Abstract
All animals use a sophisticated array of receptor proteins to sense their external and internal environments. Major advances have been made in recent years in understanding the molecular and genetic bases for sensory transduction in diverse modalities, indicating that both metabotropic and ionotropic pathways are important in sensory functions. Here, I review the historical background and recent advances in understanding the roles of a relatively newly discovered family of receptors, the degenerin/epithelial sodium channels (DEG/ENaC). These animal-specific cation channels show a remarkable sequence and functional diversity in different species and seem to exert their functions in diverse sensory modalities. Functions for DEG/ENaC channels have been implicated in mechanosensation as well as chemosensory transduction pathways. In spite of overall sequence diversity, all family members share a unique protein topology that includes just two transmembrane domains and an unusually large and highly structured extracellular domain, that seem to be essential for both their mechanical and chemical sensory functions. This review will discuss many of the recent discoveries and controversies associated with sensory function of DEG/ENaC channels in both vertebrate and invertebrate model systems, covering the role of family members in taste, mechanosensation, and pain.
I. INTRODUCTION
The rapid advancements in molecular and genomic biology have resulted in a wealth of information about how genes and their protein products affect cellular and organismal functions, and how such functions evolved. These data also led to the realization that multiple independent protein families have often evolved to serve similar physiological functions. The complex relationship between protein structure and physiological functions highlights the importance of studying such relationships with integrative and comparative approaches.
One of the most diverse groups of proteins in terms of the relationship between protein structure and function are ion channels. These membrane-targeted proteins are found in all cell types, including prokaryotes, and are critical for maintaining the appropriate ionic gradients across all cellular barriers, including the plasma membrane and intracellular compartments (Ashcroft and ScienceDirect (Online service), 2000). This review focuses on a relatively newly discovered and enigmatic family of ion channels; degenerin/epithelial Na+ channels (DEG/ENaC). DEG/ENaC proteins form nonvoltage gated, amiloride-sensitive cation channels (Bianchi and Driscoll, 2002; Garty and Palmer, 1997). DEG/ENaC channels comprise three to nine independent subunits, which can be either hetero- or homomultimers (Benson et al., 2002; Canessa et al., 1994b; Eskandari et al., 1999; Jasti et al., 2007; Kellenberger and Schild, 2002; Zha et al., 2009b). In cases where members of the family have been characterized electrophysiologically, subunit composition was found to have a significant effect on the pharmacological and electrical properties of the channel, suggesting that subunit composition is a critical regulatory mechanism in these channels (Askwith et al., 2004; Benson et al., 2002; Chu et al., 2004; Xie et al., 2003; Zha et al., 2009a; Zhang et al., 2008).
Despite of the high diversity in the primary sequence of individual subunits, several structural constituents indicated that all members of the family have a similar protein topology (Bianchi, 2007; Bianchi and Driscoll, 2002; Corey and Garcia-Anoveros, 1996; Tavernarakis and Driscoll, 2000, 2001a). The typical DEG/ENaC subunit has two transmembrane domains, two short intracellular domains and a large extracellular loop, which is a hallmark characteristic of the DEG/ENaC protein family topology (Fig. 1.1). The DEG/ENaC family seems to be animal specific and many different members have been identified in diverse species (Fig. 1.2).
Figure 1.1.
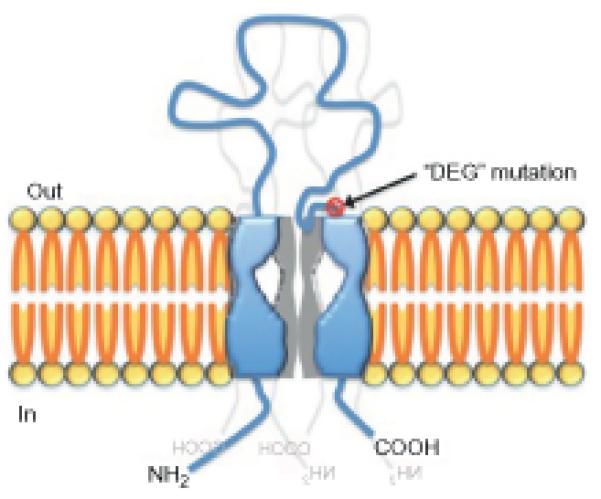
Topology of a typical DEG/ENaC channel. Each channel comprises three subunits (or multiples of three). Channels can be either homomeric or heteromeric protein complexes and are likely to include other accessory proteins. Each subunit comprises two transmembrane domains, two short intracellular domains (N terminus is typically longer than the C terminus), and an unusually large and highly structured extracellular domain. The “DEG mutation” represents an amino acid residue, which was shown to lock DEG/ ENaC channels in a constitutively open state (Snyder et al., 2000).
In the few instances where the pharmacological, structural, and biophysical properties of specific DEG/ENaC subunits have been studied, the channels have been characterized as ligand-gated, voltage insensitive, depolarizing cation channels, which seem to be more selective for Na+ over Ca2+ and K+ (Garty and Palmer, 1997). The physical cloning of various DEG/ENaC subunits enabled the identification of selective agonists and antagonists for specific subunits. In addition, natural ligands and physical stimuli were found to activate or modulate channel functions. These include (1) peptides such as members of the invertebrate FMRFamide family (Askwith et al., 2000; Green et al., 1994; Lingueglia et al., 1995; Xie et al., 2003), mammalian FFamide and SFamide peptides (Deval et al., 2003; Sherwood and Askwith, 2008, 2009), natural, and dynorphin-related opioid peptides (Sherwood and Askwith, 2009); (2) small increases in extracellular proton concentrations (Adams et al., 1998b; Benson et al., 2002; Price et al., 2001; Waldmann et al., 1997b; Xie et al., 2003; Xiong et al., 2004); (3) sulfhydryl compounds (Cho and Askwith, 2007); (4) small polyamines such as agmatine (Yu et al., 2010); and (5) mechanical stimuli (Bazopoulou et al., 2007; Lu et al., 2009; O’Hagan et al., 2005; Price et al., 2001; Simon et al., 2010; Tavernarakis and Driscoll, 2001a; Zhang et al., 2004; Zhong et al., 2010). Together, these data indicated that, like other ligand-gated ion channel families, DEG/ENaC channels have evolved to serve many different physiological functions, acting as ionotropic receptors to diverse extracellular stimuli.
Although amiloride-sensitive sodium currents from various epithelial tissues have been recorded since the early 1970s, the genes encoding for these channels, which were shown to be critical for regulating salt exchange in the kidney and blood pressure, were not identified until the early 1990s (Canessa et al., 1993, 1994a; Lingueglia et al., 1993a,b). The successful cloning of ENaC-coding genes was achieved by using expression cloning in Xenopus oocytes, which demonstrated that the mature ENaC comprises proteins from three highly related but independent genes. These genes were subsequently termed ENaCα, ENaCβ, and ENaCγ (Canessa et al., 1994b). The existence of homologous channels in invertebrates was originally debated. Nevertheless, several studies suggested the existence of amiloride-sensitive sodium currents in the leech, Xenopus, and the pond snail, suggesting these channels were not mammalian specific (Green et al., 1994; Weber et al., 1992, 1993). Later, cloning of several DEG/ENaC-like proteins from the worm Caenorhabditis elegans, and the fruit fly, Drosophila melanogaster showed that the DEG/ENaC family is likely to be ubiquitously present in animal genomes (for a comprehensive review of the early studies, see Garty and Palmer, 1997).
Subsequently, several additional members of the DEG/ENaC superfamily have been cloned from mammalian models, including several acid-sensitive ion channels (ASIC/Accn) (Price et al., 2000, 2001, 1996; Waldmann et al., 1997b, 1996; Xie et al., 2002). In contrast to ENaC-coding genes, which are transcriptionally enriched in epithelial tissues, members of the ASIC subfamily seem to be highly enriched in neuronal tissues, both centrally and peripherally (Lu et al., 2009; Xie et al., 2002). The completion of the sequencing of the human and other animal genomes revealed that mammals encode for eight to nine independent members of the DEG/ENaC protein superfamily. Surprisingly, the release of the completed genomes of the worm and the fruit fly revealed that the genomes of these invertebrates harbored a significantly larger number of independent DEG/ENaC-like genes (31 in the fruit fly and 30 in the worm), which also included several genes that can produce multiple variants due to alternative splicing and multiple transcriptional initiation sites (Bazopoulou et al., 2007; Liu et al., 2003a,b). Hence, DEG/ENaC genes represent one of the largest ion channel families in invertebrate genomes. The expansion of the DEG/ENaC protein family in these animals suggests the hypothesis that DEG/ENaC ion channels have evolved to serve a much wider range of physiological functions in invertebrates relative to their roles in mammals. Alternatively, it may suggest that DEG/ENaC subunits in invertebrates are highly specialized; each subunit is performing a narrow slice of the physiological tasks performed by mammalian family members. Our group is focused on understanding the role of DEG/ENaC channels in invertebrate physiology, which we hope will help us to resolve these two alternative hypotheses.
