Abstract
Background
Our group has shown a positive dose-response in maximal cardiorespiratory exercise capacity (VO2max) and heart rate variability (HRV) to 6 months of exercise training but no improvement in VO2max for women ≥60 years. Here, we examine the HRV response to exercise training in postmenopausal women younger and older than 60 years.
Methods
We examined 365 sedentary, overweight, hypertensive, postmenopausal women randomly assigned to sedentary control or exercise groups exercising at 50% (4 kcal/kg/week, [KKW]), 100% (8 KKW) and 150% (12 KKW) of the National Institutes of Health (NIH) Consensus Development Panel physical activity guidelines. Primary outcomes included time and frequency domain indices of HRV.
Results
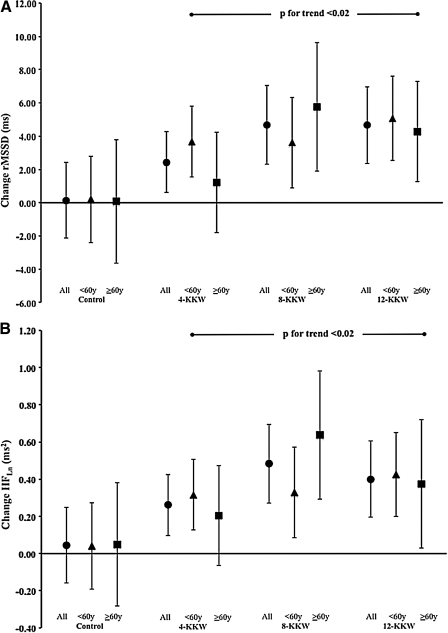
Overall, our analysis demonstrated a significant improvement in parasympathetic tone (rMSSD and high frequency power) for both age strata at 8 KKW and 12 KKW. For rMSSD, the age-stratified responses were: control, <60 years, 0.20 ms, 95% confidence interval (CI)−2.40, 2.81; ≥60 years, 0.07 ms, 95% CI −3.64, 3.79; 4 KKW, <60 years, 3.67 ms, 95% CI 1.55, 5.79; ≥60 years, 1.20 ms, 95% CI −1.82, 4.22; 8-KKW, <60 years, 3.61 ms, 95% CI 0.88, 6.34; ≥60 years, 5.75 ms, 95% CI 1.89, 9.61; and 12-KKW, <60 years, 5.07 ms, 95% CI 2.53, 7.60; ≥60 years, 4.28 ms, 95% CI 0.42, 8.14.
Conclusions
VO2max and HRV are independent risk factors for cardiovascular disease (CVD) mortality. Despite no improvement in VO2max, parasympathetic indices of HRV increased in women ≥60 years. This is clinically important, as HRV has important CVD risk and neurovisceral implications beyond cardiorespiratory function.
Introduction
The risk for cardiovascular disease (CVD) increases in women after menopause.1 As with men, maximal cardiorespiratory exercise capacity (VO2max) is protective against CVD in women.2 Accordingly, interventions aimed at improving maximal cardiorespiratory capacity carry with them a 13% reduction in CVD risk accompanying each increase in maximal metabolic equivalent (MET).3 Alterations in the autonomic nervous system, as measured by heart rate variability (HRV), are also independently associated with CVD and all-cause mortality.4,5 This relationship is important, as autonomic balance portrays the relationship between the parasympathetic and sympathetic nervous systems and is intricately connected to many factors affecting cardiovascular function.6,7 As it pertains to aging, both maximal cardiorespiratory capacity and HRV decrease after menopause, yet both are positively affected by exercise training.1,8
In a recent report detailing the primary outcomes of a large clinical trial involving exercise training in postmenopausal women, the Dose Response to Exercise in Women (DREW) trial, we demonstrated a significant dose-dependent improvement in VO2max in postmenopausal women after 6 months of exercise at ∼50%, 100%, and 150% of the National Institute of Health's (NIH) Consensus Development Panel recommendation for exercise.9,10 In a series of ancillary reports, we showed that exercise training also improved HRV and that those with improved HRV also showed improvements in circulating insulin.12,13 This latter observation is important because the autonomic nervous system is intricately linked to numerous systemwide physiologic functions, including, but not limited to, mood, depression, indices of anthropometry, respiration, digestion, sexual arousal, cognitive and executive functioning, and vagal control of the cardiovascular system.11–17 Therefore, it is conceivable that autonomic balance and VO2max may be affected differently from one another concomitant to exercise training.
