Abstract
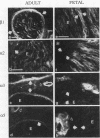
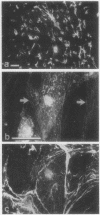
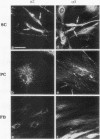
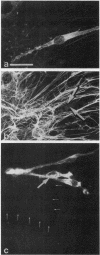
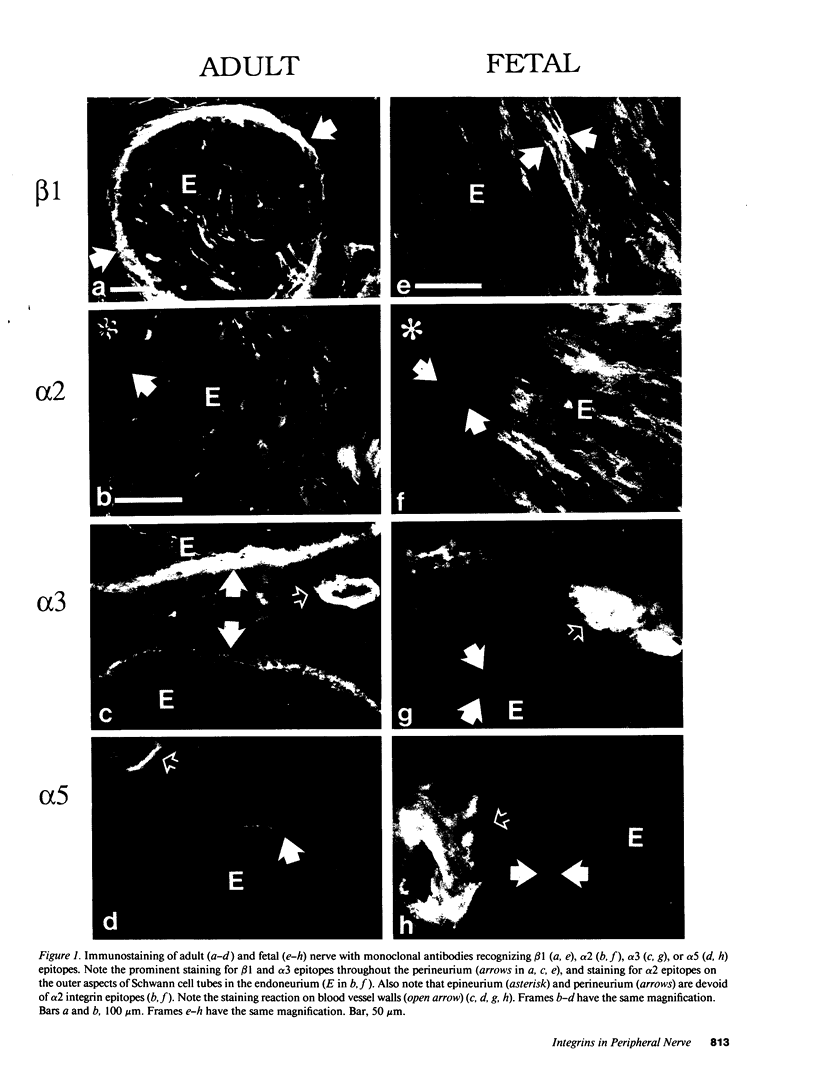
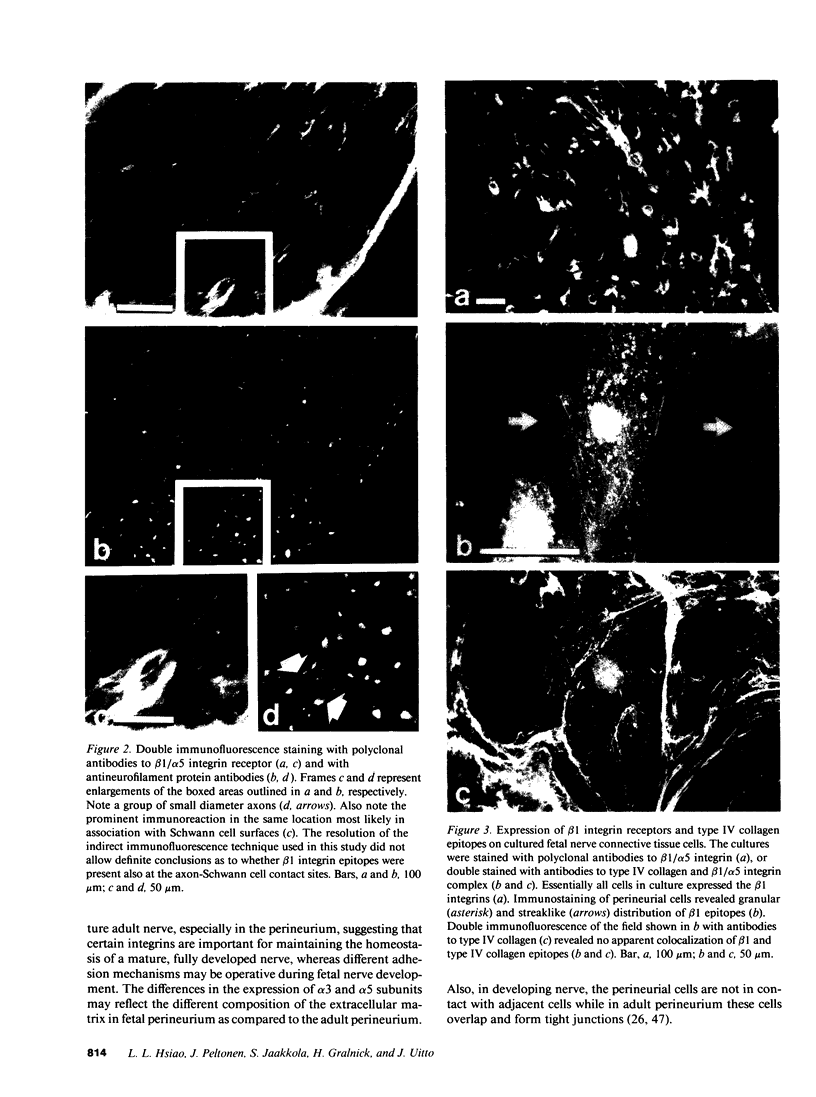
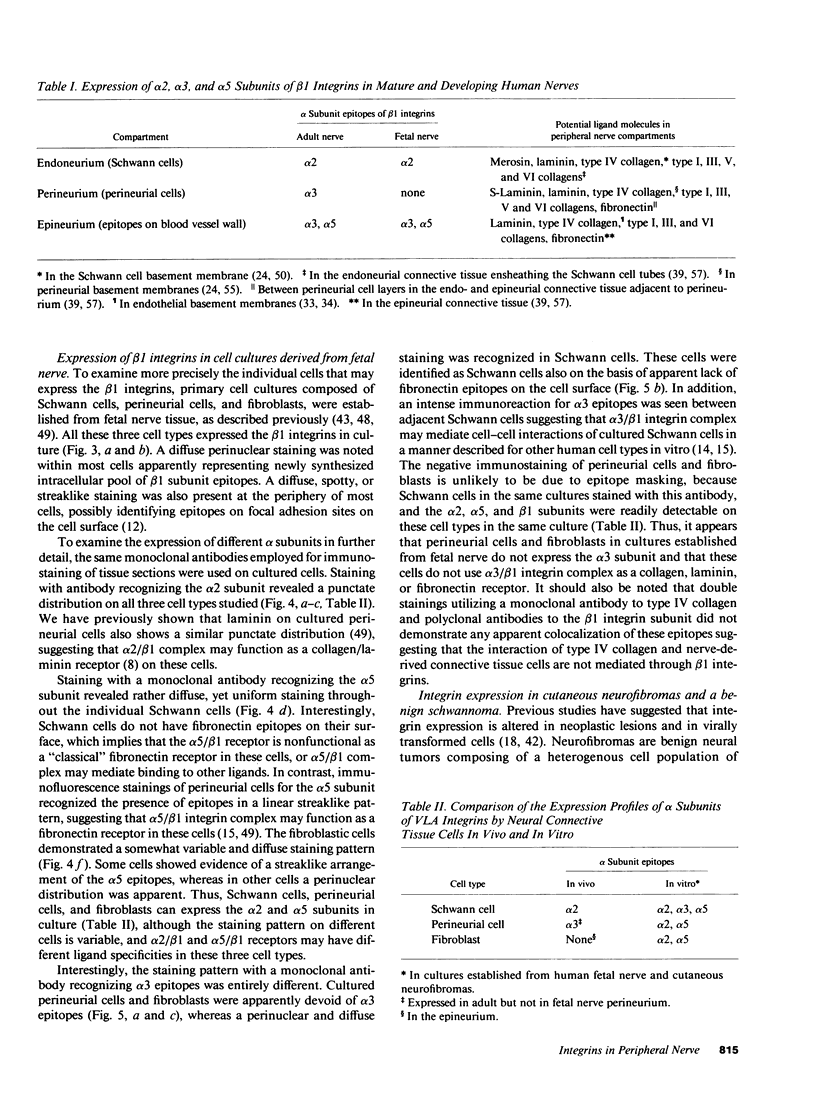
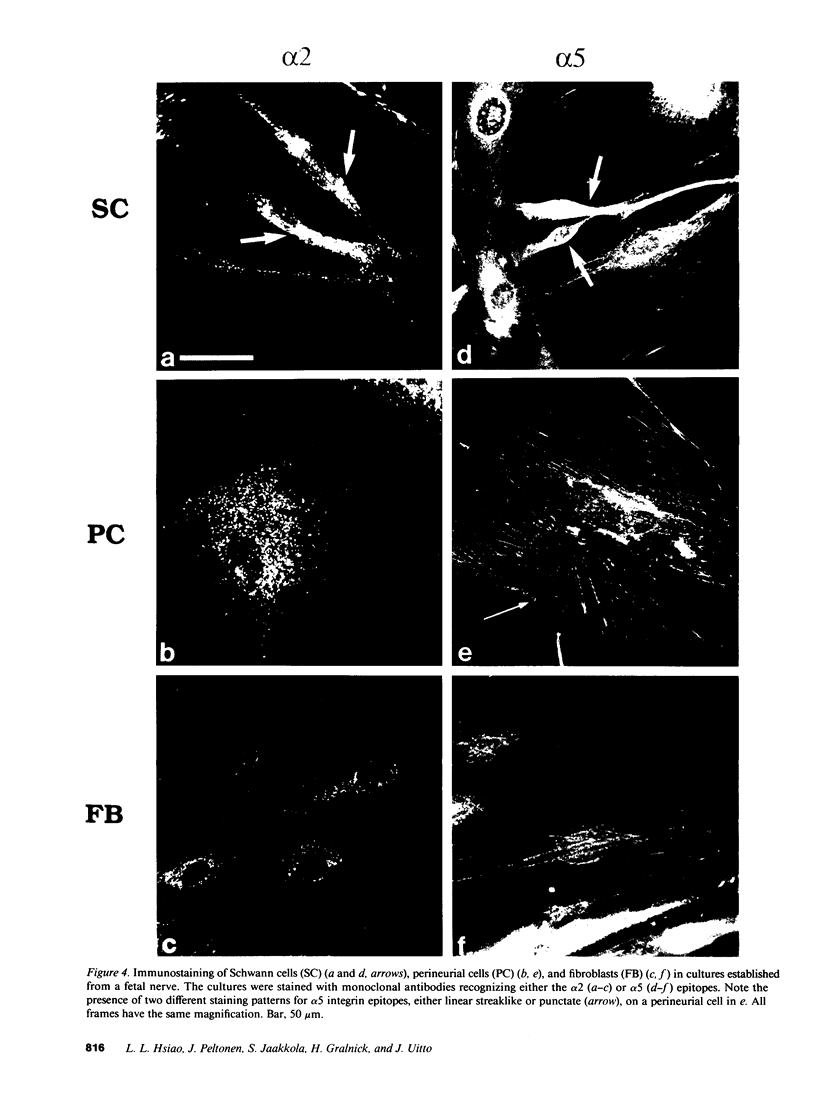
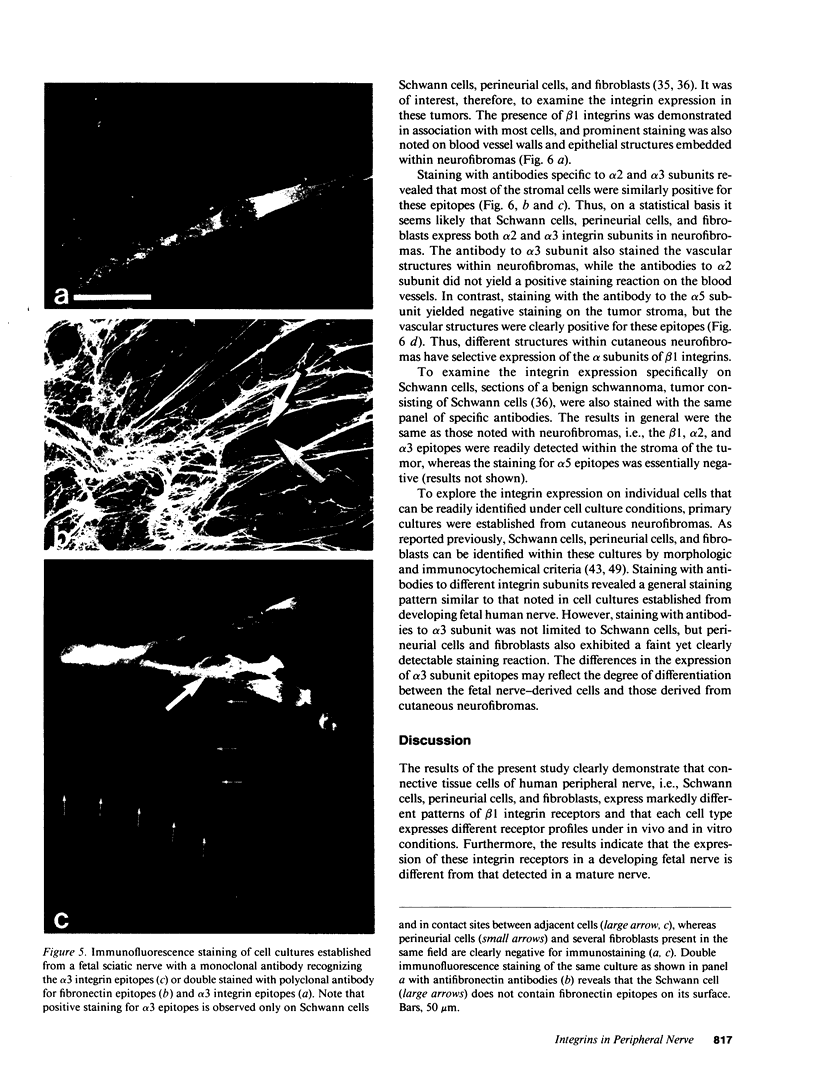
Strikingly selective expression patterns of beta 1, alpha 2, alpha 3, and alpha 5 integrin subunits were revealed in endoneurium, perineurium, and epineurium of fetal and adult human peripheral nerve by immunostaining with specific antibodies. The alpha 2 subunit was expressed only on Schwann cells both in fetal and adult nerve, whereas the alpha 3 epitopes were expressed exclusively in the adult tissue and were primarily present on perineurial cells. The alpha 5 epitopes were expressed only on the innermost cell layer of perineurium of fetal and adult nerve. The tumor cells within schwannomas and cutaneous neurofibromas expressed both alpha 2 and alpha 3 subunits, indicating that Schwann cells have the potential to express also the alpha 3 subunit in vivo. Cell cultures established from human fetal nerve and neurofibromas revealed expression of the alpha 2 and alpha 5 epitopes on Schwann cells, perineurial cells, and fibroblasts, whereas only Schwann cells contained the alpha 3 epitopes which were occasionally concentrated on the adjacent Schwann cells at cell-cell contacts. Our findings emphasize that nerve connective tissue cells change their profiles for expression of extracellular matrix receptors under conditions which have different regulatory control signals exerted by, for example, axons, humoral factors, or the extracellular matrix of the peripheral nerve. This plasticity may play an important role during nerve development and in neoplastic processes affecting the connective tissue compartments of peripheral nerve.