Although members of the DEG/ENaC superfamily are easily recognized by their unique protein topology (Fig. 1.1), identifying the relationships between family members across distant species based on protein sequence alone is hampered by the poor overall sequence conservation of the extracellular loop domain. Hence, protein alignment analyses alone were not powerful enough to draw physiological homology conclusions (Fig. 1.2). Consequently, newly identified family members typically require physiological analyses de novo.
The best physiologically characterized members of the family are the three mammalian ENaC genes (Garty and Palmer, 1997; Horisberger and Chraibi, 2004). The mammalian ENaC channels are typically found at the apical membrane of epithelial cells where they play an essential role in regulating sodium gradients across epithelial barriers in a variety of tissues (Snyder, 2005; Snyder et al., 1995; Voilley et al., 1994). Mutations in ENaC subunits can lead to disorders such as Liddle’s syndrome, which is a rare form of genetically inherited hypertension syndrome (Snyder et al., 1995). ASIC represent the other major mammalian branch of the DEG/ENaC family (Waldmann et al., 1999). These channels are enriched in peripheral and central neurons and are highly sensitive to changes in extracellular proton concentrations (Bassilana et al., 1997; Waldmann et al., 1997a; Wemmie et al., 2002). ASIC channels seem to play a major role in several pH-dependent physiological processes in the brain that include seizure termination (Ziemann et al., 2008), learning and memory (Askwith et al., 2004; Wemmie et al., 2002), and fear conditioning (Coryell et al., 2009, 2007; Wemmie et al., 2003; Ziemann et al., 2009). Similar central neuronal roles have also been recently identified for DEG/ENaC channels in the worm model (Voglis and Tavernarakis, 2008). How these pH-sensitive channels affect neuronal functions is still a mystery. At least some of the functions might be mediated by direct, short-term effects on synaptic plasticity, possibly by sensing microchanges in pH that are associated with the low pH environment of the lumen of synaptic vesicles. In contrast to our understanding of the role of ASIC channels in the CNS, their roles in sensory functions are still controversial, which will be discussed in details below.
Despite the advances in understanding the role of DEG/ENaC signaling in the brain, its role in peripheral neuronal functions is still poorly understood. Nevertheless, recent work in invertebrate and mammalian models indicated that members of the DEG/EnaC superfamily are playing a major role in chemosensation and mechanosensation, although the capacity in which they exert their sensory functions is still not well understood in most systems. This review focuses on the current state of research on the sensory roles of DEG/ENaC channels in diverse animal models.
The emerging interest in DEG/ENaC-dependent signaling has resulted in many studies of their functions in diverse species. As more and more individual subunits are being characterized, a complex picture is emerging in terms of the physiological roles of DEG/ENaC and their diverse gating mechanisms. Although the first DEG/ENaC channels cloned were characterized as a constitutively open sodium channels, later studies of the ENaC channel and the majority of other family members suggested that members of the family are likely acting as either classic ligand-gated ion channels (Horisberger and Chraibi, 2004) or mechanically gated channels (Bazopoulou et al., 2007; Bianchi, 2007).
II. CHEMOSENSATION
A. Salt taste
Maintaining appropriate ionic homeostasis is critical for all organisms, especially in regard to sodium, which is kept in relatively high extracellular concentrations in most animal tissues. Animals actively regulate their sodium intake via food consumption (Geerling and Loewy, 2008). In agreement with the importance of sodium, studies in rodent models indicated that some taste cells are specialized for responding to NaCl while others are less specialized and can be activated by many different solutes, suggesting that they act as detectors of osmolarity rather than directly responding to specific ions (Frank et al., 2008; Lundy and Contreras, 1999; Yoshida et al., 2009). Interestingly, some of the NaCl responsive cells in the mammalian taste epithelium are also sensitive to amiloride, which essentially blocks their responsiveness to salt (Heck et al., 1984). This amiloride sensitivity led to the hypothesis that ENaC channels might be involved in the salt taste signal transduction in mammals (Chandrashekar et al., 2010; Yoshida et al., 2009).
The first genetic evidence that DEG/ENaC signaling contributes to salt taste came from studies in the fruit fly (Liu et al., 2003b). These studies used transgenic expression of RNAi or dominant-negative alleles targeting ppk11 and ppk19, two independent DEG/ENaC encoding genes, which resulted in a reduced appetitive response to low concentrations of KCl and NaCl. The same manipulations had no effect on other taste modalities, suggesting these channels were not generic taste-related molecules.
Further support for salt sensing being genetically and cellularly independent of other taste modalities came from genetic studies in the mouse, which indicated that umami, sweet, and bitter sensing cells (type II taste cells) signal through G-protein coupled receptors (GPCR) (Zhang et al., 2003; Zhao et al., 2003). In contrast, the same studies clearly indicated that GPCR-independent pathways are responsible for sensing sour and salt tastes (Zhang et al., 2003).
Although the contribution of amiloride-sensitive sodium currents to salt taste is now generally accepted, other findings challenged these conclusions. These included studies showing that some NaCl responsive taste cells were not inhibited by amiloride in primary cultures. The receptor responsible for the amiloride-insensitive salt responses was recently identified as a variant of the TRP channel VR-1, a ligand-gated channel involved in the response to noxious heat and capsaicin (the “hot” compound in chili peppers; Lyall et al., 2005). Other ligand-gated ion channels that have been recently implicated in amiloride-insensitive salt taste are P2X2 and P2X3 ATP receptors (Eddy et al., 2009).
Despite the incomplete understanding of the role of ENaC signaling in salt taste, a recent study of a conditional ENaCα subunit knockout in the taste epithelia of mice resulted in animals that did not respond behaviorally or physiologically to a wide range of sodium concentrations, strongly supporting the primary role of ENaC signaling in mediating mammalian salt taste (Chandrashekar et al., 2010).
In contrast to other taste modalities, the amiloride-sensitive taste cells, which are responsive to appetitive levels of NaCl, are likely represented by type I taste cells (Vandenbeuch et al., 2008). These are surprising findings since these cells were previously assumed to act as nonexcitatory support taste cells that are not directly involved in taste transduction (Pumplin et al., 1997). These findings raise an interesting problem in terms of how salt taste is coded by the nervous system if indeed Type I cells, which do not form synaptic connections (Finger et al., 2000), are responsible for detecting salt taste via ENaC-dependent mechanisms (Vandenbeuch et al., 2008).
B. Sour taste
The molecular identity of the mammalian sour receptor is still controversial (Dotson, 2010). Early studies in rodent models suggested that the sour-taste receptor acts as a sodium channel, which can be partially blocked by amiloride (Ugawa et al., 1998). The subsequent cloning of the sensitive pH-gated members of the ASIC, which are members of the DEG/ENaC family, suggested that these channels might be the elusive sour-taste receptor (Shimada et al., 2006; Ugawa, 2003; Ugawa et al., 2003). Further analyses of the possible candidate ASIC channels involved in sour-taste transduction indicated that the sour-taste channel is possibly formed by heterodimerization of two alternatively spliced isoforms of the ASIC2 channel, ASIC2a and ASIC2b (Ugawa et al., 2003). Neurophysiological characterizations of these channels in Xenopus oocytes showed that they had pH-dependent sodium currents that were very similar to the currents evoked by low pH in taste buds in rats (Ugawa et al., 2003). Further, immunohistochemical studies in rats showed that ASIC2 channels are enriched in a subpopulation of taste receptor cells that are responsive to acid stimuli (Lin et al., 2004, 2002; Ugawa et al., 1998). Surprisingly, studies of ASIC2 in the mouse model indicated that the channel was not expressed in taste receptor cells, and homozygous ASIC2 knockout mice showed normal appetitive and physiological response to acids (Richter et al., 2004). These studies challenged the possible universal role of ASIC channels in sensing sour ligands.
