Abstract
In the early postpartum period, mother and infant navigate a critical neuroendocrine transition from pregnancy to lactation. Two major clinical problems that occur during this transition are failed lactation and perinatal mood disorders. These disorders often overlap in clinical settings. Failed lactation is common. Although all major medical organizations recommend 6 months of exclusive breastfeeding, only 13% of women in the United States achieve this recommendation. Perinatal mood disorders affect 10% of mothers, with substantial morbidity for mother and child. We hypothesize that shared neuroendocrine mechanisms contribute to both failed lactation and perinatal mood disorders. In this hypothesis article, we discuss data from both animal models and clinical studies that suggest neuroendocrine mechanisms that may underlie these two disorders. Research to elucidate the role of these underlying mechanisms may identify treatment strategies both to relieve perinatal depression and to enable women to achieve their infant feeding goals.
Introduction
Failed lactation and perinatal depression are two common disorders with major public health consequences. All major medical organizations recommend that mothers breastfeed exclusively for at least 6 months,1 but only 13% of women in the United States achieve this goal, resulting in an estimated $13 billion in excess costs, due primarily to infection-related infant morbidity and mortality.2 Perinatal depression, defined as a major depressive episode3 that occurs either during pregnancy or within the first 6 months postpartum, affects 10%–15% of mothers and can have devastating consequences for mother, child, and family.4 In both clinical practice and observational studies, these two conditions frequently occur together, but the direction of this association is unclear. Higher rates of formula feeding have been documented among depressed mothers,5 however, and longitudinal data suggest that the onset of depression usually precedes weaning.6
Failed lactation is difficult to capture in epidemiologic studies because a variety of factors may lead a mother to wean. However, data suggest that weaning is often involuntary. In a recent longitudinal study of U.S. women, less than half breastfed their infants as long as they had wanted to.7 We define failed lactation as weaning earlier than the mother desires because of physiologic difficulties, such as pain, difficult latch, or concerns about milk supply.8
We hypothesize that failed lactation and perinatal mood disorders share a common pathophysiologic basis. In the weeks after birth, the maternal neuroendocrine system undergoes an abrupt transition as hypothalamic and other central neuroregulatory systems adjust to loss of placental hormones, declining binding globulin levels, and the stressor of caring for a new infant. In this article, we present our hypothesis that this neuroendocrine transition may contribute both to breastfeeding difficulties and to perinatal mood disorders, and we propose a blueprint for future investigations of these conditions. In animal studies, authors have elucidated the role of lactogenic hormones, progesterone withdrawal, adrenal and autonomic stress reactivity, and thyroid homeostasis in both lactation physiology and maternal behavior, which serves as a proxy for postpartum mood disorders. Clinical studies also provide evidence for these pathways in the pathophysiology of both failed lactation and perinatal depression. In the text that follows, we discuss clinical and preclinical evidence of overlapping mechanisms in lactogenic hormones, ovarian hormones, stress reactivity systems, pain perception, and thyroid homeostasis (Table 1 and Fig. 1). We also address the potential effects of maternal depression on fetal/infant development as it relates to establishment of lactation. We conclude with areas for future research to elucidate the pathophysiology and define optimal treatment for perinatal depression associated with breastfeeding difficulties. Understanding the shared neuroendocrine mechanisms associated with these conditions may suggest new avenues for treatment of these common and morbid complications of the postpartum period.
Table 1.
Proposed Neuroendocrine Mechanisms Linking Perinatal Depression with Lactation Failure
| Neuroendocrine pathway | Hypothesized shared pathophysiology |
|---|---|
| Gonadal/placental steroids | |
| Progesterone | Withdrawal of progesterone leads to onset of milk synthesis but may also trigger mood instability. Allopregnanolone withdrawal may interfere with establishment of breastfeeding in mothers who are sensitive to neurosteroid fluctuations. |
| Estrogen | Estrogen stimulates differentiation of breast tissue during pregnancy, and levels fall after birth with suppression of the hypothalamic-pituitary-ovarian axis. Low estrogen levels may contribute to perinatal mood instability in some women. |
| Lactogenic hormones | |
| Oxytocin | Acute or chronic stress can interfere with oxytocin, inhibiting both milk transfer and mother-infant bonding. In addition, differences in oxytocin receptor distribution may alter the effect of oxytocin on behavior. |
| Prolactin | Low serotonin levels may lead to both insufficent prolactin release and perinatal mood disorders, and low prolactin may increase maternal anxiety. |
| Stress reactivity | |
| Hypothalamic-pituitary-adrenal (HPA) axis | Loss of HPA axis homeostasis in women with perinatal mood disorders may interfere with both the stress-attenuating effects of lactation and the physiology of milk production. |
| Autonomic nervous system | Animal studies suggest that oxytocin-rich parasympathetic centers in the brainstem subserve mothering and feeding behavior. Dysregulation of these pathways may contribute to both perinatal mood disorders and diminished breastfeeding. |
| Pain perception | Differences in central nociception pathways may predispose women to both severe breastfeeding-associated pain and perinatal depression. |
| Hypothalamic-pituitary-thyroid axis | Thyroxin is necessary for normal lactation-associated oxytocin release. Hypothyroxinemia may contribute to both perinatal mood disorders and low milk supply. |
| Infant development | Gestation in the setting of maternal depression or anxiety may affect infant temperament and delay oromotor development, which can impede the infant's ability to latch and lead to breastfeeding difficulties. |
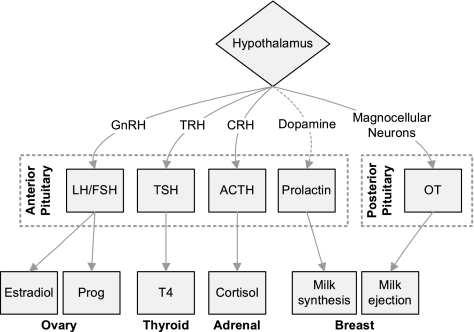
FIG. 1.
Pituitary hormones implicated in lactation and mood disorders Solid lines upregulate and dashed lines downregulate function. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; DA, dopamine; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; OT, oxytocin; Prog, progesterone; T4, tetraiodothyronine; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone.