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aumailley M., Mann K., von der Mark H., Timpl R. Cell attachment properties of collagen type VI and Arg-Gly-Asp dependent binding to its alpha 2(VI) and alpha 3(VI) chains. Exp Cell Res. 1989 Apr;181(2):463–474. doi: 10.1016/0014-4827(89)90103-1. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Bunge M. B., Williams A. K., Wood P. M., Uitto J., Jeffrey J. J. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J Cell Biol. 1980 Jan;84(1):184–202. doi: 10.1083/jcb.84.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson D. J., Mahan J. T. Keratinocyte migration and the extracellular matrix. J Invest Dermatol. 1988 May;90(5):623–628. doi: 10.1111/1523-1747.ep12560762. [DOI] [PubMed] [Google Scholar]
- Dziadek M., Edgar D., Paulsson M., Timpl R., Fleischmajer R. Basement membrane proteins produced by Schwann cells and in neurofibromatosis. Ann N Y Acad Sci. 1986;486:248–259. doi: 10.1111/j.1749-6632.1986.tb48078.x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion and the molecular processes of morphogenesis. Annu Rev Biochem. 1985;54:135–169. doi: 10.1146/annurev.bi.54.070185.001031. [DOI] [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. Structural requirements for the stimulation of neurite outgrowth by two variants of laminin and their inhibition by antibodies. J Cell Biol. 1988 Apr;106(4):1299–1306. doi: 10.1083/jcb.106.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984 Jul;3(7):1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrig K., Leivo I., Engvall E. Merosin and laminin. Molecular relationship and role in nerve-muscle development. Ann N Y Acad Sci. 1990;580:276–280. doi: 10.1111/j.1749-6632.1990.tb17936.x. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Hemler M. E. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAMBLE H. J. COMPARATIVE ELECTRON-MICROSCOPIC OBSERVATIONS ON THE CONNECTIVE TISSUES OF A PERIPHERAL NERVE AND A SPINAL NERVE ROOT IN THE RAT. J Anat. 1964 Jan;98:17–26. [PMC free article] [PubMed] [Google Scholar]