Subsequent studies identified members of the polycystic kidney disease (PKD) genes as possible candidates for the “universal” mammalian sour-taste receptor (Chandrashekar et al., 2009; Huang et al., 2006; Ishii et al., 2009; Ishimaru et al., 2006; Kataoka et al., 2008). Nevertheless, recent knockout models of PKD1L3, one of the two PKD genes implicated in sour taste, were shown to have normal sour-taste behaviors and physiology, challenging the role of PKD-like genes in sensing sour taste (Nelson et al., 2010). These puzzling and conflicting data may suggest that multiple independent pathways, which are possibly different in different mammalian species, detect sour taste. Alternatively, it is possible that redundant, independent molecular mechanisms underlie sour taste. Further support for the redundancy model in humans comes from a recent study of sour ageusia (inability to detect low pH in ingested foods) in two individuals, which showed genetic mutations and reduced expression of both ASIC- and PKD-related proteins in sour-taste buds (Huque et al., 2009).
C. Other
In contrast to the limited taste repertoire in vertebrates, insects seem to have evolved a gustatory system that responds to a wide spectrum of chemicals, which do not necessarily overlap with the five canonical taste modalities (sweet, bitter, umami, salt, and sour). One such striking example is the sensing of “water” taste in Drosophila. Two recent studies indicated that flies have a specific population of gustatory receptor neurons that directly respond to the taste of water. Further, these studies implicated DEG/ENaC signaling in water sensing by using either (1) a combination of pharmacology and reversed genetics approach (Chen et al., 2010) or (2) a functional genomics approach to identify genes that are highly expressed in the proboscis of flies (Cameron et al., 2010). Both studies identified ppk28, a member of the DEG/ENaC family in flies, as the molecular receptor for water. Specifically, these studies showed that ppk28 is necessary for water detection, and that expression of ppk28 in nonwater sensing gustatory receptor neurons was sufficient to confer water sensitivity, indicating that ppk28 is likely the water receptor. The mechanism by which a DEG/ENaC channel like ppk28 can detect water molecules is still unknown. As will be discussed below, the possible role of DEG/ENaC channels in mechanosensory functions may suggest that ppk28 senses water by detecting mechanical changes in membrane physical properties in response to changes in external osmolarity.
Insects also rely extensively on their chemosensory systems for detecting social signals underlying behaviors such as courtship, aggression, and aggregation. Lounge lizard (llz/ppk25), a member of the DEG/ENaC family in Drosophila, was shown to be expressed in chemosensory-related accessory cells that are specific to the male forelegs, and to contribute to male courtship behavior (Ben-Shahar et al., 2010, 2007; Lin et al., 2005). Although a direct role for llz/ppk25 in sensing pheromones has not been demonstrated, data suggest that it might contribute to male courtship behavior (Lin et al., 2005). Surprisingly, an independent study indicated that genetic ablation of llz-expressing cells had no effect on male courtship behaviors (Ben-Shahar et al., 2010). Interestingly, llz represents a subfamily of several DEG/ENaC subunits in Drosophila, suggesting that several different subunits might be playing a role in social communication in insects. Consequently, more studies are required to establish llz and other similar DEG/ ENaC subunits in signaling pathways underlying pheromonal sensing.
llz is expressed in nonneuronal sheath cells, suggesting that its putative effects on courtship, are not mediated by direct response to pheromones, or that the sheath, glia-related cells are also acting as nonneuronal sensory cells (Ben-Shahar et al., 2010, 2007). Further support for this hypothesis came from a recent study in C. elegans in which ACD-1, a DEG/ENaC subunit, was shown to be expressed in chemosensory-related glia cells, and to contribute to acid avoidance behavior, as well as attraction to the amino acid lysine (Wang and Bianchi, 2009; Wang et al., 2008). Together, these data indicate that the contribution of some DEG/ENaC subunits to chemosensation via nonneuronal sensory pathways might be more prevalent than previously thought.
III. MECHANOSENSATION
A. C. elegans
All organisms seem to have evolved on mechanisms to sense mechanical stimuli, and in most cases, physiological studies indicated that the mechanosensory complex acts as a cation channel (for a recent review, see Arnadottir and Chalfie, 2010). Yet, the molecular identities of the proteins responsible for sensing mechanical stimuli are still mostly unknown (Christensen and Corey, 2007; Corey, 2006). The difficulty in identifying the mechanosensory conducting channels is likely the result of functional redundancies in mechanosensory systems, which complicate genetic studies. In addition, the low number of conducting channels per each individual mechanosensitive cell has made biochemical approaches for their isolation difficult. Nevertheless, in the past few years, researchers have identified at least some of the ion channels that underlie mechanosensory functions. The best examples to date are the classic screens for mec mutations in C. elegans, which have identified 15 genes that are defective in their response to gentle touch (Brown et al., 2007; Chelur et al., 2002; Cueva et al., 2007; Goodman et al., 2002; O’Hagan et al., 2005). This discovery led to the identification of a sensory protein complex involved in the touch sensation of worms (O’Hagan et al., 2005). Interestingly, two of the mec genes, mec-4 and mec-10, were shown to be members of the DEG/ENaC family (Goodman and Schwarz, 2003), and mutations in the mec-4/mec-10 complex led to a decrease in neuronal intracellular Ca2+ levels in response to gentle touch (Bianchi et al., 2004; Brown et al., 2007; Cueva et al., 2007). Further characterization of the mec-4 channel by using the in vivo whole-cell patch-clamp technique showed that the mec-4 complex was responsible for conducting the mechanical stimulus in mechanosensory neurons, which mediate light touch (Brown et al., 2007; Cueva et al., 2007; Nelson et al., 2010). To date, the mec-4/mec-10 complex in C. elegans is one of the only bone fide examples of a eukaryotic, molecularly and genetically defined, mechanically activated ion channel. The only other well-established ionotropic mechanosensors are members of the TRPN subfamily of the transient receptor potential channels in flies and worms (Kang et al., 2010; Lee et al., 2010).
Identifying the DEG/ENaC mechanosensitive channel also led to the isolation of other conserved components of the mechanosensory transduction. These included intra- and extracellular components of the DEG/ENaC-dependent mechanosensitive protein complexes (for a comprehensive review, see Chalfie, 2009). For example, mec-2, a gene that encodes for a stomatin-like protein, was shown to be important for the function of the mechanosensory complex by modulating the mec-4/mec-10 DEG/ENaC channel (Goodman et al., 2002). This discovery led to studies showing that a stomatin-domain protein is also important for light mechanosensation in mammals (Fricke et al., 2000; Huang et al., 1995; Martinez-Salgado et al., 2007; Price et al., 2004). These comparative investigations indicated that at least some mechanosensory complexes are likely to be conserved across distant animal species, highlighting the value of studying these important questions in genetically tractable model organisms, using a comparative and integrative approaches.
How DEG/ENaC channels might exert their mechanosensory functions is still unknown. The current prevailing model for the mechanical activation of DEG/ENaC sensory complexes hypothesizes that the highly structured extracellular domain of some DEG/ENaC channels could interact with extracellular matrix proteins, while the short intracellular domains are likely to interact with constituents of the cytoskeleton. Upon deflection of the animal’s outer surfaces, the pressure on the anchored extracellular domain results in a protein conformational changes that lead to the opening of the channel’s pore (Fig. 1.3).
B. Drosophila
The identification of members of the DEG/ENaC family in mechanosensitivity screens in C. elegans led to several studies that attempted to identify mechanosensitive DEG/ENaC subunits in the nervous systems of other model organisms. Despite initial enthusiasm, whether DEG/ENaC channels are playing a mechanosensory role in other species is still debatable. However, recent work in Drosophila suggests that functional DEG/ENaC signaling is required for the function of mechanosensitive, nociceptive neurons in the larval stage; mutations in ppk, a DEG/ENaC subunit that is expressed in class IV mechanonociceptive multidendritic sensory neurons (Adams et al., 1998a), were shown to affect locomotion (Ainsley et al., 2003), and contribute to the sensation of harsh mechanical and thermal stimuli (Hwang et al., 2007; Tracey et al., 2003). Taking advantage of the power of Drosophila genetics and novel tools for in vivo activation of neurons, a subsequent study suggested that genetic disruptions of ppk were essential for the response to harsh mechanical stimuli (Zhong et al., 2010). Although these studies fell short of showing that ppk acts as a sensory channel, they did support an essential role for DEG/ENaC in the mechanosensory transduction pathway.