The answer to this question is important to the aging process in women, as in one of our ancillary reports stratified by age, we found that regardless of exercise dose, women aged >60 showed an attenuated response in VO2max to exercise training, where the extent that VO2max changed was not deemed statistically significant.18 Based on this finding, some may question the utility of recommending low-to-moderate exercise in an older population if VO2max is held as the gold standard determinant of exercise training efficacy. However, we believe that VO2max is the resultant sum of numerous physiologic system adaptations. It should also hold that any component associated with systemwide improvements may also be affected by exercise participation even if maximal VO2max is not.19,20 It is, therefore, conceivable that HRV may improve in older participants independent of a change in VO2max. Although several small sample studies have shown an improvement in HRV accompanying an exercise intervention in older participants, no such data exist in larger interventions using carefully monitored doses of exercise energy expenditure.21–23 Given our collective observations with DREW, we asked the question whether women ≥60 years who exhibit an attenuated response in VO2max also show a similar attenuation in their HRV response to exercise training.
Materials and Methods
Study design
The complete design, methods, and primary outcomes of our study are presented elsewhere.9,24 Briefly, the DREW study was a randomized, dose-response exercise training trial complying with the Declaration of Helsinki comparing a nonexercise control group with three groups exercising at incremental doses (50%, 100%, and 150%) of the minimal NIH Consensus Development Panel's recommendation for energy expenditure.10 The Cooper Institute and Pennington Biomedical Research Center's institutional review boards reviewed DREW annually. The primary outcomes for the DREW study included VO2max based on the average of two baseline and two follow-up exercise tests and resting blood pressure. Ancillary studies have examined HRV as well as the VO2max response of women in various age groups.18–20
Participants
After an initial evaluation and run-in period, we randomized 464 postmenopausal women (45–75 years) to one of three exercise groups or a nonexercise control group for a 6-month intervention period. The exercise intensity for this study was fixed at 50% of measured VO2max, which is considered to be low-to-moderate exercise intensity. During the exercise portion of the study, there were distinct and separate intervention and assessment teams, and all assessment staff were blinded to participant randomization assignment. The exercise testing and exercise training laboratories were on separate floors of the building. We regularly reminded participants not to discuss their randomization assignments with assessment team members.
Study participants were sedentary (exercising <20 minutes, <3 days/weeks, <8000 steps/day assessed over the course of 1 week), overweight or obese (body mass index [BMI] 25.0–43.0 kg/m2), and had a systolic blood pressure of 120–160 mm Hg. We excluded women who had a history of stroke, heart attack, or any serious medical condition that prevented them from adhering to the protocol or exercising safely. Our current analysis is limited to 365 women who successfully completed both VO2max and HRV testing. Of these, 118 women were ≥60 years. After baseline testing, which included HRV measurements, participants were randomized to their respective treatment groups.
Heart rate variability
Although many indices of HRV exist, the time and frequency domain indices are the most common. For the time domain, the root mean square of successive RR intervals (rMSSD) represents parasympathetic nervous system activity, and the standard deviation of normal beat-to-beat (RR) intervals (SDNN) is a global index encompassing both the sympathetic and parasympathetic nervous systems. For the frequency domain, high frequency (HF, 0.15–0.40 Hz) power has been linked to vagal activity, whereas the role of other indices is less clear.6 Nonetheless, we also calculated additional frequency domain spectra, such as low frequency power (LF, 0.04–0.15 Hz), very low frequency power (VLF, 0.0033–0.04 Hz), and total frequency power (PT, 0.00–0.40 Hz).