Gonadal and Lactogenic Hormones
During pregnancy, estrogen and progesterone stimulate differentiation of breast ducts and lobules in anticipation of lactation. In the early postpartum period, falling progesterone triggers the onset of milk synthesis, and infant suckling stimulates production of oxytocin and prolactin. These hormones control the synthesis and secretion of milk, and they also impact maternal mood. Progesterone withdrawal may be the cause of the baby blues in the first few days postpartum, and in some women, this transition triggers a prolonged depressive episode. In animal models, both oxytocin and prolactin are critical for maternal caretaking, and antagonists cause disruption of mothering behavior.9–11
Progesterone
The postpartum fall in progesterone triggers onset of milk production, releasing the gestational progesterone blockade of prolactin-induced transcription of milk proteins.12 This precipitous decrease also affects mood through progesterone's neurosteroid metabolite, allopregnanolone. Allopregnanolone is a potent gamma-aminobutyric acid (GABA) agonist, and during pregnancy, rising progesterone leads to elevated central allopregnanolone levels, which inhibit stress reactivity.13 After birth, falling allopregnanolone levels necessitate rapid changes in GABA receptor expression. In a knockout mouse model, postpartum animals lacking one of the GABA subunits exhibit anxiety and poor pup caretaking behavior, but virgin animals are unaffected, suggesting that GABA function is sensitive to changing progesterone levels in the peripartum period.14 In postpartum women, lower levels of allopregnanolone are associated with depressive symptoms in the early postpartum period.15 Further, women with a history of depression have reduced conversion of oral progesterone to allopregnanolone16 and reduced allopregnanolone levels in response to a standardized stressor.17 In some individuals, allopregnanolone appears to have a biphasic effect, with anxiogenic effects at low doses and calming effects at higher doses.18 These findings support the hypothesis that in sensitive individuals, fluctuations in progesterone and allopregnanolone may contribute to perinatal depression.
A link between postpartum mood instability and onset of lactation was described as early as 1875, when George Savage wrote: “About two days after delivery some women become excited, sleepless, and incoherent.…This is called ‘milk fever,’ and coincides with the beginning of the flow of milk.”19 For many women, this transient mood instability, referred to as the baby blues,20 will resolve spontaneously. More severe and persistent mood symptoms will result in postpartum depression (PPD), defined as a major depressive episode with onset in the acute postpartum period.21,22 Because allopregnanolone withdrawal coincides with the onset of milk production, we speculate that women who are sensitive to neurosteroid fluctuations may have greater difficulty navigating feeding challenges in the first few days after birth, and these difficulties may lead to premature weaning.
Estrogen
During pregnancy, estrogen stimulates elaboration of ductal tissue in the breast.12 Estrogen levels fall after birth and remain low during lactation because of suppression of the hypothalamic-pituitary-ovarian axis. These low levels appear to be important for early milk production, as pharmacologic doses of estrogen can inhibit milk synthesis after birth.12 We speculate that this physiologic hypogonadism during lactation may play a role in the development of PPD in women vulnerable to fluctuations of gonadal steroids. Bloch et al.23 have demonstrated that women with a history of PPD demonstrate abnormal responses to physiologic fluctuations in gonadal steroids, and postpartum estrogen supplementation, which reverses the postpartum decline in estrogen levels, can improve depressive symptoms.24 In a small, open-label study of sublingual 17β-estradiol for PPD,25 the authors reported no adverse reactions in 13 breastfeeding infants, although data on milk production was not presented. Further studies are needed to determine the role of lactational hypogonadism in PPD and the impact of estrogen supplementation on breastfeeding outcomes.
Oxytocin
Oxytocin is critical in breastfeeding physiology because it stimulates let down, the contraction of myoepithelial cells that transfers milk to the areola for the suckling infant.12 Oxytocin also plays a vital role in the onset of maternal behaviors in a variety of animal models.26,27 This distinctly mammalian neuropeptide binds to receptors at widespread sites within the brain, where it affects maternal behavior necessary for offspring survival. Central oxytocin activity decreases aggressive responses toward offspring and increases aggressive/defensive behaviors against others who may present infanticidal threats. These behavioral changes are correlated with greater oxytocin receptor expression in the paraventricular nucleus (PVN), medial preoptic area, lateral septum, and central nucleus of the amygdala28 and stand in striking contrast to those shown by virgin females, who are naturally indifferent, avoidant, or even hostile to young. Experimental manipulations attest to the causal role of oxytocin in initiation of maternal behaviors. Oxytocin knockout mice display both infanticide and overly aggressive responses to intruders.29 Oxytocin administration into the cerebral ventricles of virgin rats or sheep induces mothering behavior,30–33 whereas oxytocin antagonists impair the normal onset of maternal behavior after parturition.9 These findings underscore the importance of intact oxytocin pathways for maternal behavior after partuition.
Data from human studies suggest that disruptions in oxytocin homeostasis may affect both maternal mood and breastfeeding success. Authors recently reported an association between low oxytocin during pregnancy and PPD.34 During lactation, stressors can inhibit oxytocin activity. In clinical studies, pain and stress inhibit milk transfer, and this inhibition can be overcome with exogenously administered oxytocin.35 Birth-related stressors may also impact oxytocin levels. Nissen et al.36 found that emergent cesarean birth was associated with diminished oxytocin production during suckling, compared with vaginal birth, and this association was stronger among anxiety-prone women.37 These findings suggest that both stressors and anxiety are correlated with diminished oxytocin release, which may, in turn, affect milk let down and perinatal mood.
Prolactin
The hormone prolactin stimulates milk synthesis in the mammary epithelial cell. Prolactin levels are elevated in pregnancy and in the immediate postpartum period. Basal levels fall with prolonged lactation, although suckling continues to induce peak levels that are twice as high as baseline levels.38
Prolactin also plays a central role in maternal behavior in animal models. In rodents, knockout or antagonism of central prolactin receptors impairs maternal behaviors,10,11,39 whereas cerebroventricular infusion induces maternal behavior in virgin females.40 In addition, pregnancy-induced prolactin elevations appear necessary for neurogenesis of cells involved in olfactory learning important for pup recognition. Deficits or blockade of prolactin during pregnancy increases maternal anxiety and impairs maternal behavior after parturition.41 In primates, greater levels of urinary prolactin accompany greater levels of maternal behaviors,42 and prolactin levels increase in males and females engaging in alloparenting nurturing behaviors.