C. Mammals
One of the most central challenges still remaining in sensory biology is the identification of the elusive mammalian hair cell transduction channel essential for the conduction of auditory signals (Corey, 2006; Gillespie and Muller, 2009). Early observations suggested that the drug amiloride can block sodium currents in the chick hair cell, suggesting that the conducting channel might be an amiloride-sensitive ion channel (Jorgensen and Ohmori, 1988). The cloning of the amiloride-sensitive ENaC channels from hair cells and the emerging role of DEG/ENaC signaling in mechanosensation in worms further strengthened this hypothesis (Chalfie et al., 1993). Despite the reports of the expression of various ENaC and ASIC channel subunits in mammalian hair cells, several lines of evidence emerged to suggest that DEG/ENaC channels are not likely to act as the hair cell transduction channel. First, the amiloride sensitivity of the hair cell channel is much higher than what was observed for DEG/ENaC channels in other epithelial tissues (Jorgensen and Ohmori, 1988). Second, the biophysical properties of DEG/ENaC channels are not consistent with the previously characterized biophysical parameters of the hair cell channel (Corey, 2006; Kellenberger and Schild, 2002). Third, knockouts of either the ENaCα (Rusch and Hummler, 1999) or ASIC2 channel in neonatal hair cells in mice did not result in any significant auditory or hair cell physiological impairments (Peng et al., 2004; Roza et al., 2004). To date, no direct evidence for the roles of ASIC1 or ASIC3 in hearing transduction have been reported. Nevertheless, it is now generally accepted that members of the DEG/ENaC channel family are not the mechanosensory channels responsible for mammalian hearing (Corey, 2006).
Although there is a lack of evidence to support a role for DEG/ENaC channels in the mammalian auditory signal transduction, it is possible that members of the family contribute to other types of mechanosensation. Genetic studies support the hypothesis that eukaryotic mechanosensation is mediated by ion channel receptors from multiple and independent protein families (Arnadottir and Chalfie, 2010).
Several ENaC and ASIC channels are expressed in subcutaneous mechanosensory structures, suggesting they might act as the mechanosensory transducer in the skin (Drummond et al., 2000; Price et al., 2000, 2001). Yet, genetic studies of these channels resulted in conflicting results. The first knockout (KO) model of ASIC2 (also called ACCN1 or BNaC1), a subunit that is enriched in the skin mechanosensitive neuronal fibers, indicated that the lack of DEG/ENaC signaling could lead to a mild reduction in the mechanosensitive response of fast adapting mechanoreceptors in the skin (Price et al., 2001). Yet, subsequent studies using independent ASIC2 and ASIC 3 KO models did not find any associations between ASIC functions and mechanosensitive currents in mammalian sensory neurons (Drew et al., 2004; Roza et al., 2004). Further, a transgenic model that expresses a dominant-negative form of ASIC3, which abolished most of the ASIC-like currents in DRG sensory neurons, also showed increased sensitivity to mechanical stimuli (Mogil et al., 2005). Together, these findings suggest that ASIC-dependent signaling might play a role in the mammalian skin mechanosensation, possibly as an important modulator but not necessarily as the mechanotransducing channel itself.
Whether other mammalian DEG/ENaC subunits might act as subcutaneous mechanosensitive channels is still poorly understood. At least one report indicated that both β and γENaC subunits are expressed in medium and large number of DRG neurons that project to the mechanosensitive Merkel cells and Meissner-like corpuscles present in the rat footpad (Finger et al., 2000). Surprisingly, the αENaC subunit was not present in these neurons, suggesting that a different DEG/ENaC subunit might be responsible for the sodium currents in these cells. In contrast to the human genome, which contains four independent genes that encode ENaC subunits, the rat genome has only three (Ji et al., 2006; Le and Saier, 1996). These data suggest that in rodents the βENaC and γENaC subunits could form a mechanosensitive channel independent of the αENaC protein, possibly by heterodimerization with one of the ASIC subunits present in DRG neurons (Benson et al., 2002; Xie et al., 2002).
Mechanosensitive channels also play a role in regulating the vertebrate blood pressure, most likely by regulating the baroreceptive sensory response to change in aortic pressure (Chapleau et al., 1995b; Cunningham et al., 1995; Ma et al., 2002). Arterial tension is sensed by specialized sensory neurons, which have their cell bodies in the nodus ganglion (Ma et al., 2002; Snitsarev et al., 2002). Previous reports indicated that rat nodus neurons are sensitive to mechanical stimuli in primary cultures as well as in vivo in response to glass probe stimuli or hypoosmotic buffers (Chapleau et al., 1995a,b; Cunningham et al., 1997; Kraske et al., 1998; Snitsarev et al., 2007; Sullivan et al., 1997) and exhibit a mechanical stimulus-dependent increase in intracellular Ca2+ levels (Chapleau et al., 1995b; Cunningham et al., 1995). Further, these currents were blocked by gadolinium, a trivalent cation, which is thought to directly block mechanosensitive channels (Chapleau et al., 1995a; Kraske et al., 1998). Cell-attached patch-clamp studies indicated that the putative mechanosensitive channels in the rat nodus neurons act as nonselective, voltage-independent cation channels (Cunningham et al., 1995). Although the molecular identity of the barosensitive channels in nodus neurons is still controversial, several studies suggest that DEG/ENaC signaling might contribute to their response to mechanical stimuli. First, RT-PCR studies indicated that all three ENaC subunits are expressed in nodus neurons (Drummond et al., 2000, 1998, 2001). Second, immunohistochemical localization of the γENaC subunit indicated that the channel is enriched at the sensory neurites of nodus neurons, which innervate the aortic arch (Drummond et al., 2000). Further support for the possible role of ENaC signaling came from studies of the effects of benzamil, an amiloride analog, on the carotid reflex response, which showed dose-dependent inhibition of mechanosensitivity (Drummond et al., 2001). Yet, genetic evidence that the ENaC channel is directly involved in regulating the arterial pressure reflex is still lacking.
Recently, the mammalian ASIC2 channel has emerged as a possible component of the baroreceptor complex and for the control of circulation pressure (Lu et al., 2009). This study showed that all three ASIC subunits, including all their alternatively spliced isoforms, are expressed in nodus sensory neurons. Further, immunostaining of the nodus ganglion suggested that different neuronal populations expressed the ASIC2 subunit and either ASIC1 or ASIC3. These data supported the hypothesis that different sensory neuronal populations express receptors with different properties (Lu et al., 2009). Studies of ASIC2 knockout mice showed significant baroreflex impairments by measuring the reflex response electrophysiologically. In contrast, ASIC2 transgenic mice showed hypersensitive arterial baroreception, further supporting the premise that ASIC2 signaling is a critical component of the baroresponse reflex in mammals (Lu et al., 2009).
IV. PERIPHERAL PAIN
Although the general role of DEG/ENaC signaling in eukaryotic mechanosensation is still controversial, the data discussed above indicated that, at least in invertebrates, DEG/ENaC subunits are playing an important role in the function of mechanically activated sensory neurons, often in the context of mechanical and thermal nociceptive stimuli (Albeg et al., 2010; Bounoutas and Chalfie, 2007; Chatzigeorgiou et al., 2010; Chelur et al., 2002; Goodman et al., 2002; Roza et al., 2004; Suzuki et al., 2003; Tavernarakis and Driscoll, 2001a,b; Zhang et al., 2004; Zhong et al., 2010). Although a similar role for DEG/ENaC channels in mammalian mechanical and thermal nociceptive responses is still poorly understood (Askwith et al., 2001; Drew et al., 2004; Page et al., 2004; Price et al., 2000, 2001), roles for these channels in other forms of mammalian pain perception are starting to emerge. These studies have been discussed recently in comprehensive reviews and hence will be described here briefly (Deval et al., 2010; Sluka et al., 2009; Wemmie et al., 2006).
