of the thorax, head and abdomen (mean of each flowering plant and day) in dependence on season (a) and ambient temperature (b) in two different years (for year 1996 see Kovac & Schmaranzer, 1996). The horizontal lines are mean thorax values of two seasons (spring and summer) or ranges of ambient temperature (Ta). Values of the thorax temperature for the dandelion is marked with pink circles. Mean values between seasons (a) and between ranges of Ta (b) are significantly different (P < 0.05, see Results).
Testing the difference between the spring and summer with all values (means per stay and bee, including values in sun and shade) confirmed the seasonal effect [spring: Tth = 35.4 ± 3.0°C, N = 880; summer: Tth = 32.7 ± 2.9°C, (N = 786); Mann–Whitney/Wilcoxon's test, P < 0.0001; W = 172258.0; anova, main factor season: P ≪ 0.0001, F-ratio = 508.17, d.f. = 1; covariate Ta: P ≪ 0.0001, F-ratio = 378.86, d.f. = 1; covariate radiation: P ≪ 0.0001, F-ratio = 220.27, d.f. = 1; N = 1666].
Type of flower
Table 1 gives an overview of body temperature and environmental parameters for each measuring day and plant divided in three classes of solar radiation (<200 W m−2, 200–500 W m−2, >500 W m−2). It is of special interest that bees measured on the same day in the same environment and similar Ta at different plants could exhibit remarkable differences in their thorax temperature. For example, bees foraging in the shade at apricot blossoms (Prunus armenica L.) had an average thorax temperature of 36.6 ± 1.8°C (N = 112, Ta = 18.2°C, radiation = 177 W m−2), whereas the thorax temperature of bees foraging on lady's smock (Cardamine pratensis L.) was only 34.7 ± 2.9°C (N = 59, Ta = 18.4°C, radiation = 186 W m−2; Mann–Whitney/Wilcoxon's test, P < 0.0001; W = 4580.0). Water-collecting bees a few metres away from the nectar foragers had the highest thorax temperatures (Tth = 37.1 ± 2.3°C, N = 200, Ta = 18.0°C, radiation = 151 W m−2; water versus Cardamine: Mann–Whitney/Wilcoxon's test, P < 0.0001; W = 3329.5; water versus Prunus: Mann–Whitney/Wilcoxon's test, not significant; W = 9924.5. The thorax temperature of water-collecting bees was most of the time higher than that of nectar-foraging bees on the same day and place. Another example of strongly differing thoracic temperatures were found in bees foraging in the sun on Ranunculus (Tth = 37.2 ± 1.3°C, N = 84, Ta = 23.6°C, radiation = 966 W m−2) and on Crepis (Tth = 33.2 ± 1.1°C, N = 58, Ta = 23.2°C, radiation = 722 W m−2; Mann–Whitney/Wilcoxon's test, P < 0.0001; W = 77.5; for further examples see Table 1).
Discussion
Ambient temperature and radiation
For a comprehensive description of an insect's thermoregulatory performance, it is of great advantage to investigate the entire range of ambient temperature to which it is likely to be exposed in its natural environment. Infrared thermography enabled us to measure the temperature of all three body parts of undisturbed foragers and revealed new knowledge about their thermoregulatory behaviour. An interesting result was that the bees regulated the Tth at a rather constant level in a broad range of Ta (10–27 °C) on average but showed a strong variation at a certain Ta, depending on the plants from which they were foraging (Fig. 3). To our knowledge, there are only two similar investigations on this topic (Heinrich, 1979a; Kovac & Schmaranzer, 1996). Heinrich's study (1979a) is a pioneer work in this field. He measured A. mellifera mellifera foraging from Eucalyptus sp. and A. m. adansonii foraging from B. pilosa and Petraea volubilis (in the shade). The bees exhibited a thoracic (core) temperature of ∼30.5–33 °C at ambient temperatures of 11–22 °C. The (surface) Tth of our foragers measured in the shade was clearly higher (Fig. 3), with average values ranging from ∼35 to 33 °C. Kovac and Schmaranzer (1996) reported even higher mean thorax surface temperatures of 35–38 °C in bees foraging nectar from several plants. Water foragers measured in the same environment (Kovac et al., 2010) regulated the thorax to another 2–3 °C higher (Ta = 10–27°C; Fig. 3). At a Ta above 27 °C, the Tth increased somewhat more steeply in the nectar foragers than in the water foragers. At these high ambient temperatures, the bees' main problem seems not to be that their body temperature is too low. Rather, the dissipation of excessive heat becomes more important. More bees returning to the hive were shown to carry a fluid droplet at these temperatures (Cooper et al., 1985). Such droplets have a considerable cooling effect not only on the head but also on the thorax (Heinrich, 1979a,b). We suggest that cooling was more difficult for the nectar than for the water foragers because their head temperature became higher at a Ta above ∼27 °C (anova, P < 0.0001; F-ratio = 296.07; d.f. = 3; Fig. 3).