Suckling-induced response to prolactin may play a role in low milk supply and perinatal mood disorders. In animal studies, inhibition of both serotonin43 and endogenous opioids44 blocks suckling-induced prolactin release and milk transfer. Prolonged stress appears to decrease the effect of endogenous opioids on prolactin secretion,45 suggesting that tonic hypothalamic-pituitary-adrenal (HPA) axis activation may inhibit prolactin production. In human studies, both obesity46 and family history of alcoholism47 are associated with blunted prolactin response to suckling. These findings are consistent with data showing that stressors affect prolactin physiology. For example, in a longitudinal study of mothers of preterm infants,48 salivary amylase was inversely correlated with prolactin area under the curve (AUC), suggesting that alpha-adrenergic activity inhibits prolactin release. Diminished prolactin response to meals and to thyrotropin-releasing hormone (TRH) administration has also been described in functional hypothalamic amenorrhea.49 These data suggest that defects in serotonin and stress reactivity pathways may be associated with both maternal mood and prolactin production, leading to undesired weaning because of diminished milk supply.
Stress Reactivity
Both the HPA axis and the autonomic nervous system are implicated in lactation physiology, and converging evidence suggests that depression and anxiety arise from aberrations in these stress reactivity pathways. Depression-related stress reactivity may, therefore, interfere with lactation, leading to premature weaning.
Hypothalamic-Pituitary-Adrenal Axis
Cortisol in a necessary cofactor for milk production. In mammary epithelial cells, circulating glucocorticoids bind with cytosolic receptors, and these complexes act synergistically with prolactin to initiate nuclear transcription and subsequent synthesis of milk proteins.12 At the same time, cortisol levels decrease during breastfeeding episodes,50 and the HPA response to physical stress is diminished in breastfeeding women compared with bottle-feeding women.51 These findings are consistent with studies demonstrating attenuated stress response in lactating animals,52,53 which appears to be mediated by both oxytocin and prolactin.54,55
A marked transition in HPA axis function occurs with partuition. During pregnancy, placental production of corticotrophin-releasing hormone (CRH) upregulates the maternal HPA axis, leading to marked increases in circulating cortisol56 and suppression of hypothalamic CRH secretion. This hypothalamic suppression normally recovers gradually after childbirth, resulting in a period of blunted HPA axis activity.57 In women with perinatal mood disorders, this transition is characterized by a markedly reduced adrenocorticotropic hormone (ACTH) response to CRH in the anterior pituitary and concomitant hypocortisolism.57,58
These aberrations in HPA reactivity may play a role in both PPD and lactation failure. Inadequate circulating cortisol may directly impact milk production at the level of the mammary epithelium, and disruption of oxytocin and prolactin may diminish the stress-attenuating effects of breastfeeding, reducing maternal enjoyment of breastfeeding and leading to earlier weaning.
Autonomic Nervous System
Parasympathetic pathways also play an important role in lactation and maternal mood. Animal studies suggest that oxytocin receptor-rich vagal nerve nuclei in the central periaquaductal gray govern maternal behavior and lactation success. Lesions in this region decreased dam postures necessary for efficient nursing, reduced pup weight gain, and increased maternal aggression in response to an intruder challenge.59 These findings suggest that parasympathetic pathways modulate both nursing behavior and postpartum stress reactivity. Studies in nursing mothers show increased parasympathetic and decreased sympathetic activation compared with bottle-feeding or mixed-feeding mothers,51,60–62 suggesting that successful breastfeeding requires—and reinforces—parasympathetic activation.
Autonomic nervous system physiology changes with the transition from pregnancy to lactation. Sympathetic activity decreases over the course of gestation, manifested by declining stress-induced changes in heart rate, blood pressure, and salivary alpha-amylase.63,64 These changes appear to be attenuated in depressed pregnant women, who have higher heart rates and lower parasympathetic heart rate variability compared with nondepressed women.65 No studies, to our knowledge, have measured sympathetic/parasympathetic balance in PPD, but major depression and anxiety are associated with reduced parasympathetic control in nonpregnant populations.66,67 Studies linking diminished parasympathetic activity with depression and anxiety suggest that autonomic dysregulation may predispose women to mood disorders. This sympathetic/parasympathetic imbalance may also impair lactation physiology, contributing to failed lactation.
Pain Perception
Breastfeeding-associated pain is a common reason for early weaning, affecting one third of mothers who discontinue breastfeeding before 1 month.68 We have found that early breastfeeding pain is associated with subsequent diagnosis of perinatal depression.69
Pain perception is, in essence, a central reaction to a sensory stressor and, thus, represents a special case of stress reactivity. Depression-related differences in pain perception may further link perinatal mood disorders with failed lactation. Indeed, low oxytocin levels are associated with diminished pain thresholds.70 Prior studies have linked depressive symptoms with childbirth-associated pain and catastrophization,71 which describes a cognitive and emotional response that assumes adverse circumstances can and will get progressively worse. These tendencies are associated with both depression and chronic pain and may be mediated, in part, by catastrophizing-associated differences in central nociception pathways.72 Such differences in central nociception pathways may predispose women to both perinatal depression and severe breastfeeding-associated pain, leading to early weaning.
Thyroid
In animal models, reducing maternal thyroid hormone levels lowers oxytocin levels, diminishes milk ejection, and reduces milk quality.73,74 Human studies similarly show a link between clinical hypothyroidism and diminished milk production,75 and daily milk production is directly correlated with circulating thyroxine and triiodothyronine (T4).76 Thyroid dysregulation is also implicated in perinatal mood disorders. Both total T4 and thyroid-binding globulin rise during pregnancy, but free T4 levels remain within the normal range.77 After delivery, both estrogen and thyroid-binding globulin levels decrease, with a concomitant reduction in total T4.78 Aberrations in this transition may manifest as depression or anxiety. Anxiety symptoms are increased among women with thyroid dysfunction,79 and low-normal levels of total and free thyroxine during late pregnancy are associated with postpartum depressive symptoms.80,81 Other authors have also reported an association between thyroid disturbance and mood and anxiety symptoms in the perinatal period.82 Postpartum hypothyroxinemia may, therefore, contribute to both perinatal mood disorders and reduced milk supply, predisposing women to early weaning.