The cloning of various ASIC subunits from mammalian genomes, and the discovery that some ASICs are highly sensitive proton receptors, led to studies that tested whether they might represent the nociceptive acid receptors that were originally described physiologically in the early 1980s in free nerve endings of somatosensory neurons in rodents (Krishtal and Pidoplichko, 1981a,b). There are currently at least six known mammalian ASIC subunits, which are transcribed via alternative splicing from four independent genetic loci (ASIC1-4, sometimes referred to as ACCN1-4; Lingueglia, 2007). Not all subunits show acid activated currents as homomultimers in heterologous expression systems (Askwith et al., 2004; Wemmie et al., 2006), possibly indicating that some subunit combinations might either have a different gating mechanism, are missing other proteins for their functions in vitro, or that some in vitro expressed subunits do not actually assemble in vivo, and hence do not represent physiologically relevant channels. Expression studies of DRG nociceptive neurons in rodents indicated that all known ASIC subunits are expressed in these tissues, further supporting the hypothesis that at least some of these proteins are acting as the elusive peripheral pain-related acid receptors (Waldmann et al., 1997b). Since the transient activation threshold of acid-evoked currents, and the channel’s electrokinetics, are strongly affected by specific subunit compositions, the presence of all subunits in various DRG neurons supported a role for differential subunit expression as a mechanism for establishing diverse acid sensitivity threshold, as is expected from behavioral studies of pain response to acid stimuli in vivo (Askwith et al., 2001; Benson et al., 2002; Donier et al., 2008; Xie et al., 2003).
Among the various identified ASIC channels in DRG neurons, the ASIC3 homomeric channels show the highest pH sensitivities and hence were speculated to comprise the main acid sensing pain receptor in variety of different DRG and trigeminal sensory neurons (Deval et al., 2008; Hattori et al., 2009; Ikeuchi et al., 2008, 2009; Walder et al., 2010). Yet, ASIC3 knockout mice or transgenic animals that expressed an ASIC3 dominant-negative allele did not show a lower response to acidic pain relative to wild types (Mogil et al., 2005; Price et al., 2001). In fact, the ASIC3 knockout showed small but significant increase in responses to acid pain stimulus. Surprisingly, the data obtained in mouse models did not agree with pharmacological data that was obtained from human and rat investigations; several pharmacological studies suggested that general ASIC antagonists such as amiloride can block acid-induced pain in healthy human subjects (Ugawa et al., 2002), and similarly, other ASIC antagonists such as A-317567 (Dube et al., 2005) or the somewhat ASIC3 selective toxin APETx2 had significant effects on cutaneous pain in rats. These pharmacological findings were further validated with in vivo siRNA studies (Deval et al., 2008). These conflicting data suggested that ASIC3 channels play a desensitizing role in pain sensory neurons in the mouse but might play a sensitizing role in human and rat neurons. These contradictory data from very close species such as the mouse and the rat suggest that ASIC3 is acting as the acid receptor in the periphery, but that the behavioral phenotypes observed in the mouse are due to its activity somewhere else in the pain circuit. Alternatively, it may suggest that although ASIC3 is a highly sensitive acid receptor, it is not playing the role of a nociceptive receptor in cutaneous pain neurons, but rather that it is acting as a modulatory factor for other still unknown acid receptors. These alternative hypotheses could be sorted out by the development of conditional knockout models of ASIC3, which will target its deletion to specific populations of DRG neurons. Further, the recent developments in rat knockout technologies (Jacob et al., 2010) will enable us to test the role of ASIC3 in rat pain with genetic rather than only pharmacological approaches. Regardless, these studies indicate the importance of studying DEG/ENaC signaling in multiple different species, since even under very similar physiological contexts, different species might utilize the same signaling pathways in contrasting manners.
V. CONCLUSIONS
Degenerin/epithelial sodium channels are emerging as important molecular players in animal sensory biology. Their possible role in mediating nociceptive behaviors in both invertebrates and vertebrates suggest that these channels evolved to serve such functions early in the metazoan radiation. One puzzling aspect of DEG/ENaC diversification is the large number of independent subunits present in invertebrate genomes relative to mammalian genomes. To my knowledge, no other ligand-gated ion channel families show such striking invertebrate-vertebrate dichotomy, suggesting these channels play special roles in invertebrate biology. As the significance of DEG/ENaC signaling in the mammalian nervous system becomes more apparent, the importance of developing novel models for studying DEG/ENaC signaling in genetically tractable models such as Drosophila and C. elegans should lead to the development of new understandings of how these channels exert their functions, what other proteins are playing a role in DEG/ENaC signaling, and how DEG/ENaC signaling affects neuronal physiology at both central and peripheral neurons, as well as in nonneuronal cell types.
Figure 1.2.
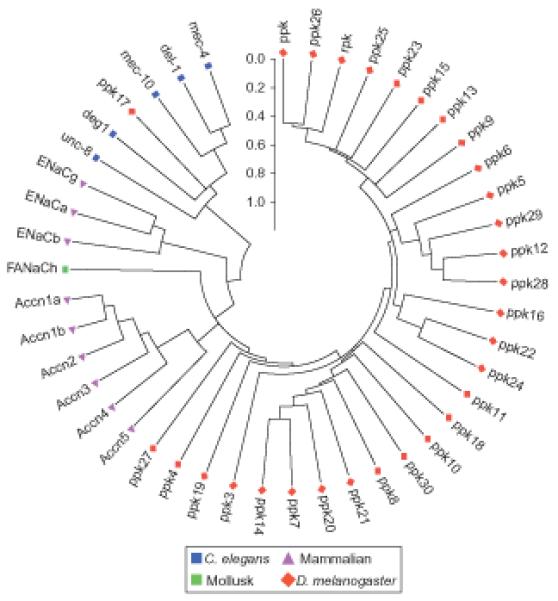
Molecular phylogenetic anaylsis of DEG/ENaC protein sequences. Evolutionary analyses were conducted in MEGA5 using default parameters (Tamura et al., 2007). The evolutionary history was inferred by using the Maximum Likelihood method based on the JTT matrix-based model (Jones et al., 1992). The tree with the highest log likelihood (− 9302.3626) is shown. Initial tree(s) for the heuristic search were obtained automatically as follows. When the number of common sites is less than 100, or less than one-fourth of the total number of sites, the maximum parsimony method was used; otherwise BIONJ method with MCL distance matrix was used. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 45 amino acid sequences, which included representatives from Drosophila (rpk, and all ppk genes), C. elegans (mec-4, del-1, mec-10, deg-1, unc-8), mouse (Accn and ENaC genes), and the FMRFamide-gated channel from the pond snail (FaNaCh). All sequences were downloaded from the NCBI database, using the most updated reference sequence for each protein. All positions containing gaps and missing data were eliminated. There were a total of 86 positions in the final dataset.
Figure 1.3.
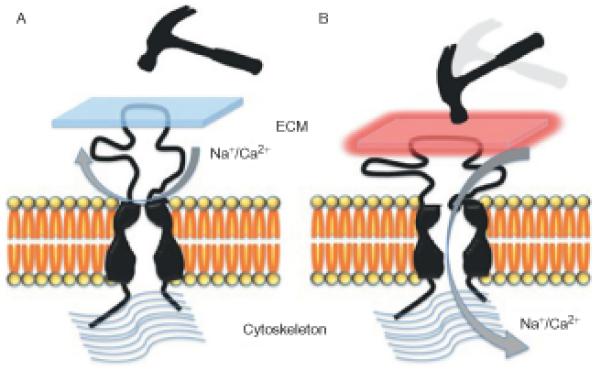
A model for the possible role of DEG/ENaC channels in the response to mechanical stimuli. (A) The large extracellular domain is attached to the extracellular matrix (ECM) either directly or possibly via other linker proteins. The short intracellular domains are attached directly or via other proteins to the cytoskeleton. (B) Upon mechanical pressure, the extracellular domain compresses, which results in opening of the pore, leading to influx of cations, which depolarizes the sensory cell. Currently, there are no conclusive data to support any of the models proposed for the mechanical gating of DEG/ENaC channels.
