Abstract
Introduction
Duchenne muscular dystrophy (DMD) is a severe, muscle-wasting disease caused by mutations in the dystrophin gene. The mdx mouse is the first and perhaps the most commonly used animal model for study of DMD. Both male and female mdx mice are used. However, it is not completely clear whether gender influences contraction and the passive mechanical properties of mdx skeletal muscle.
Methods
We compared isometric tetanic forces and passive forces of the extensor digitorum longus muscle between male and female mdx mice.
Results
At age 6 months, female mdx mice showed better-preserved specific tetanic force. Interestingly, at 20 months of age, female mdx muscle appeared stiffer.
Conclusions
Our results suggest that gender may profoundly influence physiological measurement outcomes in mdx mice. Gender should be considered when using the mdx model.
Keywords: dystrophin, EDL, gender, mdx, muscle force
Duchenne muscular dystrophy (DMD) is an X-linked, recessive, muscle-wasting disease. It is caused by mutations in the dystrophin gene.1 The mdx mouse is the most commonly used model for studying the pathogenesis of DMD and developing new therapies.2 Dystrophin expression is abolished in mdx mice due to a nonsense point mutation in exon 23 of the dystrophin gene.3 In contrast to humans, mdx mice are only mildly affected. Importantly, they are fertile.4 As a result, both male and female mdx mice are readily available for preclinical studies. Although it is generally accepted that gender may profoundly influence animal physiology, very few studies have explored the impact of gender on disease progression and therapeutic outcome in mdx mice, as reviewed by Grounds et al.5 In fact, some studies have grouped mdx mice of opposite genders into one group.6–8
Change in muscle mechanical properties is a major clinical symptom and a critical therapeutic endpoint. However, male and female differences in muscle contractile and mechanical properties have not been studied in mdx mice. In this study we compared isometric tetanic force generation and passive (elastic and viscous) properties of the extensor digitorum longus (EDL) muscle between genders in mdx and C57Bl/10 (BL10) mice. We observed a significant difference in body weight, EDL muscle weight, and EDL muscle cross-sectional area (CSA) between male and female mice. Although nominal differences were detected in active and passive contractile properties between male and female BL10 mice, gender-associated changes were observed in mdx animals. Specifically, female mdx mice exhibited significantly higher specific tetanic force than male mdx mice at 6 months of age. Although 20-month-old male and female mdx mice yielded similar specific tetanic force, the absolute tetanic force was significantly reduced in the females at this age. In addition, 20-month-old female mdx EDL muscle was stiffer than that of aged-matched male mdx muscle. Interestingly, female mdx muscle also showed a higher hydroxyproline content than males. Taken together, our data suggest that gender may profoundly influence the physiological properties of mdx skeletal muscle.
METHODS
Experimental Mice
All animal experiments were approved by the animal care and use committee of the University of Missouri and were in accordance with NIH guidelines. Experimental mice (mdx and control BL10) were purchased from the Jackson Laboratory (Bar Harbor, Maine). Sample size data are provided in Table 1.
Table 1.
Morphometric properties of the experimental animals.
| 6-month-old males | 6-month-old females | 20-month-old males | 20-month-old females | ||
|---|---|---|---|---|---|
| BL10 | n | 10 | 9 | 10 | 10 |
| Body weight (g) | 32.03 ± 0.57 | 25.67 ± 0.67* | 37.44 ± 0.64‡ | 39.96 ± 1.18‡ | |
| EDL weight (mg) | 13.90 ± 0.77 | 10.97 ± 0.40 | 13.00 ± 0.18 | 11.86 ± 0.30* | |
| CSA (mm2) | 2.12 ± 0.12 | 1.78 ± 0.06 | 2.12 ± 0.03 | 1.88 ± 0.05* | |
| Lo (mm) | 14.09 ± 0.04 | 13.18 ± 0.05* | 13.14 ± 0.04‡ | 13.54 ± 0.04*,‡ | |
| mdx | n | 13 | 9 | 19 | 15 |
| Body weight (g) | 35.44 ± 0.42† | 27.38 ± 0.71* | 31.12 ± 0.56†,‡ | 22.48 ± 0.48*,†,‡ | |
| EDL weight (mg) | 16.73 ± 0.42 | 13.56 ± 0.48* | 15.95 ± 0.33† | 11.11 ± 0.35*,‡ | |
| CSA (mm2) | 2.57 ± 0.07† | 2.05 ± 0.07* | 2.42 ± 0.05† | 1.79 ± 0.06* | |
| Lo (mm) | 13.93 ± 0.05 | 14.18 ± 0.16† | 14.13 ± 0.10† | 13.28 ± 0.04*,‡ |
EDL, extensor digitorum longus; CSA, cross-sectional area; Lo, optimal length.