We examined each participant's HRV between 6:30 am and 11:00 am for 25 minutes in a semidark room at 22–23°C after a 12-hour fast inclusive of abstinence from caffeine and alcohol (12 hour) and exercise (48 hour). We controlled each participant's respiration rate using a metronome at a fixed rate of 12 breaths per minute (0.2 Hz). The RR interval measurements were conducted at the same time (±1 hour) of day for each participant during the baseline and posttest assessment periods. To assess HRV, we used a two-channel ECG signal detected by a Polar Heart Rate Monitor transmitted online to a PC through Polar Advantage Interface receiver. All data were filtered to eliminate ectopic beats and artifacts. Additionally, an RR interval tachogram was displayed for manual editing, and areas of ectopy or artifacts were identified and removed by manual deletion. Each edited RR interval was replaced with an average value. Segments containing >15% of edited RR intervals were interpreted as premature beats and were excluded from data analysis.25
Statistical analysis
We used a generalized linear model to analyze the influences of the differing doses of exercise training on HRV characteristics. We focused our primary analysis on the time domain index of rMSSD, as it is sensitive to short periods of recording time.6 We also examined the frequency domain index HFLn as a means of examining parasympathetic activity. We adjusted all our outcomes among the randomization groups for select specified covariates, including baseline HRV, race, and use of antidepressant medication. Given that this is an ancillary analysis in a large cohort, we first reproduced the findings of our primary outcome study and other ancillary reports from which we formed our current research question. The results were consistent, so we undertook further analysis to answer the question of whether age affects HRV improvements associated with exercise training. To accomplish this, we subdivided women by age into those <60 years and ≥60 years of age, given our recent report showing that this latter age group was unresponsive to exercise training as determined by changes in Vo2max.18 Within-group differences between baseline and follow-up are reported as mean and 95% confidence intervals (95% CI). Knowing that age was significant for baseline Vo2max.from our previous report, we added baseline Vo2max.as a covariate to our analysis to examine if lower fitness levels at the start of training influenced our current analyses. If present, age group interactions were explored for pairwise comparisons between the exercise training groups vs. the control group using a Dunnett-Hsu post-hoc assessment. The Dunnett-Hsu test allows for specific multiple pairwise comparisons while still protecting against type I statistical errors. Between-group differences at baseline and follow-up in prevalence were examined using chi-square tests. All reported p values are two-sided (p<0.05). All analyses were performed using SPSS version 19.0 (Somers, NY).
Results
We show the clinical and demographic data for our cohort in Table 1. Similar to our previous reports, we observed that VO2max increased significantly with increasing exercise dose of exercise: control, −2.86 mL/kg/min, 95% CI −0.67,0.10); 4 kcal/kg/weeks, (KKW) 0.64 mL/kg/min, 95% CI 0.35, 0.98; 8 KKW, 1.41 mL/kg/min, 95% CI 1.01, 1.81; and 12 KKW, 1.60 mL/kg/min, 95% CI 1.22, 1.98. The rMSSD of HRV reflecting parasympathetic activity, also increased (Fig. 1A). These results were confirmed by our analysis of HFLn. We also observed that when stratified by age, VO2max was lower in participants ≥60 years (p<0.001) and that women > age 60 did not demonstrate a significant improvement in VO2max regardless of exercise dose. Based on these confirmatory findings for our previous reports, we further explored the age subgroup analysis finding that each time and frequency domain measurement of HRV was significantly lower in women ≥60 years (p<0.02). After exercise training, however, we observed that rMSSD (Fig. 1B) and HFLn both improved significantly in the 8 KKW and 12 KKW groups regardless of age. Although women <60 years showed a significant improvement for these two parameters after 4 KKW exercise training exposure, women ≥60 years did not. None of our findings were influenced by baseline VO2max (p=0.2).