References
- Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J. Cell Biol. 1998a;140:143–152. doi: 10.1083/jcb.140.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CM, Snyder PM, Price MP, Welsh MJ. Protons activate brain Na channel 1 by inducing a conformational change that exposes a residue associated with neurode-generation. J. Biol. Chem. 1998b;273:30204–30207. doi: 10.1074/jbc.273.46.30204. [DOI] [PubMed] [Google Scholar]
- Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, Adams CM, Welsh MJ, Johnson WA. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr. Biol. 2003;13:1557–1563. doi: 10.1016/s0960-9822(03)00596-7. [DOI] [PubMed] [Google Scholar]
- Albeg A, Smith C, Chatzigeorgiou M, Feitelson DG, Hall DH, Schafer WR, Miller DM, 3rd, Treinin M. C. elegans multi-dendritic sensory neurons: Morphology and function. Mol. Cell. Neurosci. 2010;46:308–317. doi: 10.1016/j.mcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM. Ion Channels and Disease Channelopathies. Academic Press; San Diego: 2000. ScienceDirect (Online service) p. xxi.p. 481. [Google Scholar]
- Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron. 2000;26:133–141. doi: 10.1016/s0896-6273(00)81144-7. [DOI] [PubMed] [Google Scholar]
- Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc. Natl. Acad. Sci. USA. 2001;98:6459–6463. doi: 10.1073/pnas.111155398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J. Biol. Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- Bassilana F, Champigny G, Waldmann R, de Weille JR, Heurteaux C, Lazdunski M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. J. Biol. Chem. 1997;272:28819–28822. doi: 10.1074/jbc.272.46.28819. [DOI] [PubMed] [Google Scholar]
- Bazopoulou D, Voglis G, Tavernarakis N. The role of DEG/ENaC ion channels in sensory mechanotransduction. In: Wang DH, editor. Molecular Sensors for Cardiovascular Homeostasis. Springer; USA: 2007. pp. 3–31. [Google Scholar]
- Ben-Shahar Y, Nannapaneni K, Casavant TL, Scheetz TE, Welsh MJ. Eukaryotic operon-like transcription of functionally related genes in Drosophila. Proc. Natl. Acad. Sci. USA. 2007;104:222–227. doi: 10.1073/pnas.0609683104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar Y, Lu B, Collier DM, Snyder PM, Schnizler M, Welsh MJ. The Drosophila gene CheB42a is a novel modifier of Deg/ENaC channel function. PLoS One. 2010;5:e9395. doi: 10.1371/journal.pone.0009395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. USA. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L. Mechanotransduction: Touch and feel at the molecular level as modeled in Caenorhabditis elegans. Mol. Neurobiol. 2007;36:254–271. doi: 10.1007/s12035-007-8009-5. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Driscoll M. Protons at the gate: DEG/ENaC ion channels help us feel and remember. Neuron. 2002;34:337–340. doi: 10.1016/s0896-6273(02)00687-6. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Gerstbrein B, Frokjaer-Jensen C, Royal DC, Mukherjee G, Royal MA, Xue J, Schafer WR, Driscoll M. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: Implications for necrosis initiation. Nat. Neurosci. 2004;7:1337–1344. doi: 10.1038/nn1347. [DOI] [PubMed] [Google Scholar]
- Bounoutas A, Chalfie M. Touch sensitivity in Caenorhabditis elegans. Pflugers Arch. 2007;454:691–702. doi: 10.1007/s00424-006-0187-x. [DOI] [PubMed] [Google Scholar]
- Brown AL, Fernandez-Illescas SM, Liao Z, Goodman MB. Gain-of-function mutations in the MEC-4 DEG/ENaC sensory mechanotransduction channel alter gating and drug blockade. J. Gen. Physiol. 2007;129:161–173. doi: 10.1085/jgp.200609672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am. J. Physiol. 1994a;267:C1682–C1690. doi: 10.1152/ajpcell.1994.267.6.C1682. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994b;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Chalfie M. Neurosensory mechanotransduction. Nat. Rev. Mol. Cell Biol. 2009;10:44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Driscoll M, Huang M. Degenerin similarities. Nature. 1993;361:504. doi: 10.1038/361504a0. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapleau MW, Cunningham JT, Sullivan MJ, Wachtel RE, Abboud FM. Structural versus functional modulation of the arterial baroreflex. Hypertension. 1995a;26:341–347. doi: 10.1161/01.hyp.26.2.341. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Hajduczok G, Sharma RV, Wachtel RE, Cunningham JT, Sullivan MJ, Abboud FM. Mechanisms of baroreceptor activation. Clin. Exp. Hypertens. 1995b;17:1–13. doi: 10.3109/10641969509087050. [DOI] [PubMed] [Google Scholar]
- Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, Kindt KS, Hwang SW, Miller DM, 3rd, reinin M, Driscoll M, et al. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat. Neurosci. 2010;13:861–868. doi: 10.1038/nn.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen LROH, Chalfie M. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature. 2002;420:669–673. doi: 10.1038/nature01205. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wang Q, Wang Z. The amiloride-sensitive epithelial Na+ channel PPK28 is essential for drosophila gustatory water reception. J. Neurosci. 2010;30:6247–6252. doi: 10.1523/JNEUROSCI.0627-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Askwith CC. Potentiation of acid-sensing ion channels by sulfhydryl compounds. Am. J. Physiol. Cell Physiol. 2007;292:C2161–C2174. doi: 10.1152/ajpcell.00598.2006. [DOI] [PubMed] [Google Scholar]
- Christensen AP, Corey DP. TRP channels in mechanosensation: Direct or indirect activation? Nat. Rev. Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J. Neurosci. 2004;24:8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP. What is the hair cell transduction channel? J. Physiol. 2006;576:23–28. doi: 10.1113/jphysiol.2006.116582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Garcia-Anoveros J. Mechanosensation and the DEG/ENaC ion channels. Science. 1996;273:323–324. doi: 10.1126/science.273.5273.323. [DOI] [PubMed] [Google Scholar]
- Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol. Psychiatry. 2007;62:1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, et al. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J. Neurosci. 2009;29:5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva JG, Mulholland A, Goodman MB. Nanoscale organization of the MEC-4 DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 2007;27:14089–14098. doi: 10.1523/JNEUROSCI.4179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Wachtel RE, Abboud FM. Mechanosensitive currents in putative aortic baroreceptor neurons in vitro. J. Neurophysiol. 1995;73:2094–2098. doi: 10.1152/jn.1995.73.5.2094. [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Wachtel RE, Abboud FM. Mechanical stimulation of neurites generates an inward current in putative aortic baroreceptor neurons in vitro. Brain Res. 1997;757:149–154. doi: 10.1016/s0006-8993(97)00153-4. [DOI] [PubMed] [Google Scholar]
- Deval E, Baron A, Lingueglia E, Mazarguil H, Zajac JM, Lazdunski M. Effects of neuropeptide SF and related peptides on acid sensing ion channel 3 and sensory neuron excitability. Neuropharmacology. 2003;44:662–671. doi: 10.1016/s0028-3908(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27:3047–3055. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Gasull X, Noel J, Salinas M, Baron A, Diochot S, Lingueglia E. Acid-sensing ion channels (ASICs): Pharmacology and implication in pain. Pharmacol. Ther. 2010;128:549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Donier E, Rugiero F, Jacob C, Wood JN. Regulation of ASIC activity by ASIC4— New insights into ASIC channel function revealed by a yeast two-hybrid assay. Eur. J. Neurosci. 2008;28:74–86. doi: 10.1111/j.1460-9568.2008.06282.x. [DOI] [PubMed] [Google Scholar]
- Dotson CD. The search for mechanisms underlying the sour taste evoked by acids continues. Chem. Senses. 2010;35:545–547. doi: 10.1093/chemse/bjq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J. Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond HA, Price MP, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron. 1998;21:1435–1441. doi: 10.1016/s0896-6273(00)80661-3. [DOI] [PubMed] [Google Scholar]
- Drummond HA, Abboud FM, Welsh MJ. Localization of beta and gamma subunits of ENaC in sensory nerve endings in the rat foot pad. Brain Res. 2000;884:1–12. doi: 10.1016/s0006-8993(00)02831-6. [DOI] [PubMed] [Google Scholar]
- Drummond HA, Welsh MJ, Abboud FM. ENaC subunits are molecular components of the arterial baroreceptor complex. Ann. N. Y. Acad. Sci. 2001;940:42–47. doi: 10.1111/j.1749-6632.2001.tb03665.x. [DOI] [PubMed] [Google Scholar]
- Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117:88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Eddy MC, Eschle BK, Barrows J, Hallock RM, Finger TE, Delay ER. Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647. Chem. Senses. 2009;34:789–797. doi: 10.1093/chemse/bjp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari S, Snyder PM, Kreman M, Zampighi GA, Welsh MJ, Wright EM. Number of subunits comprising the epithelial sodium channel. J. Biol. Chem. 1999;274:27281–27286. doi: 10.1074/jbc.274.38.27281. [DOI] [PubMed] [Google Scholar]