Infant Development
Breastfeeding can be described as a two-person, single-organ system. Infant suckling stimulates production of both oxytocin and prolactin, and infant demand drives milk supply. If the breast is not emptied regularly, engorgement occurs, downregulating prolactin receptors in the mammary epithelium and reducing milk production.83 Successful lactation, therefore, requires mature infant suck-swallow patterns; maternal depression may affect this oromotor development. Exposure to elevated CRH during pregnancy has been linked with differences in neurologic maturation84 and neuromuscular development.85 At birth, neonates born to depressed mothers exhibit different suckling responses than those born to euthymic mothers.86
Maternal depression may also affect breastfeeding outcomes via its influence, in utero, on physiologic processes that underlie developing infant temperament and behavior. Maternal depression during pregnancy may have a profound effect on offspring development.87 For example, maternal psychologic symptoms lead to distinguishable differences in fetal heart rate response to stress by the end of pregnancy,88 such that fetuses of more anxious mothers showed significant increases in heart rate during a stressor, whereas fetuses of less anxious mothers showed nonsignificant decreases in heart rate. Thus, even before birth, fetuses of more anxious mothers show greater stress reactivity than those of nonanxious mothers.
These differences continue to be observed after birth. Infants of depressed mothers have physiologic and biochemical profiles that mimic their mothers during pregnancy, including lower levels of dopamine and serotonin, elevated norepinephrine and cortisol, and greater relative right frontal electroencephalogram (EEG).89–91 In addition, elevated maternal depressive symptoms during pregnancy are related to lower vagal tone in newborns90 and in 3–6-month-old infants at rest and during interactions with their mothers.92,93 Taken together, these results suggest that infants of depressed mothers may develop maladaptive biobehavioral regulatory patterns, which are known to underlie characteristics associated with negative temperament.
One of the most extensively studied physiologic pathways from maternal depression in utero to infant outcomes is placental CRH (pCRH). In some94,95 but not all96 studies, midpregnancy pCRH has been found to be an early predictor of postpartum and prenatal depression. pCRH has also been associated with preterm labor, reduced birth weight, and slow growth rate in infants.97–101 High late-pregnancy cortisol levels are also associated with increased crying, fussing, and negative reactivity and facial expressions in infants, as well as maternal ratings of more difficult temperament.102,103
The role of infant temperament in feeding outcomes is not well understood, but there is evidence of a relationship between difficult temperament and reduced breastfeeding during the first half-year of life.104,105 Jones et al.106 assessed maternal-infant behavior and development in cohorts of depressed and nondepressed women who were breastfeeding and bottle-feeding, and they concluded that maternal depression affects both infant temperament and infant EEG asymmetry, which, in turn, reduced breastfeeding duration. Thus, gestation in the setting of maternal depression or anxiety may impact infant development, leading to breastfeeding difficulties and early weaning.
Treatment Implications and Future Research
Failed lactation and perinatal depression are common conditions, and these two disorders frequently occur together. Clinicians seeing women for either mood disorders or lactation difficulties should be aware of this overlap and assess women with breastfeeding problems for depression, as well as refer women with depressive symptoms for breastfeeding support from an experienced provider, such as an International Board-Certified Lactation Consultant.107
Studies linking acute and chronic stress with diminished levels of lactogenic hormones and low milk supply suggest that relaxation techniques could be helpful for improving both mood and breastfeeding success. For example, in a randomized controlled trial in mothers of neonatal intensive care unit (NICU) infants, mothers assigned to use relaxation tapes for 1 week produced 63% more milk (90.1 vs. 55.4 mL) during a pumping session compared with mothers in the control group.108 Psychotherapy may also be beneficial. Berga et al.109 demonstrated that cognitive behavioral therapy (CBT) can correct hypothalamic amenorrhea, suggesting that CBT can improve stress-associated hypothalamic dysfunction.
Studies are needed to determine if assessing circulating neuroendocrine markers and correcting low or high levels can improve mood or breastfeeding outcomes. Future research should also determine if pharmacotherapy for perinatal depression affects production of lactogenic hormones, milk production, and breastfeeding outcomes. Mammary gland serotonin plays a role in local autoregulation,110 and implantation of pellets containing the selective serotonin reuptake inhibitor (SSRI) fluoxetine in a rodent model caused local involution of lactating mammary glands. In a small study, women taking SSRIs (7 of 8, 88%) were more likely to report delayed secretory activation than women not taking SSRIs (184 of 423, 44%).111 Women taking SSRIs at 2 weeks were also less likely to be breastfeeding at 12 weeks in an observational study.112 Further studies are needed to confirm these findings and determine the impact of different SSRI regimens on feeding outcomes.
Future studies must also consider the multiple factors that impact both breastfeeding intention and duration, including sociodemographic variables,113 maternal knowledge and beliefs,114 maternity care practices,115 and maternity leave.116 Finally, further studies should differentiate among phenotypes of maternal depression and anxiety. These conditions are differentially associated with withdrawn vs. intrusive parenting styles,117,118 and these interaction styles may, in turn, have differing effects on infant development and feeding outcome.
Conclusions
Failed lactation and perinatal depression are common clinical problems that incur substantial morbidity for both mothers and infants. As we have reviewed, these two conditions frequently occur together, and they may be manifestations of neuroendocrine perturbations in gonadal and lactogenic hormones, stress reactivity, thyroid homeostasis, or infant development. Further studies are needed to characterize differences in maternal neuroendocrine physiology among depressed and nondepressed mothers who are or are not breastfeeding their infants. Such research may identify treatment strategies to both relieve postpartum mood disorders and enable women to achieve their infant feeding goals.