At very low Ta, by contrast, it seems to be more important to keep the head warm. The haemolymph circulation from the warm thorax (Heinrich, 1979b, 1980a; Coelho, 1991a,b) provided the head with enough heat to prevent the Thd from falling below ∼20 °C, which seems to be necessary for the proper functioning of physiological and neural processes. Regulation of the Tth at a high level even at low Ta allows the bees to keep the Thd at a level high enough to guarantee a high suction speed at unlimited sources (Kovac et al., 2010). In nectar foragers a high nectar suction speed is generally not as important because the nectar is not available in an unlimited amount. Nectar foragers usually get only small portions of nectar per blossom and then have to fly or walk to the next blossom.
The temperature of the nectar foragers' abdomen was mostly below that of water foragers, probably because of the lower thorax temperature and perhaps because water foragers foraged much closer to the nest in the present study (Fig. 3). Heinrich (1980b, 1993) suggested that bees use a series of aortic loops in the petiole as a counter-current heat exchanger to prevent heat leakage to the abdomen in the cold. We agree with this opinion. However, the amount of heat reaching the abdomen may differ considerably. In contrast to the present study, where the abdominal temperature was not much elevated above the ambient temperature, it was considerably increased at low ambient temperatures in other previous investigations (Kovac & Schmaranzer, 1996; Kovac et al., 2010).
Digby (1955) investigated the factors affecting the temperature excess of dead or anaesthetised insects in artificial sunlight under laboratory conditions and found the temperature excess to vary directly with the radiation strength. This applies to living insects only in the ectothermic state. Foraging honeybees, however, are always endothermic at medium to low Ta (Figs 3; Heinrich, 1979a; Schmaranzer & Stabentheiner, 1988; Waddington, 1990; Kovac & Schmaranzer, 1996; Kovac et al., 2010). On average the thorax temperature excess was higher in our measurements on A. mellifera carnica than in an investigation on A. m. mellifera and A. m. adansonii by Heinrich (1979a). An important result of our investigation was that the bees used the heat gain from the sun to enhance their body temperature. This enables a quicker exploitation of the flowers because a high body temperature not only increases suction speed (Kovac et al., 2010) but also increases the bee's agility (Crailsheim et al., 1999; Stabentheiner & Crailsheim, 1999; Stabentheiner et al., 2003b) and flight muscle power output (Coelho, 1991a). However, at a high Ta of ∼30 °C our bees probably were only weakly endothermic. The thorax temperature excess in sunshine of ∼8 °C above ambient air was only ∼1.5 °C higher than the abdominal excess. The finding that in shade the thorax temperature excess was only ∼3.5 °C confirms that they were only weekly endothermic. At these high ambient temperatures the bees foraging in the sunshine are able to reach the optimal upper level of Tth for force production and takeoff of 38–39 °C (Coelho, 1991a) without much endothermic effort.
We often observed that bees preferred flowers in sunshine to flowers in shade. Our measurements of the blossom surface temperature (Fig. 4) showed that the solar radiation elevated their temperature by about 4 °C above the ambient air. Dyer et al. (2006) found that floral temperature can serve as an additional reward for pollinator insects when nutritional rewards are also available. However, we cannot exclude from our results that bees preferred the warmer flowers in sunshine owing to greater amounts of nectar secretion, because the production and concentration of nectar depends on ambient temperature and relative humidity (e.g. Beutler, 1953; Shuel, 1970; Núñez, 1977; Corbet et al., 1979a,b, 1993; Szabo, 1984; Corbet, 2003).