- GAMBLE H. J., EAMES R. A. AN ELECTRON MICROSCOPE STUDY OF THE CONNECTIVE TISSUES OF HUMAN PERIPHERAL NERVE. J Anat. 1964 Oct;98:655–663. [PMC free article] [PubMed] [Google Scholar]
- Gamble H. J., Breathnach A. S. An electron-microscope study of human foetal peripheral nerves. J Anat. 1965 Jul;99(Pt 3):573–584. [PMC free article] [PubMed] [Google Scholar]
- Harkin J. C. Pathology of nerve sheath tumors. Ann N Y Acad Sci. 1986;486:147–154. doi: 10.1111/j.1749-6632.1986.tb48070.x. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hunter D. D., Shah V., Merlie J. P., Sanes J. R. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989 Mar 16;338(6212):229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jaakkola S., Peltonen J., Riccardi V., Chu M. L., Uitto J. Type 1 neurofibromatosis: selective expression of extracellular matrix genes by Schwann cells, perineurial cells, and fibroblasts in mixed cultures. J Clin Invest. 1989 Jul;84(1):253–261. doi: 10.1172/JCI114148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola S., Peltonen J., Uitto J. J. Perineurial cells coexpress genes encoding interstitial collagens and basement membrane zone components. J Cell Biol. 1989 Mar;108(3):1157–1163. doi: 10.1083/jcb.108.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Kaufmann R., Frösch D., Westphal C., Weber L., Klein C. E. Integrin VLA-3: ultrastructural localization at cell-cell contact sites of human cell cultures. J Cell Biol. 1989 Oct;109(4 Pt 1):1807–1815. doi: 10.1083/jcb.109.4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhofer D., Languino L. R., Ruoslahti E., Pierschbacher M. D. Alpha 2 beta 1 integrins from different cell types show different binding specificities. J Biol Chem. 1990 Jan 15;265(2):615–618. [PubMed] [Google Scholar]