Acknowledgments
This work was supported by NIH 5K12HD050113-04 (to A.M.S.), K01DA019949-01A1 (to KG), K23MH085165 (to S.M.-B.), and UL1RR025747 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.American College of Obstetricians Gynecologists. Committee opinion No. 361: Breastfeeding: Maternal and infant aspects. Obstet Gynecol. 2007;109:479–480. doi: 10.1097/00006250-200702000-00064. [DOI] [PubMed] [Google Scholar]
- 2.Bartick M. Reinhold A. The burden of suboptimal breastfeeding in the United States: A pediatric cost analysis. Pediatrics. 2010;125:e1048–1056. doi: 10.1542/peds.2009-1616. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Assocation: Diagnostic, statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Assocation; 1994. [Google Scholar]
- 4.Flynn HA. Davis M. Marcus SM. Cunningham R. Blow FC. Rates of maternal depression in pediatric emergency department and relationship to child service utilization. Gen Hosp Psychiatry. 2004;26:316–322. doi: 10.1016/j.genhosppsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 5.O'hara MW. Swain AM. Rates and risk of postpartum depression: A meta-analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- 6.Dennis C-L. McQueen K. The relationship between infant-feeding outcomes and postpartum depression: A qualitative systematic review. Pediatrics. 2009;123:e736–751. doi: 10.1542/peds.2008-1629. [DOI] [PubMed] [Google Scholar]
- 7.Infant Feeding Practices Study II Table 3.35. Percent of mothers who breastfed their babies as long as they wanted to, by breastfeeding duration and selected demographics1, among mothers who completely stopped breastfeeding and pumping milk for their baby during the study. 2009. www.cdc.gov/ifps/results/ch3/table3-35.htm. [Mar 22;2010 ]. www.cdc.gov/ifps/results/ch3/table3-35.htm
- 8.Li R. Fein SB. Chen J. Grummer-Strawn LM. Why mothers stop breastfeeding: Mothers' self-reported reasons for stopping during the first year. Pediatrics. 2008;122(Suppl 2):S69–76. doi: 10.1542/peds.2008-1315i. [DOI] [PubMed] [Google Scholar]
- 9.Insel TR. Young L. Wang Z. Molecular aspects of monogamy. Ann NY Acad Sci. 1997;807:302–316. doi: 10.1111/j.1749-6632.1997.tb51928.x. [DOI] [PubMed] [Google Scholar]
- 10.Lucas BK. Ormandy CJ. Binart N. Bridges RS. Kelly PA. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102–4107. doi: 10.1210/endo.139.10.6243. [DOI] [PubMed] [Google Scholar]
- 11.Torner L. Neumann ID. The brain prolactin system: Involvement in stress response adaptations in lactation. Stress. 2002;5:249–257. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- 12.Pang WW. Hartmann PE. Initiation of human lactation: Secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211–221. doi: 10.1007/s10911-007-9054-4. [DOI] [PubMed] [Google Scholar]
- 13.Brunton PJ. McKay AJ. Ochedalski T, et al. Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci. 2009;29:6449–6460. doi: 10.1523/JNEUROSCI.0708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maguire J. Mody I. GABA(A)R plasticity during pregnancy: Relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nappi RE. Petraglia F. Luisi S. Polatti F. Farina C. Genazzani AR. Serum allopregnanolone in women with postpartum “blues.”. Obstet Gynecol. 2001;97:77–80. doi: 10.1016/s0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- 16.Klatzkin RR. Morrow AL. Light KC. Pedersen CA. Girdler SS. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31:1208–1219. doi: 10.1016/j.psyneuen.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Klatzkin RR. Morrow AL. Light KC. Pedersen CA. Girdler SS. Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biol Psychol. 2006;71:2–11. doi: 10.1016/j.biopsycho.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Andréen L. Nyberg S. Turkmen S. van Wingen G. Fernández G. Bäckström T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology. 2009;34:1121–1132. doi: 10.1016/j.psyneuen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Savage G. Observations on the insanity of pregnancy and childbirth. Guys Hosp Rep. 1875;20:83–117. [Google Scholar]
- 20.O'Hara MW. Schlechte JA. Lewis DA. Wright EJ. Prospective study of postpartum blues. Biologic and psychosocial factors. Arch Gen psychiatry. 1991;48:801–806. doi: 10.1001/archpsyc.1991.01810330025004. [DOI] [PubMed] [Google Scholar]
- 21.Yonkers KA. Vigod S. Ross LE. Diagnosis, pathophysiology, and management of mood disorders in pregnant and postpartum women. Obstet Gynecol. 2011;117:961–977. doi: 10.1097/AOG.0b013e31821187a7. [DOI] [PubMed] [Google Scholar]
- 22.Gaynes BN. Gavin N. Meltzer-Brody S, et al. Perinatal depression: Prevalence, screening accuracy, and screening outcomes summary. Evid Rep Technol Assess. 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloch M. Schmidt PJ. Danaceau M. Murphy J. Nieman L. Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 24.Moses-Kolko EL. Berga SL. Kalro B. Sit DK. Wisner KL. Transdermal estradiol for postpartum depression: A promising treatment option. Clin Obstet Gynecol. 2009;52:516–529. doi: 10.1097/GRF.0b013e3181b5a395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahokas A. Kaukoranta J. Wahlbeck K. Aito M. Estrogen deficiency in severe postpartum depression: Successful treatment with sublingual physiologic 17beta-estradiol: A preliminary study. J Clin Psychiatry. 2001;62:332–336. doi: 10.4088/jcp.v62n0504. [DOI] [PubMed] [Google Scholar]
- 26.Carter S. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 27.Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 28.Champagne F. Meaney MJ. Like mother, like daughter: Evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- 29.Ragnauth AK. Devidze N. Moy V, et al. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4:229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 30.Fahrbach S. Morrell J. Pfaff D. Role of oxytocin in the onset of estrogen-facilitated maternal behavior. In: Amico J, editor; Robinson A, editor. Oxytocin: Clinical laboratory studies. Amsterdam: Elsevier; 1985. pp. 372–388. [Google Scholar]
- 31.Kendrick K. Keverne E. Control of synthesis and release of oxytocin in the sheep brain. Ann NY Acad Sci. 1992;652:102–121. doi: 10.1111/j.1749-6632.1992.tb34349.x. [DOI] [PubMed] [Google Scholar]