Seasonal variability and type of plant
A great part of collected nectar and pollen is used to provide for the brood and young bees of the colonies. Brood rearing and colony development proceed in a special periodicity. In Central Europe the majority of the brood is reared in the spring until the beginning of the summer (e.g. Seeley, 1985; Wille, 1985; Winston, 1987; Liebig, 1994; Imdorf et al., 1996). During this time, colonies need huge amounts of nectar and pollen. The presence of a brood stimulates the foraging behaviour of the bees (Pankiw et al., 2004). We had presumed that bees foraging in the spring are better motivated and should therefore have a higher Tth (Dyer & Seeley, 1987; Stabentheiner & Schmaranzer, 1987; Schmaranzer & Stabentheiner, 1988). In Fig. 5a the mean Tth of each investigated plant is plotted against the date of measurement. The average Tth in the first period from March to June (Tth = 35.2°C) was significantly higher than that in the second period from July to September (Tth = 31.4°C). Results of Kovac and Schmaranzer (1996) lead to the same conclusion (see Fig. 5a). A similar relation between Tth and season was also found in dancing nectar and pollen foragers after their return to the hive (Stabentheiner, 2001). Plotting the Tth (average of investigated flowers on a day) in dependence on ambient temperature and dividing the Ta range into two classes of 12–20 and 20–30 °C did not show the great difference as reported by Kovac and Schmaranzer (1996; see Fig. 5b). This suggests season to be more important than ambient temperature for the observed high Tth in spring, which was confirmed by anova.
A further important result of the present study was to show the great variability in Tth on different plants that cannot be explained by differences in Ta and radiation (Table 1). An impressive example is bees foraging on crowfoot (Ranunculus) and hawksbeard (Crepis) at the same time in sunshine. Their Tth differed by 4 °C (Table 1). Another example is bees foraging from apricot blossoms (P. armenica) or lady's smock (C. pradensis) in the shade. They displayed a difference of 1.9 °C. This can only be explained by assuming different motivational states of the foragers which is related with the profitability of the source. In addition, the present results demonstrate that the body temperature at a certain plant may differ considerably at different dates. In dandelion, for example, average values per day were 37.7, 36.0, and 35.9 °C (Fig. 5; Table 1). This may have been caused by differences in nectar production as well as by differences in foraging motivation.
The thorax temperature and energy expenditure of sucrose-foraging honeybees varies markedly in direct response to the richness of food rewards and their distance from the hive (e.g. Stabentheiner & Schmaranzer, 1986, 1987, 1988; Dyer & Seeley, 1987; Schmaranzer & Stabentheiner, 1988; Waddington, 1990; Stabentheiner & Hagmüller, 1991; Underwood, 1991; Balderrama et al., 1992; Stabentheiner et al., 1995; Stabentheiner, 1996, 2001; Moffatt & Núñez, 1997; Moffatt, 2000, 2001; Sadler & Nieh, 2011). The observed thorax temperatures of nectar foragers (means ∼34–36 °C) are a bit lower than those of bees foraging 0.25–0.5 m sucrose solution (means ∼35–38 °C, Schmaranzer & Stabentheiner, 1988). However, these latter bees received sufficient food in unlimited flow at an artificial feeding place. Nectar foraging at a similar molarity is probably not as attractive because the nectar amount and flow rate of blossoms depends on ambient temperature, humidity, and other parameters. Bees have to visit many blossoms in a greater area to collect the same quantity, which requires a higher total energy expenditure to fill their crop. This probably reduces their foraging motivation and, as a consequence, the thorax temperature. The observation that a reduction of the flow rate of artificial flowers reduces the foragers' energy turnover and thus their thorax temperature (Moffatt & Núñez, 1997; Moffatt, 2000, 2001) supports this suggestion. The foragers modulate their behaviour in relation to nectar source profitability: as profitability increases, the tempo of foraging and the intensity of dancing increase (Seeley et al., 1991). The motivation of foragers is influenced by both the reward at the source and the demand in the hive (Seeley, 1986, 1992; Seeley & Tovey, 1994; Stabentheiner, 2001). The relative importance of these two parameters in bees foraging from flowers remains to be investigated. However, predicting the profitability of flowers for the visiting bee is very difficult as Goulson (1999) stated in his review: ‘Flowers typically exhibit a patchy distribution at a number of levels; flowers are often clustered into inflorescences, several flowers or inflorescences may be clustered on each plant, and the plants themselves are likely to be patchily distributed. Superimposed on this distribution, rewards per flower vary greatly between plants of a single species and between flowers on a single plant owing to genetic and environmental influences on reward production rates and also in response to the pattern of depletion of rewards by foragers' (for detailed literature see Goulson, 1999). In addition, the demand for nectar and pollen in a colony and between colonies may change in time and differ considerably.