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants
| |
All (n=365) |
<60 years (n=247) |
≥60 years (n=118) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age, years | 57.52 | 6.2 | 54.06 | 3.5 | 64.80 | 4.2 |
| Hematology | ||||||
| Total cholesterol (mmol/L) | 5.23 | 0.78 | 5.27 | 0.76 | 5.13 | 0.81 |
| HDL (mmol/L) | 1.49 | 0.37 | 1.48 | 0.36 | 1.52 | 0.40 |
| LDL (mmol/L) | 3.05 | 0.69 | 3.07 | 0.69 | 3.00 | 0.71 |
| Glucose (mmol/L) | 5.27 | 0.55 | 5.27 | 0.50 | 5.29 | 0.64 |
| hsCRP (nmol/L) | 53.83 | 53.67 | 54.11 | 53.79 | 53.19 | 55.21 |
| Anthropometry | ||||||
| Height (cm) | 162.80 | 5.8 | 163.34 | 5.8 | 161.96 | 5.8 |
| Weight (kg) | 83.99 | 11.8 | 85.02 | 11.9 | 82.56 | 11.7 |
| BMI (kg/m2) | 31.63 | 3.7 | 32.23 | 5.7 | 31.44 | 3.8 |
| Waist circumference (cm) | 100.70 | 11.4 | 99.96 | 10.6 | 102.56* | 12.6 |
| Hip circumference (cm) | 114.71 | 9.1 | 114.78 | 8.9 | 114.86 | 9.3 |
| Blood pressure | ||||||
| Resting heart rate (b/min) | ||||||
| Systolic BP (mm Hg) | 139.56 | 13.2 | 138.56 | 12.8 | 141.74 | 13.8 |
| Diastolic BP (mm Hg) | 80.90 | 8.5 | 82.28 | 8.2 | 78.03 | 8.6 |
| Cardiovascular | ||||||
| Vo2max (mL/kg/min) | 15.59 | 2.9 | 16.08 | 2.9 | 14.45* | 2.6 |
| Vo2max (L/min) | 1.30 | 0.3 | 1.36 | 0.2 | 1.24* | 0.2 |
| Maximum RER | 1.13 | 0.1 | 1.14 | 0.9 | 1.14 | 0.1 |
| Maximum heart rate (b/min) | 151 | 16 | 155 | 15 | 144* | 17 |
| Time domain for HRV (ms) | ||||||
| rMSSD | 22.99 | 11.7 | 23.99 | 11.55 | 20.91* | 11.74 |
| SDNN | 32.87 | 11.5 | 34.18 | 11.72 | 30.14* | 10.59 |
| Frequency domain for HRV (ms2) | ||||||
| TotalLn | 6.26 | 0.8 | 6.37 | 0.8 | 6.03* | 0.9 |
| VLFLn | 4.62 | 0.8 | 4.69 | 0.83 | 4.45* | 0.81 |
| LFLn | 5.29 | 0.9 | 5.38 | 0.89 | 5.10* | 0.97 |
| HFLn | 5.12 | 1.1 | 5.25 | 1.04 | 4.84* | 1.13 |
Significantly different from <60 years.
BMI, body mass index; HDL, high-denisty lipoprotein; HRV, heart rate variability; hsCRP, high sensitivity c-reactive protein; LDL, low-density lipoprotein; LF, low frequency; RER, respiratory exchange ratio; rMSSD, root mean square of successive beat-to-beat intervals; SD, standard deviation; SDNN, SD of normal RR intervals; VO2max, maximal cardiorespiratory exercise capacity.
FIG. 1.
Data represent changes the root mean square of successive RR intervals (rMSSD) indicative of changes in parasympathetic nervous system activity for the entire Dose Response to Exercise in Women (DREW) cohort (A) and when stratified by age (B) denoting younger (<60 years) and older (≥60 years) women. KKW, kcals/kg/week.