- Finger TE, Restrepo D, Silver WL. The Neurobiology of Taste and Smell. 2nd edn Wiley-Liss; New York: 2000. [Google Scholar]
- Frank ME, Lundy RF, Jr., Contreras RJ. Cracking taste codes by tapping into sensory neuron impulse traffic. Prog. Neurobiol. 2008;86:245–263. doi: 10.1016/j.pneurobio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke B, Lints R, Stewart G, Drummond H, Dodt G, Driscoll M, von During M. Epithelial Na+ channels and stomatin are expressed in rat trigeminal mechanosensory neurons. Cell Tissue Res. 2000;299:327–334. doi: 10.1007/s004419900153. [DOI] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: Function, structure, and regulation. Physiol. Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp. Physiol. 2008;93:177–209. doi: 10.1113/expphysiol.2007.039891. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Muller U. Mechanotransduction by hair cells: Models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Schwarz EM. Transducing touch in Caenorhabditis elegans. Annu. Rev. Physiol. 2003;65:429–452. doi: 10.1146/annurev.physiol.65.092101.142659. [DOI] [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O’Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- Green KA, Falconer SW, Cottrell GA. The neuropeptide Phe-Met-Arg-Phe-NH2 (FMRFamide) directly gates two ion channels in an identified Helix neurone. Pflugers Arch. 1994;428:232–240. doi: 10.1007/BF00724502. [DOI] [PubMed] [Google Scholar]
- Hattori T, Chen J, Harding AM, Price MP, Lu Y, Abboud FM, Benson CJ. ASIC2a and ASIC3 heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ. Res. 2009;105:279–286. doi: 10.1161/CIRCRESAHA.109.202036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- Horisberger JD, Chraibi A. Epithelial sodium channel: A ligand-gated channel? Nephron Physiol. 2004;96:37–41. doi: 10.1159/000076406. [DOI] [PubMed] [Google Scholar]
- Huang M, Gu G, Ferguson EL, Chalfie M. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature. 1995;378:292–295. doi: 10.1038/378292a0. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huque T, Cowart BJ, Dankulich-Nagrudny L, Pribitkin EA, Bayley DL, Spielman AI, Feldman RS, Mackler SA, Brand JG. Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PLoS One. 2009;4:e7347. doi: 10.1371/journal.pone.0007347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137:662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Kolker SJ, Sluka KA. Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J. Pain. 2009;10:336–342. doi: 10.1016/j.jpain.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Misaka T, Kishi M, Kaga T, Ishimaru Y, Abe K. Acetic acid activates PKD1L3-PKD2L1 channel—A candidate sour taste receptor. Biochem. Biophys. Res. Commun. 2009;385:346–350. doi: 10.1016/j.bbrc.2009.05.069. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob HJ, Lazar J, Dwinell MR, Moreno C, Geurts AM. Gene targeting in the rat: Advances and opportunities. Trends Genet. 2010;26:510–518. doi: 10.1016/j.tig.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Ji HL, Su XF, Kedar S, Li J, Barbry P, Smith PR, Matalon S, Benos DJ. Delta-subunit confers novel biophysical features to alpha beta gamma-human epithelial sodium channel (ENaC) via a physical interaction. J. Biol. Chem. 2006;281:8233–8241. doi: 10.1074/jbc.M512293200. [DOI] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992;8:275. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Jorgensen F, Ohmori H. Amiloride blocks the mechano-electrical transduction channel of hair cells of the chick. J. Physiol. 1988;403:577–588. doi: 10.1113/jphysiol.1988.sp017265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sevigny J, Kinnamon JC, Finger TE. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem. Senses. 2008;33:243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol. Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kraske S, Cunningham JT, Hajduczok G, Chapleau MW, Abboud FM, Wachtel RE. Mechanosensitive ion channels in putative aortic baroreceptor neurons. Am. J. Physiol. 1998;275:H1497–H1501. doi: 10.1152/ajpheart.1998.275.4.H1497. [DOI] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI. Receptor for protons in the membrane of sensory neurons. Brain Res. 1981a;214:150–154. doi: 10.1016/0006-8993(81)90446-7. [DOI] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI. A “receptor” for protons in small neurons of trigeminal ganglia: Possible role in nociception. Neurosci. Lett. 1981b;24:243–246. doi: 10.1016/0304-3940(81)90164-6. [DOI] [PubMed] [Google Scholar]
- Le T, Saier MH., Jr. Phylogenetic characterization of the epithelial Na+ channel (ENaC) family. Mol. Membr. Biol. 1996;13:149–157. doi: 10.3109/09687689609160591. [DOI] [PubMed] [Google Scholar]
- Lee J, Moon S, Cha Y, Chung YD. Drosophila TRPN(NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS One. 2010;5:e11012. doi: 10.1371/journal.pone.0011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Ogura T, Kinnamon SC. Acid-activated cation currents in rat vallate taste receptor cells. J. Neurophysiol. 2002;88:133–141. doi: 10.1152/jn.2002.88.1.133. [DOI] [PubMed] [Google Scholar]
- Lin W, Burks CA, Hansen DR, Kinnamon SC, Gilbertson TA. Taste receptor cells express pH-sensitive leak K+ channels. J. Neurophysiol. 2004;92:2909–2919. doi: 10.1152/jn.01198.2003. [DOI] [PubMed] [Google Scholar]
- Lin H, Mann KJ, Starostina E, Kinser RD, Pikielny CW. A Drosophila DEG/ ENaC channel subunit is required for male response to female pheromones. Proc. Natl. Acad. Sci. USA. 2005;102:12831–12836. doi: 10.1073/pnas.0506420102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E. Acid-sensing ion channels in sensory perception. J. Biol. Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Renard S, Voilley N, Waldmann R, Chassande O, Lazdunski M, Barbry P. Molecular cloning and functional expression of different molecular forms of rat amiloride-binding proteins. Eur. J. Biochem. 1993a;216:679–687. doi: 10.1111/j.1432-1033.1993.tb18188.x. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett. 1993b;318:95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- Liu L, Johnson WA, Welsh MJ. Drosophila DEG/ENaC pickpocket genes are expressed in the tracheal system, where they may be involved in liquid clearance. Proc. Natl. Acad. Sci. USA. 2003a;100:2128–2133. doi: 10.1073/pnas.252785099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Leonard AS, Motto DG, Feller MA, Price MP, Johnson WA, Welsh MJ. Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron. 2003b;39:133–146. doi: 10.1016/s0896-6273(03)00394-5. [DOI] [PubMed] [Google Scholar]
- Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, et al. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy RF, Jr., Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J. Neurophysiol. 1999;82:2970–2988. doi: 10.1152/jn.1999.82.6.2970. [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Desimone JA. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem. Senses. 2005;30(Suppl. 1):i42–i43. doi: 10.1093/chemse/bjh104. [DOI] [PubMed] [Google Scholar]
- Ma X, Abboud FM, Chapleau MW. Analysis of afferent, central, and efferent components of the baroreceptor reflex in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R1033–R1040. doi: 10.1152/ajpregu.00768.2001. [DOI] [PubMed] [Google Scholar]
- Martinez-Salgado C, Benckendorff AG, Chiang LY, Wang R, Milenkovic N, Wetzel C, Hu J, Stucky CL, Parra MG, Mohandas N, et al. Stomatin and sensory neuron mechanotransduction. J. Neurophysiol. 2007;98:3802–3808. doi: 10.1152/jn.00860.2007. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J. Neurosci. 2005;25:9893–9901. doi: 10.1523/JNEUROSCI.2019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TM, Lopezjimenez ND, Tessarollo L, Inoue M, Bachmanov AA, Sullivan SL. Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem. Senses. 2010;35:565–577. doi: 10.1093/chemse/bjq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T, Lewin GR, Welsh MJ, et al. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–1747. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Peng BG, Ahmad S, Chen S, Chen P, Price MP, Lin X. Acid-sensing ion channel 2 contributes a major component to acid-evoked excitatory responses in spiral ganglion neurons and plays a role in noise susceptibility of mice. J. Neurosci. 2004;24:10167–10175. doi: 10.1523/JNEUROSCI.3196-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J. Biol. Chem. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, et al. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Price MP, Thompson RJ, Eshcol JO, Wemmie JA, Benson CJ. Stomatin modulates gating of acid-sensing ion channels. J. Biol. Chem. 2004;279:53886–53891. doi: 10.1074/jbc.M407708200. [DOI] [PubMed] [Google Scholar]