However, any motivation effect on body temperature is superimposed by physiological constraints. Although bees were observed to heat their thorax up to 48 °C (Stabentheiner et al., 2002, 2007) they usually exhibit a Tth below 44 °C (Stabentheiner & Schmaranzer, 1987; Schmaranzer & Stabentheiner, 1988; Kovac & Schmaranzer, 1996; Kovac et al., 2010; Fig. 3). Lower limits for takeoff and flight in this investigation were ∼27 °C, which is somewhat lower as reported by Esch (1976) and Heinrich (1993) at ∼30 °C and obviously lower as reported by Coelho (1991b) at ∼35 °C. Bees need to increase this minimum level as nectar load increases. Temperatures at takeoff, where sucrose- and water-foraging bees are heavily loaded, are usually higher than temperatures upon landing (Schmaranzer & Stabentheiner, 1988; Kovac et al., 2010).
The finding that bees that were investigated on the same day in the same location sometimes displayed very different Tth's at different plants (Table 1) supports the motivation hypothesis. However, as previously mentioned, the higher spring thorax temperatures may have been caused by a greater motivation as a result of both a higher reward and a higher demand in the colony.
Conclusion
Honeybees are always endothermic during foraging on flowers. However, at higher ambient temperatures (∼30 °C) the thoracic temperature excess is reduced to a low level and the prevention of overheating becomes more important. On average, the thorax temperature is kept rather constant at a high level in a broad range of Ta (10–27 °C) but shows a strong variation at a certain Ta, depending on the plants they are foraging from. The heat gain from solar radiation is used to elevate the thorax temperature during foraging and, in this way, probably improves the agility and speed of food exploitation. A high thorax temperature enables elevation of the head temperature and keeps the abdomen temperature some degrees above the ambient air. This improves physiological processes involved in food uptake, respiration, and energy supply. We suggest that the higher thorax temperature in spring is mainly caused by a higher foraging motivation as a result of the higher demand for nectar and pollen in the colony.
Acknowledgments
Supported by the Austrian Science Fund (FWF, P16584-B06, P20802-B16). We thank the reviewers for their constructive criticism and their helpful comments on the manuscript. We greatly appreciate the help with electronics and software by G. Stabentheiner and S. K. Hetz, with data evaluation by M. Ablasser, N. Köberl, A. Lienhard and B. Maurer, and C. Ahern for English correction.
References
- Balderrama NM, Almeida LO, Nunez JA. Metabolic rate during foraging in the honey bee. Journal of Comparative Physiology B. 1992;162:440–447. doi: 10.1007/BF00258967. [DOI] [PubMed] [Google Scholar]
- Beutler R. Nectar. Bee World. 1953;34:106–116. [Google Scholar]
- Cena K, Clark JA. Effect of solar radiation on temperatures of working honey bees. Nature. 1972;236:222–223. doi: 10.1038/newbio236222a0. [DOI] [PubMed] [Google Scholar]
- Coelho JR. The effect of thorax temperature on force production during tethered flight in the honeybee (Apis mellifera) drones, workers and queens. Physiological Zoology. 1991a;64:823–835. [Google Scholar]
- Coelho JR. Heat transfer and body temperature in honey bee (Hymenoptera: Apidae) drones and workers. Environmental Entomology. 1991b;20:1627–1635. [Google Scholar]
- Cooper PD, Schaffer WM, Buchmann SL. Temperature regulation of honey bees (Apis mellifera) foraging in the Sonoran desert. Journal of Experimental Biology. 1985;114:1–15. [Google Scholar]
- Corbet SA. Nectar sugar content: estimating standing crop and secretion rate in the field. Apidologie. 2003;34:1–110. [Google Scholar]