Female significantly different from age- and strain-matched male.
mdx is significantly different from BL10 within the same gender.
Twenty-month-old group is significantly different from 6-month-old group within the same gender and strain.
Isometric Force Assay
The maximal isometric tetanic force (Po) of the EDL muscle was measured at 150 HZ, as described elsewhere.9 Briefly, each animal was anesthetized, and the EDL muscle was gently dissected and mounted to an intact muscle test system (Aurora Scientific, Inc., Aurora, Ontario, Canada).9 The EDL was submerged in a 30°C jacketed organ bath containing Ringer buffer oxygenated with 95% O2 and 5% CO2. The Po of the EDL muscle was determined at the optimal length (Lo). Data were recorded and analyzed using LabVIEW-based software (Aurora Scientific). Time to maximal force and half-relaxation time were determined from the isometric tetanic force.
Elastic Property Assay
Elastic properties of the EDL muscle were measured in the absence of electric stimulation. Briefly, the muscle was subjected to a six-step passive stretch protocol. At each step, the EDL muscle was stretched by an increment of 10% Lo at a stretch rate of 2 cm/s.10
Viscous Property Assay
Viscous properties of the EDL muscle were determined by measuring the stress–relaxation rate (SRR) while the muscle was stretched and held at 110% Lo.10
Quantification of Hydroxyproline Content
EDL hydroxyproline content was determined as described elsewhere.10 Tendons were removed from the muscle. The EDL muscle was lyophilized overnight. The lyophilized muscle was hydrolyzed with 6N HCl and then neutralized with 10N NaOH. The lysate was oxidized with 62 mM chloramine-T and reacted with p-dimethylaminobenzaldehyde and 60% perchloric acid to develop a red–purple color. The color absorbance was measured at 558 nm, and the hydroxyproline content was determined from a standard curve.
Statistical Analysis
Data are shown as mean ± standard error of mean. Statistical analysis was carried out using SAS software, version 9 (SAS Institute, Inc., Cary, North Carolina). For multigroup comparison, a three-factor analysis of variance was performed that considered strain (BL10 and mdx), age (6 and 20 months), gender (male and female). The data show that the variability (as measured by the standard deviation) of the responses varied across different factor combinations. Consequently, heterogeneous variances were considered. The MIXED procedure of SAS was used for analysis. The REPEATED option in this procedure allows modeling of heterogeneous variances as well as dependencies of measurements made on the same animal. The models included interaction terms and, because at least some interactions were significantly different from zero for all outcomes considered, the effects of one factor were considered while holding the other two factors fixed. To account for the multiple tests done, a more stringent significance level of 0.01 was used rather than the usual 0.05. This approach to adjusting for multiple tests was used rather than the overly conservative Bonferroni adjustment commonly used. Residuals from the fitted model were examined and, in most cases, the assumption of normality was reasonable. In some cases, there were one or two outliers (an outlier is defined as the one that has a “studentized” residual of >3), and in these situations the model was re-fit with the outliers excluded. Results were reported as significant only if they appeared to be significant with and without the outliers. Statistical significance between two groups was analyzed by the Student t-test (P < 0.05 statistically significant).
RESULTS
Body Weight and Anatomic Properties of the EDL Muscle
At 6 months of age, BL10 female weight was significantly lower than that of BL10 males (Table 1). However, in 20-month-old BL10 mice, there was no significant body weight difference between males and females. At both the 6- and 20-month time-points, the female mdx mice had a significantly lower body weight than the age-matched male mdx mice (Table 1). A significant difference in body weight was observed between males, but not females, for 6-month-old BL10 and mdx mice. At 20 months of age, mdx body weight was significantly lower than that of the BL10 animals. However, females seemed to have lost more weight (mdx 44% lower than BL10), with the difference in males being less dramatic (mdx 17% lower than BL10) (Table 1).