- Pumplin DW, Yu C, Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J. Comp. Neurol. 1997;378:389–410. doi: 10.1002/(sici)1096-9861(19970217)378:3<389::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Richter TA, Dvoryanchikov GA, Roper SD, Chaudhari N. Acid-sensing ion channel-2 is not necessary for sour taste in mice. J. Neurosci. 2004;24:4088–4091. doi: 10.1523/JNEUROSCI.0653-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza C, Puel JL, Kress M, Baron A, Diochot S, Lazdunski M, Waldmann R. Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J. Physiol. 2004;558:659–669. doi: 10.1113/jphysiol.2004.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch A, Hummler E. Mechano-electrical transduction in mice lacking the alpha-subunit of the epithelial sodium channel. Hear. Res. 1999;131:170–176. doi: 10.1016/s0378-5955(99)00030-1. [DOI] [PubMed] [Google Scholar]
- Sherwood TW, Askwith CC. Endogenous arginine-phenylalanine-amide-related peptides alter steady-state desensitization of ASIC1a. J. Biol. Chem. 2008;283:1818–1830. doi: 10.1074/jbc.M705118200. [DOI] [PubMed] [Google Scholar]
- Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J. Neurosci. 2009;29:14371–14380. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Ueda T, Ishida Y, Yamamoto T, Ugawa S. Acid-sensing ion channels in taste buds. Arch. Histol. Cytol. 2006;69:227–231. doi: 10.1679/aohc.69.227. [DOI] [PubMed] [Google Scholar]
- Simon A, Shenton F, Hunter I, Banks RW, Bewick GS. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J. Physiol. 2010;588:171–185. doi: 10.1113/jphysiol.2009.182683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: A new target for pain and CNS diseases. Curr. Opin. Drug Discov. Devel. 2009;12:693–704. [PMC free article] [PubMed] [Google Scholar]
- Snitsarev V, Whiteis CA, Abboud FM, Chapleau MW. Mechanosensory transduction of vagal and baroreceptor afferents revealed by study of isolated nodose neurons in culture. Auton. Neurosci. 2002;98:59–63. doi: 10.1016/s1566-0702(02)00033-4. [DOI] [PubMed] [Google Scholar]
- Snitsarev V, Whiteis CA, Chapleau MW, Abboud FM. Mechano- and chemosensitivity of rat nodose neurones—Selective excitatory effects of prostacyclin. J. Physiol. 2007;582:177–194. doi: 10.1113/jphysiol.2007.133330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM. Minireview: Regulation of epithelial Na+ channel trafficking. Endocrinology. 2005;146:5079–5085. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- Snyder PM, Bucher DB, Olson DR. Gating induces a conformational change in the outer vestibule of ENaC. J. Gen. Physiol. 2000;116:781. doi: 10.1085/jgp.116.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ. Mechanism by which Liddle’s syndrome mutations increase activity of a human epithelial Na+ channel. Cell. 1995;83:969–978. doi: 10.1016/0092-8674(95)90212-0. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Sharma RV, Wachtel RE, Chapleau MW, Waite LJ, Bhalla RC, Abboud FM. Non-voltage-gated Ca2 influx through mechanosensitive ion channels in aortic baroreceptor neurons. Circ. Res. 1997;80:861–867. doi: 10.1161/01.res.80.6.861. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kerr R, Bianchi L, Frokjaer-Jensen C, Slone D, Xue J, Gerstbrein B, Driscoll M, Schafer WR. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 2003;39:1005–1017. doi: 10.1016/j.neuron.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Driscoll M. Caenorhabditis elegans degenerins and vertebrate ENaC ion channels contain an extracellular domain related to venom neurotoxins. J. Neurogenet. 2000;13:257–264. doi: 10.3109/01677060009084497. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Driscoll M. Degenerins. At the core of the metazoan mechano-transducer? Ann. N. Y. Acad. Sci. 2001a;940:28–41. [PubMed] [Google Scholar]
- Tavernarakis N, Driscoll M. Mechanotransduction in Caenorhabditis elegans: The role of DEG/ENaC ion channels. Cell Biochem. Biophys. 2001b;35:1–18. doi: 10.1385/CBB:35:1:01. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Jr., Wilson RI, Laurent G, Benzer S. Painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Ugawa S. Identification of sour-taste receptor genes. Anat. Sci. Int. 2003;78:205–210. doi: 10.1046/j.0022-7722.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Minami Y, Guo W, Saishin Y, Takatsuji K, Yamamoto T, Tohyama M, Shimada S. Receptor that leaves a sour taste in the mouth. Nature. 1998;395:555–556. doi: 10.1038/26882. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J. Clin. Invest. 2002;110:1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, Shimada S. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J. Neurosci. 2003;23:3616–3622. doi: 10.1523/JNEUROSCI.23-09-03616.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N. A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signalling. EMBO J. 2008;27:3288–3299. doi: 10.1038/emboj.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voilley N, Lingueglia E, Champigny G, Mattei MG, Waldmann R, Lazdunski M, Barbry P. The lung amiloride-sensitive Na channel: Biophysical properties, pharmacology, ontogenesis, and molecular cloning. Proc. Natl. Acad. Sci. USA. 1994;91:247–251. doi: 10.1073/pnas.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J. Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. J. Biol. Chem. 1996;271:10433–10436. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J. Biol. Chem. 1997a;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A protongated cation channel involved in acid-sensing. Nature. 1997b;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H(+)-gated cation channels. Ann. N. Y. Acad. Sci. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bianchi L. Insights into the molecular determinants of proton inhibition in an acid-inactivated degenerins and mammalian epithelial Na(+) channel. Biochemistry. 2009;48:10005–10013. doi: 10.1021/bi9014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Apicella A, Jr., Lee SK, Ezcurra M, Slone RD, Goldmit M, Schafer WR, Shaham S, Driscoll M, Bianchi L. A glial DEG/ENaC channel functions with neuronal channel DEG-1 to mediate specific sensory functions in C. elegans. EMBO J. 2008;27:2388–2399. doi: 10.1038/emboj.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber WM, Asher C, Garty H, Clauss W. Expression of amiloride-sensitive Na+ channels of hen lower intestine in Xenopus oocytes: Electrophysiological studies on the dependence of varying NaCl intake. Biochim. Biophys. Acta. 1992;1111:159–164. doi: 10.1016/0005-2736(92)90306-7. [DOI] [PubMed] [Google Scholar]
- Weber WM, Dannenmaier B, Clauss W. Ion transport across leech integument. I. Electrogenic Na+ transport and current fluctuation analysis of the apical Na+ channel. J. Comp. Physiol. B. 1993;163:153–159. doi: 10.1007/BF00263601. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr., et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr., Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J. Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: Advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Xie J, Price MP, Berger AL, Welsh MJ. DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J. Neurophysiol. 2002;87:2835–2843. doi: 10.1152/jn.2002.87.6.2835. [DOI] [PubMed] [Google Scholar]
- Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J. Neurophysiol. 2003;89:2459–2465. doi: 10.1152/jn.00707.2002. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, et al. Neuroprotection in ischemia: Blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Horio N, Murata Y, Yasumatsu K, Shigemura N, Ninomiya Y. NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience. 2009;159:795–803. doi: 10.1016/j.neuroscience.2008.12.052. [DOI] [PubMed] [Google Scholar]
- Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. 2010;68:61–72. doi: 10.1016/j.neuron.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J. Neurosci. 2009a;29:8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Wang R, Collier DM, Snyder PM, Wemmie JA, Welsh MJ. Oxidant regulated inter-subunit disulfide bond formation between ASIC1a subunits. Proc. Natl. Acad. Sci. USA. 2009b;106:3573–3578. doi: 10.1073/pnas.0813402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Arnadottir J, Keller C, Caldwell GA, Yao CA, Chalfie M. MEC-2 is recruited to the putative mechanosensory complex in C. elegans touch receptor neurons through its stomatin-like domain. Curr. Biol. 2004;14:1888–1896. doi: 10.1016/j.cub.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Zhang W, Bianchi L, Lee WH, Wang Y, Israel S, Driscoll M. Intersubunit interactions between mutant DEG/ENaCs induce synthetic neurotoxicity. Cell Death Differ. 2008;15:1794–1803. doi: 10.1038/cdd.2008.114. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr. Biol. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, Iii, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat. Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