- Corbet SA, Unwin DM, Prŷs-Jones OE. Humidity, nectar and insect visits to flowers, with special reference to CrataegusTilia and Echium. Ecological Entomology. 1979a;4:9–22. [Google Scholar]
- Corbet SA, Willmer PG, Beament JWL, Unwin DM, Prŷs-Jones OE. Post-secretory determinants of sugar concentration in nectar. Plant Cell and Environment. 1979b;2:293–308. [Google Scholar]
- Corbet SA, Fussell M, Ake R, Fraser A, Gunson C, Savage A, et al. Temperature and the pollinating activity of social bees. Ecological Entomology. 1993;18:17–30. [Google Scholar]
- Crailsheim K, Stabentheiner A, Hrassnigg N, Leonhard B. Oxygen consumption at different activity levels and ambient temperatures in isolated honeybees (Hymenoptera: Apidae) Entomologia Generalis. 1999;24:1–12. [Google Scholar]
- Digby PSB. Factors affecting the temperature excess of insects in sunshine. Journal of Experimental Biology. 1955;32:279–298. [Google Scholar]
- Droege G. Das Imkerbuch. Berlin, German: VEB Deutscher Landwirtschaftsverlag; 1989. DDR–1040. [Google Scholar]
- Dyer CD, Seeley TD. Interspecific comparison of endothermy in honey-bees (Apis): deviations from the expected size-related patterns. Journal of Experimental Biology. 1987;127:1–26. [Google Scholar]
- Dyer AG, Whitney HM, Arnold SEJ, Glover BJ, Chittka L. Bees associate warmth with floral colour. Nature. 2006;442:525. doi: 10.1038/442525a. [DOI] [PubMed] [Google Scholar]
- Esch H. Body temperature and flight performance of honey bees in a servomechanically controlled wind tunnel. Journal of Comparative Physiology. 1976;109:265–277. [Google Scholar]
- Goulson D. Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspectives in Plant Ecology, Evolution and Systematics. 1999;2:185–209. [Google Scholar]
- Heinrich B. Thermoregulation of African and European honeybees during foraging, attack, and hive exits and returns. Journal of Experimental Biology. 1979a;80:217–229. [Google Scholar]
- Heinrich B. Keeping a cool head: honeybee thermoregulation. Science. 1979b;205:1269–1271. doi: 10.1126/science.205.4412.1269. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Mechanisms of body-temperature regulation in honeybees, Apis mellifera. I. Regulation of head temperature. Journal of Experimental Biology. 1980a;85:61–72. [Google Scholar]
- Heinrich B. Mechanisms of body-temperature regulation in honeybees, Apis mellifera. II. Regulation of thoracic temperature at high air temperature. Journal of Experimental Biology. 1980b;85:73–87. [Google Scholar]
- Heinrich B. The Hot-Blooded Insects. Berlin, Heidelberg, Germany; London, U.K.; Paris, France: Springer-Verlag; 1993. [Google Scholar]
- Imdorf A, Rickli M, Fluri P. Massenwechsel des Bienenvolkes. FAM Publikation Sektion Bienen, Bern; 1996. [Google Scholar]
- Kleinhenz M, Bujok B, Fuchs S, Tautz J. Hot bees in empty broodnest cells: heating from within. Journal of Experimental Biology. 2003;206:4217–4231. doi: 10.1242/jeb.00680. [DOI] [PubMed] [Google Scholar]
- Kovac H, Schmaranzer S. Thermoregulation of honeybees (Apis mellifera) foraging in spring and summer at different plants. Journal of Insect Physiology. 1996;42:1071–1076. [Google Scholar]
- Kovac H, Stabentheiner A, Schmaranzer S. Thermoregulation of water foraging wasps (Vespula vulgaris and Polistes dominulus) Journal of Insect Physiology. 2009a;55:959–966. doi: 10.1016/j.jinsphys.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac H, Stabentheiner A, Brodschneider R. Contribution of honeybee drones of different age to colonial thermoregulation. Apidologie. 2009b;40:82–95. doi: 10.1051/apido/2008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac H, Stabentheiner A, Schmaranzer S. Thermoregulation of water foraging honeybees – balancing of endothermic activity with radiative heat gain and functional requirements. Journal of Insect Physiology. 2010;56:1834–1845. doi: 10.1016/j.jinsphys.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht I, Maierhofer C, Röllig M. A thermographic promenade through the Berlin Botanic Garden. Thermochimica Acta. 2006;446:4–10. [Google Scholar]