Irrespective of dystrophin expression or age, female EDL muscles consistently showed a lower weight and smaller CSA compared with males (Table 1). Comparisons between strains (mdx vs. BL10, same age and same gender) showed that mdx EDL muscle had a higher muscle weight and CSA (Table 1). A statistically significant difference was noted between the BL10 and mdx strains for 20-month-old males (Table 1). When muscle weight was compared within the same strain (either BL10 or mdx), only female mdx mice showed an age-associated muscle weight reduction, decreasing by 18% from age 6 months to 20 months.
Characterization of Isometric Tetanic Force of the EDL Muscle
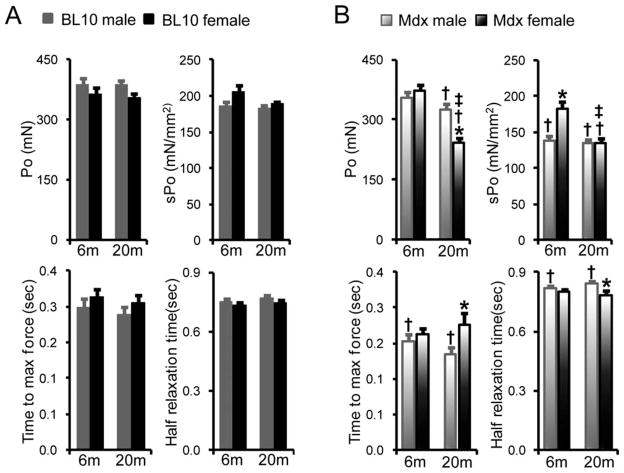
We did not detect gender differences between age-matched male and female BL10 EDL muscles for total tetanic force, specific tetanic force, time to maximal force, and half-relaxation time (Fig. 1A). Interestingly, specific tetanic force of 6-month-old female mdx EDL muscle was significantly higher than that of age-matched male mdx muscle (Fig. 1B). In 20-month-old mdx mice, females generated significantly lower total tetanic force than males. The 20-month-old mdx females took significantly more time to reach maximal force, but half-relaxation time was significantly lower than that of age-matched mdx males (Fig. 1B).
FIGURE 1.
Characterization of isometric tetanic contraction of the EDL muscle. Comparison of absolute isometric tetanic force, specific isometric tetanic force, time to maximum force, and half-relaxation time between genders in BL10 (A) and mdx (B) mice at 6 and 20 months of age. *Females significantly different from age- and strain-matched males; †mdx mice significantly different from BL10 mice within the same gender; ‡20-month-old group significantly different from 6-month-old group within the same gender and strain.
At 6 months of age, both genders produced a similar amount of total tetanic force, irrespective of dystrophin deficiency (Fig. 1A and B). Specific tetanic force was significantly reduced in 6-month-old male mdx mice compared with male BL10 mice of the same age. A similar trend was noted in 6-month-old female mice (BL10: 205 ± 9 mN/mm2; mdx: 183 ± 9 mN/mm2), but the difference did not reach statistical significance (Fig. 1A and B). Nevertheless, 6-month-old male mdx muscle showed a significantly lower time to maximal force and prolonged half-relaxation time compared with BL10 muscle of the same age. At 20 months of age, total tetanic force and specific tetanic force were significantly reduced in both male and female mdx mice compared with age-matched BL10 mice. Interestingly, a significant difference (between BL10 and mdx) in time to maximal force and half-relaxation time was found in males but not females at 20 months of age.