- 32.Kendrick K. Keverne E. Hinton M. Goode J. Oxytocin, amino acid and monoamine release in the region of the medial preoptic area and bed nucleus of the stria terminalis of the sheep during parturition and suckling. Brain Res. 1992;569:199–209. doi: 10.1016/0006-8993(92)90631-i. [DOI] [PubMed] [Google Scholar]
- 33.Levy F. Kendrick KM. Keverne EB. Piketty V. Poindron P. Intracerebral oxytocin is important for the onset of maternal behavior in inexperienced ewes delivered under peridural anesthesia. Behav Neurosci. 1992;106:427–432. doi: 10.1037//0735-7044.106.2.427. [DOI] [PubMed] [Google Scholar]
- 34.Skrundz M. Bolten M. Nast I. Hellhammer DH. Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36:1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton M. Newton NR. The let-down reflex in human lactation. J Pediatr. 1948;33:698–704. doi: 10.1016/s0022-3476(48)80075-2. [DOI] [PubMed] [Google Scholar]
- 36.Nissen E. Uvnas-Moberg K. Svensson K. Stock S. Widstrom AM. Winberg J. Different patterns of oxytocin, prolactin but not cortisol release during breastfeeding in women delivered by caesarean section or by the vaginal route. Early Hum Dev. 1996;45:103–118. doi: 10.1016/0378-3782(96)01725-2. [DOI] [PubMed] [Google Scholar]
- 37.Nissen E. Gustavsson P. Widstrom AM. Uvnas-Moberg K. Oxytocin, prolactin, milk production and their relationship with personality traits in women after vaginal delivery or cesarean section. J Psychosom Obstet Gynaecol. 1998;19:49–58. doi: 10.3109/01674829809044221. [DOI] [PubMed] [Google Scholar]
- 38.Leake RD. Waters CB. Rubin RT. Buster JE. Fisher DA. Oxytocin and prolactin responses in long-term breast-feeding. Obstet Gynecol. 1983;62:565–568. [PubMed] [Google Scholar]
- 39.Larsen CM. Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.07.233. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Bridges RS. Numan M. Ronsheim PM. Mann PE. Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci USA. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsen CM. Grattan DR. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology. 2010;151:3805–3814. doi: 10.1210/en.2009-1385. [DOI] [PubMed] [Google Scholar]
- 42.Soltis J. Wegner FH. Newman JD. Urinary prolactin is correlated with mothering and allo-mothering in squirrel monkeys. Physiol Behav. 2005;84:295–301. doi: 10.1016/j.physbeh.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Kordon C. Blake CA. Terkel J. Sawyer CH. Participation of serotonin-containing neurons in the suckling-induced rise in plasma prolactin levels in lactating rats. Neuroendocrinology. 1973;13:213–223. doi: 10.1159/000122206. [DOI] [PubMed] [Google Scholar]
- 44.Arbogast LA. Voogt JL. Endogenous opioid peptides contribute to suckling-induced prolactin release by suppressing tyrosine hydroxylase activity and messenger ribonucleic acid levels in tuberoinfundibular dopaminergic neurons. Endocrinology. 1998;139:2857–2862. doi: 10.1210/endo.139.6.6052. [DOI] [PubMed] [Google Scholar]
- 45.Kiem DT. Kanyicska B. Stark E. Fekete MI. Prolactin release induced by opiate agonists, effect of glucocorticoid pretreatment in intact and adrenalectomized rats. Neuroendocrinology. 1988;48:174–179. doi: 10.1159/000125006. [DOI] [PubMed] [Google Scholar]
- 46.Rasmussen KM. Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113:e465–471. doi: 10.1542/peds.113.5.e465. [DOI] [PubMed] [Google Scholar]
- 47.Mennella JA. Pepino MY. Breastfeeding and prolactin levels in lactating women with a family history of alcoholism. Pediatrics. 2010;125:e1162–1170. doi: 10.1542/peds.2009-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatterton RT., Jr Hill PD. Aldag JC. Hodges KR. Belknap SM. Zinaman MJ. Relation of plasma oxytocin and prolactin concentrations to milk production in mothers of preterm infants: Influence of stress. J Clin Endocrinol Metabol. 2000;85:3661–3668. doi: 10.1210/jcem.85.10.6912. [DOI] [PubMed] [Google Scholar]
- 49.Berga SL. Mortola JF. Girton L, et al. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1989;68:301–308. doi: 10.1210/jcem-68-2-301. [DOI] [PubMed] [Google Scholar]
- 50.Amico JA. Johnston JM. Vagnucci AH. Suckling-induced attenuation of plasma cortisol concentrations in postpartum lactating women. Endocr Res. 1994;20:79–87. doi: 10.3109/07435809409035858. [DOI] [PubMed] [Google Scholar]
- 51.Altemus M. Deuster PA. Galliven E. Carter CS. Gold PW. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. J Clin Endocrinol Metab. 1995;80:2954–2959. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- 52.Brunton PJ. Russell JA. Douglas AJ. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol. 2008;20:764–776. doi: 10.1111/j.1365-2826.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- 53.Slattery DA. Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neumann ID. Brain mechanisms underlying emotional alterations in the peripartum period in rats. Depress Anxiety. 2003;17:111–121. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- 55.Grattan DR. Pi XJ. Andrews ZB, et al. Prolactin receptors in the brain during pregnancy and lactation: Implications for behavior. Horm Behav. 2001;40:115–124. doi: 10.1006/hbeh.2001.1698. [DOI] [PubMed] [Google Scholar]
- 56.Nolten WE. Lindheimer MD. Rueckert PA. Oparil S. Ehrlich EN. Diurnal patterns and regulation of cortisol secretion in pregnancy. J Clin Endocrinol Metab. 1980;51:466–472. doi: 10.1210/jcem-51-3-466. [DOI] [PubMed] [Google Scholar]
- 57.Magiakou MA. Mastorakos G. Rabin D. Dubbert B. Gold PW. Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: Implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab. 1996;81:1912–1917. doi: 10.1210/jcem.81.5.8626857. [DOI] [PubMed] [Google Scholar]
- 58.Raison CL. Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 59.Lonstein JS. Stern JM. Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal, and aggressive behaviors in rats. Brain Res. 1998;804:21–35. doi: 10.1016/s0006-8993(98)00642-8. [DOI] [PubMed] [Google Scholar]