- Klemm H. Das Perineurium als Diffusionsbarriere gegenüber Peroxydase bei epi- und endoneuraler Applikation. Z Zellforsch Mikrosk Anat. 1970;108(3):431–445. [PubMed] [Google Scholar]
- Kunicki T. J., Nugent D. J., Staats S. J., Orchekowski R. P., Wayner E. A., Carter W. G. The human fibroblast class II extracellular matrix receptor mediates platelet adhesion to collagen and is identical to the platelet glycoprotein Ia-IIa complex. J Biol Chem. 1988 Apr 5;263(10):4516–4519. [PubMed] [Google Scholar]
- Larjava H., Peltonen J., Akiyama S. K., Yamada S. S., Gralnick H. R., Uitto J., Yamada K. M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990 Mar;110(3):803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Amenta P. S. The basement membrane in pathology. Lab Invest. 1983 Jun;48(6):656–677. [PubMed] [Google Scholar]
- Ohara S., Takahashi H., Ikuta F. Ultrastructural alterations of perineurial cells in the early stage of Wallerian degeneration. Lab Invest. 1986 Feb;54(2):213–221. [PubMed] [Google Scholar]
- Oldfors A. Permeability of the perineurium of small nerve fascicles: an ultrastructural study using ferritin in rats. Neuropathol Appl Neurobiol. 1981 May-Jun;7(3):183–194. doi: 10.1111/j.1365-2990.1981.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Olsen D., Nagayoshi T., Fazio M., Peltonen J., Jaakkola S., Sanborn D., Sasaki T., Kuivaniemi H., Chu M. L., Deutzmann R. Human laminin: cloning and sequence analysis of cDNAs encoding A, B1 and B2 chains, and expression of the corresponding genes in human skin and cultured cells. Lab Invest. 1989 Jun;60(6):772–782. [PubMed] [Google Scholar]
- Peltonen J., Foidart J. M., Aho H. J. Type IV and V collagens in von Recklinghausen's neurofibromas. An immunohistochemical and electrophoretical study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;47(4):291–301. doi: 10.1007/BF02890212. [DOI] [PubMed] [Google Scholar]