Discussion
The primary finding from our study demonstrates that low-to-moderate intensity exercise training increases parasympathetic activity in previously sedentary, overweight or obese, postmenopausal women regardless of age. Of particular importance to our findings is the observation that women ≥60 years of age showed a significant improvement in HRV despite demonstrating no improvement in VO2max. Unfortunately, a consensus of findings from exercise intervention trials in older individuals is difficult, as the literature to date is either cross-sectional, longitudinal, or based on conflicting results from studies using small sample sizes. In cross-sectional studies, older women undertaking high levels of physical activity (>2000 kcal/week) demonstrate higher levels of parasympathetic HRV activity than women with lower daily energy expenditures.26 A report from the Whitehall II cohort study examining how predictive variables of HRV change over time were associated with baseline behavioral, biologic, and social and psychosocial factors showed that age-adjusted and gender-adjusted participants who avoided the lowest (i.e., most adverse) quartile of HRV function were most influenced by baseline levels of exercise, BMI, cholesterol, and blood pressure.
Regarding exercise interventions, Davy et al.23 reported in 8 postmenopausal women that HRV did not improve after 12 weeks of aerobic exercise at 70% of maximal heart rate. In contrast, Stein et al.22 demonstrated an improvement in HRV characteristics for older women exercising for 12 months, and Albinet et al.21 recently showed that HRV increased in older men and women after 12 weeks of exercise training. Still, these latter two studies comprised 18 and 13 women, respectively, who were further divided into two treatment groups. Although the results of these early exercise intervention trials suggest that the effects of exercise on HRV may be equivocal, we are now able to confirm the findings of the those studies showing a positive modulation in autonomic nervous system activity accompanying exercise training in a large cohort of women ≥60 years of age. The findings of our study are clinically important because the autonomic nervous system function is associated not only with CVD risk but also with a number of health-related issues, such as mood, depression, anthropometry, extreme physical exertion, cognitive and executive functioning, and vagal control of the cardiovascular system.6,11–17,27
Overall, parasympathetic activity is higher in women than men, yet aging reduces this difference in that parasympathetic activity shifts to a lower range after menopause.28–32 Heart rate variability (HRV) is a good example of how the risk for CVD may be improved with exercise during aging as a result of changes in systems physiology independent of improvements in VO2max. For example, exercise training positively affects cognitive performance and executive function in older adults.33 This may be due in part to the observation that impaired cognitive performance, prefrontal brain control, and symptoms of depression in older women have been associated with vagal function.33–37 Thus, the improvement in parasympathetic activity in our study as higher HF power is associated with better cognitive performance, whereas low HF power is predictive of cognitive impairment in aging women.14,15,35 The role of exercise also appears to be important, as Albinet et al.21 recently demonstrated that 12 weeks of exercise significantly improved vagal-mediated indicators of HRV, specifically rMSSD, as well as cognitive function, as measured by the Wisconsin card sorting test. Although the direct link between exercise training and cognitive function is still unclear, it has been suggested that modulation of cardiac activity by the prefrontal cortex is vagally mediated and part of the neurovisceral-integrated relationship between central and peripheral nervous system interactions.36,38,39
A primary strength of the DREW study is that it was a large, well-controlled exercise training study with excellent exercise adherence and a low dropout rate. The DREW study was also well positioned to answer the research question that we have posed given the large number of women ≥60 years in our study. The DREW study has limitations because its sample is limited to sedentary, overweight or obese, postmenopausal women at a moderately elevated risk of CVD. Therefore, we do not know if the results will apply to other women or to men. Additionally, although the results of our study show that postmenopausal women can improve their HRV with exercise without improving VO2max, the clinical importance of our findings relative to future health habitus remains unclear. Furthermore, we did not measure cognitive function in DREW. Thus, we accept that the potential link between improved HRV and cognitive function is theoretical and not fully answerable at this time but should be considered as a candidate measurement in future studies examining exercise and aging in both men and women.
The important point, however, is that our current findings strongly suggest that HRV is improved in older women partaking in a low-to-moderate intensity exercise program irrespective of changes in VO2max. Although we did not observe an HRV improvement in women >60 years for the 4 KKW group, we also did not find improvement by exercising at an exercise dose >8 KKW. Specifically, 8 KKW is equal to levels of exercise recommended by the NIH Development Consensus Panel for exercise,9,10 Which are easily attainable by most people. The results of our study should help those involved in disseminating exercise advice to refine their recommendations depending on the goals of their patients.