- 60.Light KC. Smith TE. Johns JM. Brownley KA. Hofheimer JA. Amico JA. Oxytocin responsivity in mothers of infants: A preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Health Psychol. 2000;19:560–567. doi: 10.1037//0278-6133.19.6.560. [DOI] [PubMed] [Google Scholar]
- 61.Mezzacappa ES. Kelsey RM. Katkin ES. Breast feeding, bottle feeding, and maternal autonomic responses to stress. J Psychosom Res. 2005;58:351–365. doi: 10.1016/j.jpsychores.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Altemus M. Redwine LS. Leong Y-M. Frye CA. Porges SW. Carter CS. Responses to laboratory psychosocial stress in postpartum women. Psychosom Med. 2001;63:814–821. doi: 10.1097/00006842-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 63.Nierop A. Bratsikas A. Klinkenberg A. Nater UM. Zimmermann R. Ehlert U. Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary alpha-amylase responses to psychosocial stress in human pregnancy. J Clin Endocrinol Metab. 2006;91:1329–1335. doi: 10.1210/jc.2005-1816. [DOI] [PubMed] [Google Scholar]
- 64.Entringer S. Buss C. Shirtcliff EA, et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress (Amsterdam, Netherlands) 2010;13:258–268. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shea AK. Kamath MV. Fleming A. Streiner DL. Redmond K. Steiner M. The effect of depression on heart rate variability during pregnancy. A naturalistic study. Clin Autonom Res. 2008;18:203–212. doi: 10.1007/s10286-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 66.Watkins LL. Grossman P. Krishnan R. Sherwood A. Anxiety and vagal control of heart rate. Psychosom Med. 1998;60:498–502. doi: 10.1097/00006842-199807000-00018. [DOI] [PubMed] [Google Scholar]
- 67.Kemp AH. Quintana DS. Gray MA. Felmingham KL. Brown K. Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Ahluwalia IB. Morrow B. Hsia J. Why do women stop breastfeeding? Findings from the Pregnancy Risk Assessment and Monitoring System. Pediatrics. 2005;116:1408–1412. doi: 10.1542/peds.2005-0013. [DOI] [PubMed] [Google Scholar]
- 69.Watkins S. Meltzer-Brody S. Zolnoun D. Stuebe A. Early breastfeeding experiences and postpartum depression. Obstet Gynecol. 2011 doi: 10.1097/AOG.0b013e3182260a2d. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 70.Grewen KM. Light KC. Mechlin B. Girdler SS. Ethnicity is associated with alterations in oxytocin relationships to pain sensitivity in women. Ethn Health. 2008;13:219–241. doi: 10.1080/13557850701837310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flink IK. Mroczek MZ. Sullivan MJ. Linton SJ. Pain in childbirth and postpartum recovery: The role of catastrophizing. Eur J Pain. 2009;13:312–316. doi: 10.1016/j.ejpain.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 72.Gracely RH. Geisser ME. Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 73.Hapon M. Simoncini M. Via G. Jahn G. Effect of hypothyroidism on hormone profiles in virgin, pregnant and lactating rats, and on lactation. Reproduction. 2003;126:371–382. doi: 10.1530/rep.0.1260371. [DOI] [PubMed] [Google Scholar]
- 74.Hapon MB. Varas SM. Gimenez MS. Jahn GA. Reduction of mammary and liver lipogenesis and alteration of milk composition during lactation in rats by hypothyroidism. Thyroid. 2007;17:11–18. doi: 10.1089/thy.2005.0267. [DOI] [PubMed] [Google Scholar]
- 75.Miyake A. Tahara M. Koike K. Tanizawa O. Decrease in neonatal suckled milk volume in diabetic women. Eur J Obstet Gynecol Reprod Biol. 1989;33:49–53. doi: 10.1016/0028-2243(89)90077-4. [DOI] [PubMed] [Google Scholar]
- 76.Motil KJ. Thotathuchery M. Montandon CM, et al. Insulin, cortisol and thyroid hormones modulate maternal protein status and milk production and composition in humans. J Nutr. 1994;124:1248–1257. doi: 10.1093/jn/124.8.1248. [DOI] [PubMed] [Google Scholar]
- 77.Burrow GN. Fisher DA. Larsen PR. Maternal and fetal thyroid function. N Eng J Med. 1994;331:1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- 78.Yamamoto T. Amino N. Tanizawa O. Longitudinal study of serum thyroid hormones, chorionic gonadotrophin and thyrotrophin during and after normal pregnancy. Clin Endocrinol (Oxf) 1979;10:459–468. doi: 10.1111/j.1365-2265.1979.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 79.Kent GN. Stuckey BG. Allen JR. Lambert T. Gee V. Postpartum thyroid dysfunction: Clinical assessment and relationship to psychiatric affective morbidity. Clin Endocrinol (Oxf) 1999;51:429–438. doi: 10.1046/j.1365-2265.1999.00807.x. [DOI] [PubMed] [Google Scholar]
- 80.Pedersen CA. Johnson JL. Silva S, et al. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinology. 2007;32:235–245. doi: 10.1016/j.psyneuen.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 81.Pedersen CA. Stern RA. Pate J. Senger MA. Bowes WA. Mason GA. Thyroid and adrenal measures during late pregnancy and the puerperium in women who have been major depressed or who become dysphoric postpartum. J Affect Disord. 1993;29:201–211. doi: 10.1016/0165-0327(93)90034-h. [DOI] [PubMed] [Google Scholar]
- 82.Abou-Saleh MT. Ghubash R. Karim L. Krymski M. Bhai I. Hormonal aspects of postpartum depression. Psychoneuroendocrinology. 1998;23:465–475. doi: 10.1016/s0306-4530(98)00022-5. [DOI] [PubMed] [Google Scholar]
- 83.Cregan MD. Mitoulas LR. Hartmann PE. Milk prolactin, feed volume and duration between feeds in women breastfeeding their full-term infants over a 24 h period. Exp Physiol. 2002;87:207–214. doi: 10.1113/eph8702327. [DOI] [PubMed] [Google Scholar]
- 84.Class QA. Buss C. Davis EP, et al. Low levels of corticotropin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Dev Neurosci. 2008;30:419–426. doi: 10.1159/000191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ellman LM. Schetter CD. Hobel CJ. Chicz-Demet A. Glynn LM. Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hernandez-Reif M. Field T. Diego M. Differential sucking by neonates of depressed versus non-depressed mothers. Infant Behav Dev. 2004;27:465–476. [Google Scholar]