- Peltonen J., Jaakkola S., Hsiao L. L., Timpl R., Chu M. L., Uitto J. Type VI collagen. In situ hybridizations and immunohistochemistry reveal abundant mRNA and protein levels in human neurofibroma, schwannoma and normal peripheral nerve tissues. Lab Invest. 1990 Apr;62(4):487–492. [PubMed] [Google Scholar]
- Peltonen J., Jaakkola S., Lebwohl M., Renvall S., Risteli L., Virtanen I., Uitto J. Cellular differentiation and expression of matrix genes in type 1 neurofibromatosis. Lab Invest. 1988 Dec;59(6):760–771. [PubMed] [Google Scholar]
- Peltonen J., Jaakkola S., Virtanen I., Pelliniemi L. Perineurial cells in culture. An immunocytochemical and electron microscopic study. Lab Invest. 1987 Nov;57(5):480–488. [PubMed] [Google Scholar]
- Peltonen J., Larjava H., Jaakkola S., Gralnick H., Akiyama S. K., Yamada S. S., Yamada K. M., Uitto J. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Invest. 1989 Dec;84(6):1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen J., Penttinen R., Larjava H., Aho H. J. Collagens in neurofibromas and neurofibroma cell cultures. Ann N Y Acad Sci. 1986;486:260–270. doi: 10.1111/j.1749-6632.1986.tb48079.x. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E., Sundelin J., Lind P., Peterson P. A. The cell attachment domain of fibronectin. Determination of the primary structure. J Biol Chem. 1982 Aug 25;257(16):9593–9597. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rettig W. J., Murty V. V., Mattes M. J., Chaganti R. S., Old L. J. Extracellular matrix-modulated expression of human cell surface glycoproteins A42 and J143. Intrinsic and extrinsic signals determine antigenic phenotype. J Exp Med. 1986 Nov 1;164(5):1581–1599. doi: 10.1084/jem.164.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Röyttä M., Salonen V., Peltonen J. Reversible endoneurial changes after nerve injury. Acta Neuropathol. 1987;73(4):323–329. doi: 10.1007/BF00688254. [DOI] [PubMed] [Google Scholar]