- Liebig G. Entwicklung von Bienenvölkern – Ergebnisse des Forschungsprogrammes “Volksentwicklung”. Gesellschaft der Freunde der Landesanstalt für Bienenkunde der Universität Hoheheim, Festschrift Hohenheim aktuell; 1994. [Google Scholar]
- Moffatt L. Changes in the metabolic rate of the foraging honeybee: effect of the carried weight or of the reward rate? Journal of Comparative Physiology A. 2000;186:299–306. doi: 10.1007/s003590050430. [DOI] [PubMed] [Google Scholar]
- Moffatt L. Metabolic rate and thermal stability during honeybee foraging at different reward rates. Journal of Experimental Biology. 2001;204:759–766. doi: 10.1242/jeb.204.4.759. [DOI] [PubMed] [Google Scholar]
- Moffatt L, Núñez JA. Oxygen consumption in the foraging honeybee depends on the reward rate at the food source. Journal of Comparative Physiology B. 1997;167:36–42. doi: 10.1007/s003600050045. [DOI] [PubMed] [Google Scholar]
- Nieh JC, Leon A, Cameron S, Vandame R. Hot bumble bees at good food: thoracic temperature of feeding Bombus wilmattae foragers is tuned to sugar concentration. Journal of Experimental Biology. 2006;209:4185–4192. doi: 10.1242/jeb.02528. [DOI] [PubMed] [Google Scholar]
- Núñez JA. Nectar flow by melliferous flora and gathering flow by Apis mellifera ligustica. Journal of Insect Physiology. 1977;23:265–275. [Google Scholar]
- Pankiw T, Roman R, Sagili RR, Zhu-Salzman K. Pheromone-modulated behavioral suites influence colony growth in the honey bee (Apis mellifera) Naturwissenschaften. 2004;91:575–578. doi: 10.1007/s00114-004-0568-y. [DOI] [PubMed] [Google Scholar]
- Sadler N, Nieh JC. Honey bee forager thoracic temperature inside the nest is tuned to broad-scale differences in recruitment motivation. Journal of Experimental Biology. 2011;214:469–475. doi: 10.1242/jeb.049445. [DOI] [PubMed] [Google Scholar]
- Schmaranzer S. Thermoregulation of water collecting honeybees (Apis mellifera) Journal of Insect Physiology. 2000;46:1187–1194. doi: 10.1016/s0022-1910(00)00039-1. [DOI] [PubMed] [Google Scholar]
- Schmaranzer S, Stabentheiner A. Variability of the thermal behaviour of honeybees on a feeding place. Journal of Comparative Physiology B. 1988;158:135–141. [Google Scholar]
- Schmid-Hempel P, Kacelnik A, Houston AI. Honeybees maximize efficiency by not filling their crop. Behavioral Ecology and Sociobiology. 1985;17:61–66. [Google Scholar]
- Seeley TD. Survival of honeybees in cold climates: the critical timing of colony growth and reproduction. Ecological Entomology. 1985;120:826–888. [Google Scholar]
- Seeley TD. Social foraging by honeybees: how colonies allocate foragers among patches of flowers. Behavioral Ecology and Sociobiology. 1986;19:343–354. [Google Scholar]
- Seeley TD. The tremble dance of the honey bee: message and meanings. Behavioral Ecology and Sociobiology. 1992;31:375–383. [Google Scholar]
- Seeley TD. The Wisdom of the Hive. Cambridge, Massachusetts: Harvard University; 1995. [Google Scholar]
- Seeley TD, Tovey CA. Why search time to find a food storer bee accurately indicates the relative rates of nectar collecting and nectar processing in honey bee colonies. Animal Behavior. 1994;47:311–316. [Google Scholar]
- Seeley TD, Camazine S, Sneyd J. Collective decision-making in honey bees – how colonies choose among nectar sources. Behavioral Ecology and Sociobiology. 1991;28:277–290. [Google Scholar]
- Shuel RW. Current research on nectar. Bee World. 1970;51:63–68. [Google Scholar]
- Stabentheiner A. Effect of foraging distance on the thermal behaviour of honeybees during dancing, walking and trophallaxis. Ethology. 1996;102:360–370. [Google Scholar]