Acknowledgments
This work was funded by grant HL66262 from the National Institutes of Health. We thank Life Fitness (Schiller Park, IL) for providing exercise equipment.
Disclosure Statement
C.P.E. has received honoraria for lectures from scientific, educational, and community groups. T.S.C. has received honoraria for lectures from scientific, educational, and community groups, served as a consultant for Trestle Tree Inc, and has a book in publication from which he will receive royalties. S.N.B. reports receiving book royalties from Human Kinetics; honoraria for service on the Medical Advisory Boards for Matria Health Care, Magellan Health Services, and Jenny Craig; and honoraria for lectures from scientific, educational, and community groups. He also reports that the University of North Texas pays him as an Executive Lecturer; he gives these fees to the University of South Carolina Educational Foundation or to other nonprofit groups. During the past 5-year period, he has received a research grant from Jenny Craig.
References
- 1.International position paper on women's health, menopause: A comprehensive approach. National Heart, Lung, and Blood Institute NIH Publication No. 02–3284. 2000. www.nhlbi.nih.gov/health/prof/heart/other/wm_menop.htm. [May 17;2007 ]. www.nhlbi.nih.gov/health/prof/heart/other/wm_menop.htm
- 2.Blair SN. Kohl HW., 3rd Paffenbarger RS., Jr Clark DG. Cooper KH. Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 3.Kodama S. Saito K. Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji H. Larson MG. Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji H. Venditti FJ., Jr Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 6.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 7.Dewey FE. Freeman JV. Engel G, et al. Novel predictor of prognosis from exercise stress testing: Heart rate variability response to the exercise treadmill test. Am Heart J. 2007;153:281–288. doi: 10.1016/j.ahj.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Brockbank CL. Chatterjee F. Bruce SA. Woledge RC. Heart rate and its variability change after the menopause. Exp Physiol. 2000;85:327–330. [PubMed] [Google Scholar]
- 9.Church TS. Earnest CP. Skinner JS. Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: A randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 10.NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. Physical activity and cardiovascular health. JAMA. 1996;276:241–246. [PubMed] [Google Scholar]
- 11.Earnest CP. Jurca R. Church TS. Chicharro JL. Hoyos J. Lucia A. Relation between physical exertion and heart rate variability characteristics in professional cyclists during the Tour of Spain. Br J Sports Med. 2004;38:568–575. doi: 10.1136/bjsm.2003.005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao YY. Lovejoy JC. Sparti A. Bray GA. Keys LK. Partington C. Autonomic activity assessed by heart rate spectral analysis varies with fat distribution in obese women. Obes Res. 1996;4:55–63. doi: 10.1002/j.1550-8528.1996.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 13.Gutin B. Howe C. Johnson MH. Humphries MC. Snieder H. Barbeau P. Heart rate variability in adolescents: Relations to physical activity, fitness, and adiposity. Med Sci Sports Exerc. 2005;37:1856–1863. doi: 10.1249/01.mss.0000175867.98628.27. [DOI] [PubMed] [Google Scholar]
- 14.Gianaros PJ. Van Der Veen FM. Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen AL. Johnsen BH. Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48:263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein AA. Deuster PA. Kop WJ. Heart rate variability as a predictor of negative mood symptoms induced by exercise withdrawal. Med Sci Sports Exerc. 2007;39:735–741. doi: 10.1249/mss.0b013e31802f590c. [DOI] [PubMed] [Google Scholar]