- Saga S., Chen W. T., Yamada K. M. Enhanced fibronectin receptor expression in Rous sarcoma virus-induced tumors. Cancer Res. 1988 Oct 1;48(19):5510–5513. [PubMed] [Google Scholar]
- Salonen V., Aho H., Röyttä M., Peltonen J. Quantitation of Schwann cells and endoneurial fibroblast-like cells after experimental nerve trauma. Acta Neuropathol. 1988;75(4):331–336. doi: 10.1007/BF00687785. [DOI] [PubMed] [Google Scholar]
- Salonen V., Lehto M., Vaheri A., Aro H., Peltonen J. Endoneurial fibrosis following nerve transection. An immunohistological study of collagen types and fibronectin in the rat. Acta Neuropathol. 1985;67(3-4):315–321. doi: 10.1007/BF00687818. [DOI] [PubMed] [Google Scholar]
- Salonen V., Peltonen J., Röyttä M., Virtanen I. Laminin in traumatized peripheral nerve: basement membrane changes during degeneration and regeneration. J Neurocytol. 1987 Oct;16(5):713–720. doi: 10.1007/BF01637662. [DOI] [PubMed] [Google Scholar]
- THOMAS P. K. The connective tissue of peripheral nerve: an electron microscope study. J Anat. 1963 Jan;97:35–44. [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Strominger J. L., Hemler M. E. The very late antigen family of heterodimers is part of a superfamily of molecules involved in adhesion and embryogenesis. Proc Natl Acad Sci U S A. 1987 May;84(10):3239–3243. doi: 10.1073/pnas.84.10.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. 1989 Apr 1;180(3):487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- Uitto J., Matsuoka L. Y., Chu M. L., Pihlajaniemi T., Prockop D. J. Connective tissue biochemistry of neurofibromas. Ann N Y Acad Sci. 1986;486:271–286. doi: 10.1111/j.1749-6632.1986.tb48080.x. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]