- Stabentheiner A. Thermoregulation of dancing bees: thoracic temperature of pollen and nectar foragers in relation to profitability of foraging and colony need. Journal of Insect Physiology. 2001;47:385–392. doi: 10.1016/s0022-1910(00)00132-3. [DOI] [PubMed] [Google Scholar]
- Stabentheiner A, Crailsheim K. The effect of activity level and ambient temperature on thermoregulation in isolated honeybees (Hymenoptera: Apidae) Entomologia Generalis. 1999;24:13–21. [Google Scholar]
- Stabentheiner A, Hagmüller K. Sweet food means ‘hot dancing’ in honey bees. Naturwissenschaften. 1991;78:471–473. [Google Scholar]
- Stabentheiner A, Schmaranzer S. Thermografie bei bienen: körpertemperaturen am futterplatz und im ‘bienenbart’. Verhandlungen der Deutschen. Zoologischen Gesellschaft. 1986;79:417–418. [Google Scholar]
- Stabentheiner A, Schmaranzer S. Thermographic determination of body temperatures in honey bees and hornets: calibration and applications. Thermology. 1987;2:563–572. [Google Scholar]
- Stabentheiner A, Schmaranzer S. Flight-related thermobiological investigations of honeybees (Apis mellifera carnica) In: Nachtigall W, editor. BIONA-report 6. Stuttgart, Germany; New York, New York: Akademie der Wissenschaften und Literatur zu Mainz, Gustav Fischer; 1988. pp. 89–102. [Google Scholar]
- Stabentheiner A, Kovac H, Hagmüller K. Thermal behavior of round and wagtail dancing honeybees. Journal of Comparative Physiology B. 1995;165:433–444. [Google Scholar]
- Stabentheiner A, Kovac H, Schmaranzer S. Honeybee nestmate recognition: the thermal behaviour of guards and their examinees. Journal of Experimental Biology. 2002;205:2637–2642. doi: 10.1242/jeb.205.17.2637. [DOI] [PubMed] [Google Scholar]
- Stabentheiner A, Pressl H, Papst T, Hrassnigg N, Crailsheim K. Endothermic heat production in honeybee winter clusters. Journal of Experimental Biology. 2003a;206:353–358. doi: 10.1242/jeb.00082. [DOI] [PubMed] [Google Scholar]
- Stabentheiner A, Vollmann J, Kovac H, Crailsheim K. Oxygen consumption and body temperature of active and resting honeybees. Journal of Insect Physiology. 2003b;49:881–889. doi: 10.1016/S0022-1910(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Stabentheiner A, Kovac H, Schmaranzer S. Thermal behaviour of honeybees during aggressive interactions. Ethology. 2007;113:995–1006. doi: 10.1111/j.1439-0310.2007.01403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabentheiner A, Kovac H, Brodschneider R. Honeybee colony thermoregulation – regulatory mechanisms and contribution of Individuals in dependence on age, location and thermal stress. PLoS ONE. 2010;5:e8967. doi: 10.1371/journal.pone.0008967. DOI: 10.1371/journal.pone.0008967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone GN, Willmer PG. Endothermy and temperature regulation in bees: a critique of “grab and stab” measurement of body temperature. Journal of Experimental Biology. 1989;143:211–223. [Google Scholar]
- Szabo TI. Nectar secretion in dandelion. Journal of Apicultural Research. 1984;23:204–208. [Google Scholar]
- Underwood B. Thermoregulation and energetic decision-making by the honeybees Apis cerana, Apis dorsata and Apis laboriosa. Journal of Experimental Biology. 1991;157:19–34. [Google Scholar]
- Waddington KD. Foraging profits and thoracic temperatures of honey bees (Apis mellifera) Journal of Comparative Physiology B. 1990;160:325–329. [Google Scholar]
- Wille H. Weitere Ergebnisse über den Brutrhythmus von Bienenvölkern. Schweizerische Bienenzeitungg. 1985;108:327–343. [Google Scholar]
- Willmer PG, Unwin DM. Field analyses of insect heat budgets: reflectance, size and heating rates. Oecologia. 1981;50:250–255. doi: 10.1007/BF00348047. [DOI] [PubMed] [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Cambridge, Massachusetts: Harvard University Press; 1987. [Google Scholar]