- 17.Karavidas MK. Lehrer PM. Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback. 2007;32:19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- 18.Earnest CP. Blair SN. Church TS. Age attenuated response to aerobic conditioning in postmenopausal women. Eur J Appl Physiol. 2010;110:75–82. doi: 10.1007/s00421-010-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earnest CP. Lavie CJ. Blair SN. Church TS. Heart rate variability characteristics in sedentary postmenopausal women following six months of exercise training: The DREW study. PLoS One. 2008;3:e2288. doi: 10.1371/journal.pone.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earnest CP. Poirier P. Carnethon MR. Blair SN. Church TS. Autonomic function and change in insulin for exercising postmenopausal women. Maturitas. 2010;65:284–291. doi: 10.1016/j.maturitas.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albinet CT. Boucard G. Bouquet CA. Audiffren M. Increased heart rate variability and executive performance after aerobic training in the elderly. Eur J Appl Physiol. 2010;109:617–624. doi: 10.1007/s00421-010-1393-y. [DOI] [PubMed] [Google Scholar]
- 22.Stein PK. Ehsani AA. Domitrovich PP. Kleiger RE. Rottman JN. Effect of exercise training on heart rate variability in healthy older adults. Am Heart J. 1999;138:567–576. doi: 10.1016/s0002-8703(99)70162-6. [DOI] [PubMed] [Google Scholar]
- 23.Davy KP. Willis WL. Seals DR. Influence of exercise training on heart rate variability in post-menopausal women with elevated arterial blood pressure. Clin Physiol. 1997;17:31–40. doi: 10.1046/j.1365-2281.1997.01010.x. [DOI] [PubMed] [Google Scholar]
- 24.Morss GM. Jordan AN. Skinner JS, et al. Dose Response to Exercise in Women Aged 45–75 yr (DREW): Design and rationale. Med Sci Sports Exerc. 2004;36:336–344. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- 25.Huikuri HV. Seppanen T. Koistinen MJ, et al. Abnormalities in beat-to-beat dynamics of heart rate before the spontaneous onset of life-threatening ventricular tachyarrhythmias in patients with prior myocardial infarction. Circulation. 1996;93:1836–1844. doi: 10.1161/01.cir.93.10.1836. [DOI] [PubMed] [Google Scholar]
- 26.Reland S. Ville NS. Wong S. Senhadji L. Carre F. Does the level of chronic physical activity alter heart rate variability in healthy older women? Clin Sci (Lond) 2004;107:29–35. doi: 10.1042/CS20030405. [DOI] [PubMed] [Google Scholar]
- 27.Hugdahl K. Cognitive influences on human autonomic nervous system function. Curr Opin Neurobiol. 1996;6:252–258. doi: 10.1016/s0959-4388(96)80080-8. [DOI] [PubMed] [Google Scholar]
- 28.Evans JM. Ziegler MG. Patwardhan AR, et al. Gender differences in autonomic cardiovascular regulation: Spectral, hormonal, and hemodynamic indexes. J Appl Physiol. 2001;91:2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- 29.Kuo TB. Lin T. Yang CC. Li CL. Chen CF. Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999;277:H2233–2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- 30.Eaker ED. Chesebro JH. Sacks FM. Wenger NK. Whisnant JP. Winston M. Cardiovascular disease in women. Circulation. 1993;88:1999–2009. doi: 10.1161/01.cir.88.4.1999. [DOI] [PubMed] [Google Scholar]
- 31.Fluckiger L. Boivin JM. Quilliot D. Jeandel C. Zannad F. Differential effects of aging on heart rate variability and blood pressure variability. J Gerontol A Biol Sci Med Sci. 1999;54:B219–224. doi: 10.1093/gerona/54.5.b219. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz JB. Gibb WJ. Tran T. Aging effects on heart rate variation. J Gerontol. 1991;46:M99–106. doi: 10.1093/geronj/46.3.m99. [DOI] [PubMed] [Google Scholar]
- 33.Colcombe S. Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 34.Etnier JL. Nowell PM. Landers DM. Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Kim DH. Lipsitz LA. Ferrucci L, et al. Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: Women's Health and Aging Study I. J Am Geriatr Soc. 2006;54:1751–1757. doi: 10.1111/j.1532-5415.2006.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thayer JF. Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Kim CK. McGorray SP. Bartholomew BA, et al. Depressive symptoms and heart rate variability in postmenopausal women. Arch Intern Med. 2005;165:1239–1244. doi: 10.1001/archinte.165.11.1239. [DOI] [PubMed] [Google Scholar]
- 38.Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Thayer JF. Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]