- 87.DiPietro JA. Novak MF. Costigan KA. Atella LD. Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. 2006;77:573–587. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 88.Monk C. Sloan RP. Myers MM, et al. Fetal heart rate reactivity differs by women's psychiatric status: An early marker for developmental risk? J Am Acad Child Adolesc Psychiatry. 2004;43:283–290. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 89.Field T. Diego MA. Dieter J, et al. Depressed, withdrawn, and intrusive mothers' effects on their fetuses and neonates. Infant Behav Dev. 2001;24:27–39. [Google Scholar]
- 90.Jones NA. Field T. Fox NA. Davalos M. Lundy B. Hart S. Newborns of mothers with depressive symptoms are physiologically less developed. Infant Behav Dev. 1998;21:537–541. [Google Scholar]
- 91.Lundy BL. Jones NA. Field T, et al. Prenatal depression effects on neonates. Infant Behav Dev. 1999;22:119–129. [Google Scholar]
- 92.Field T. Fox NA. Pickens J. Nawrocki T. Relative right frontal EEG activation in 3- to 6-month-old infants of “depressed” mothers. Dev Psychol. 1995;31:358–363. [Google Scholar]
- 93.Pickens J. Field T. Facial expressivity in infants of depressed mothers. Dev Psychol. 1993;29:986–988. [Google Scholar]
- 94.Rich-Edwards JW. Mohllajee AP. Kleinman K, et al. Elevated midpregnancy corticotropin-releasing hormone is associated with prenatal, but not postpartum, maternal depression. J Clin Endocrinol Metab. 2008;93:1946–1951. doi: 10.1210/jc.2007-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yim IS. Glynn LM. Dunkel Schetter C. Hobel CJ. Chicz-Demet A. Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66:162–169. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meltzer-Brody S. Stuebe A. Dole N. Savitz D. Rubinow D. Thorp J. Elevated corticotropin releasing hormone (CRH) during pregnancy and risk of postpartum depression (PPD) J Clin Endocrinol Metab. 2011;96:E40–47. doi: 10.1210/jc.2010-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Field T. Diego M. Dieter J, et al. Prenatal depression effects on the fetus and the newborn. Infant Behav Dev. 2004;27:216–229. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 98.Field T. Diego M. Hernandez-Reif M. Gil K. Vera Y. Prenatal maternal cortisol, fetal activity and growth. Int J Neurosci. 2005;115:423–429. doi: 10.1080/00207450590521082. [DOI] [PubMed] [Google Scholar]
- 99.Field T. Hernandez-Reif M. Diego M. Figueiredo B. Schanberg S. Kuhn C. Prenatal cortisol, prematurity and low birthweight. Infant Behav Dev. 2006;29:268–275. doi: 10.1016/j.infbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 100.Wadhwa PD. Porto M. Garite TJ. Chicz-DeMet A. Sandman CA. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179:1079–1085. doi: 10.1016/s0002-9378(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 101.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 102.Davis EP. Glynn LM. Schetter CD. Hobel C. Chicz-Demet A. Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 103.de Weerth C. van Hees Y. Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 104.Niegel S. Ystrom E. Hagtvet KA. Vollrath ME. Difficult temperament, breastfeeding, and their mutual prospective effects: The Norwegian Mother and Child Cohort Study. J Dev Behav Pediatr. 2008;29:458–462. doi: 10.1097/dbp.0b013e3181877a88. [DOI] [PubMed] [Google Scholar]
- 105.Vandiver TA. Relationship of mothers' perceptions and behaviors to the duration of breastfeeding. Psychol Rep. 1997;80:1375–1384. doi: 10.2466/pr0.1997.80.3c.1375. [DOI] [PubMed] [Google Scholar]
- 106.Jones NA. McFall BA. Diego MA. Patterns of brain electrical activity in infants of depressed mothers who breastfeed and bottle feed: The mediating role of infant temperament. Biol Psychol. 2004;67:103–124. doi: 10.1016/j.biopsycho.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 107.International Board Certified Lactation Consultant in the Americas: How to qualify. www.americas.iblce.org/how-to-qualify. [Jun 7;2011 ]. www.americas.iblce.org/how-to-qualify
- 108.Feher SDK. Berger LR. Johnson JD. Wilde JB. Increasing breast milk production for premature infants with a relaxation/imagery audiotape. Pediatrics. 1989;83:57–60. [PubMed] [Google Scholar]
- 109.Berga SL. Marcus MD. Loucks TL. Hlastala S. Ringham R. Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril. 2003;80:976–981. doi: 10.1016/s0015-0282(03)01124-5. [DOI] [PubMed] [Google Scholar]
- 110.Matsuda M. Imaoka T. Vomachka AJ, et al. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell. 2004;6:193–203. doi: 10.1016/s1534-5807(04)00022-x. [DOI] [PubMed] [Google Scholar]
- 111.Marshall AM. Nommsen-Rivers LA. Hernandez LL, et al. Serotonin transport and metabolism in the mammary gland modulates secretory activation and involution. J Clin Endocrinol Metab. 2010;95:837–846. doi: 10.1210/jc.2009-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bogen DL. Hanusa BH. Moses-Kolko E. Wisner KL. Are maternal depression or symptom severity associated with breastfeeding intention or outcomes? J Clin Psychiatry. 2010;71:1069–1078. doi: 10.4088/JCP.09m05383blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grummer-Strawn LM. Scanlon KS. Fein SB. Infant feeding and feeding transitions during the first year of life. Pediatrics. 2008;122:S36–42. doi: 10.1542/peds.2008-1315d. [DOI] [PubMed] [Google Scholar]
- 114.Stuebe AM. Bonuck K. What predicts intent to breastfeed exclusively? Breastfeeding knowledge, attitudes, and beliefs in a diverse urban population. Breastfeed Med. 2011 doi: 10.1089/bfm.2010.0088. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.DiGirolamo AM. Grummer-Strawn LM. Fein SB. Effect of maternity-care practices on breastfeeding. Pediatrics. 2008;122:S43–49. doi: 10.1542/peds.2008-1315e. [DOI] [PubMed] [Google Scholar]
- 116.Ogbuanu C. Glover S. Probst J. Liu J. Hussey J. The effect of maternity leave length and time of return to work on breastfeeding. Pediatrics. 2011;127:e1414–1427. doi: 10.1542/peds.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feldman R. Granat A. Pariente C. Kanety H. Kuint J. Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- 118.Field T. Diego M. Hernandez-Reif M. Ascencio A. Prenatal dysthymia versus major depression effects on early mother-infant interactions: A brief report. Infant Behav Dev. 2009;32:129–131. doi: 10.1016/j.infbeh.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]