Characterization of EDL Muscle Passive Properties
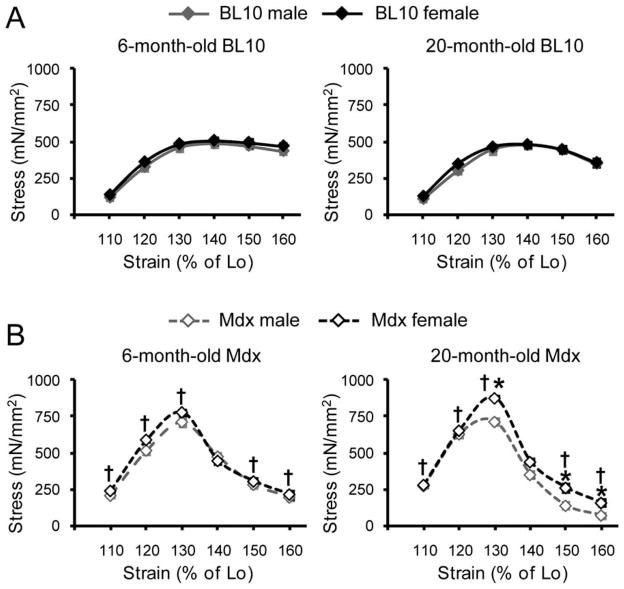
Comparison of stress–strain curve profiles between genders in BL10 mice revealed minimal alteration at both 6 and 20 months of age (Fig. 2A). Consistent with our recent report,10 mdx EDL muscles were much stiffer (Fig. 2B). Specifically, mdx muscle yielded significantly higher stress at strains of 110–130% Lo when compared with BL10 muscle (Fig. 2B).10 Furthermore, a rapid post-peak stress drop was seen in mdx muscle but not in BL10 muscle (Fig. 2B).10 Although 6-month-old male and female mdx mice had similar stress–strain curves, differences were observed between male and female mdx at 20 months of age (Fig. 2B). Twenty-month-old female mdx mice yielded significantly higher peak stress, and post-peak stress retention was significantly higher at strains of 150% and 160% Lo.
FIGURE 2.
Comparison of the stress–strain relationship between genders in BL10 (A) and mdx (B) mice. The elastic property of the EDL muscle at 6 and 20 months was characterized by the stress–strain relationship developed while straining the muscle until 160% Lo in an increment of 10% Lo. *Females significantly different from age- and strain-matched males; †mdx mice significantly different from BL10 mice within the same gender.
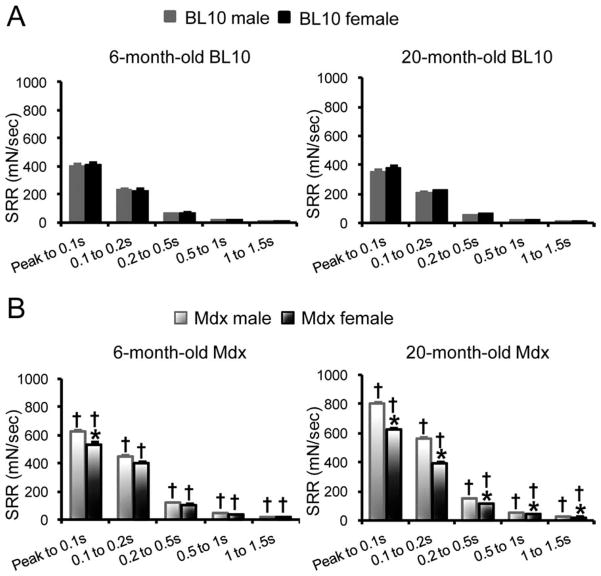
The viscous properties were determined by SRR (Fig. 3). Age- and gender-matched mdx mice showed a significantly higher SRR than that of BL10 mice (Fig. 3A and B). At 6 months of age, female mdx mice demonstrated a significantly lower SRR compared with male mdx mice within the first 100 ms post-peak. At 20 months of age, female mdx mice showed significantly lower SRR than age-matched male mdx mice across the entire measurement range (from peak to 1.5 s) (Fig. 3B). No significant difference in SSR was observed between male and female BL10 mice (Fig. 3A).
FIGURE 3.
Comparison of the stress–strain relaxation rate of the EDL muscle in BL10 (A) and mdx (B) mice. The viscous properties of the EDL muscle at 6 and 20 months was determined by the stress–relaxation rate. *Females significantly different from age- and strain-matched males; †mdx mice significantly different from BL10 mice within the same gender.
Quantification of Fibrosis by Hydroxyproline Content
In the BL10 EDL muscle, the amount of collagen did not change significantly (Fig. 4A). The hydroxyproline content was significantly increased in mdx mice (Fig. 4). We also noticed gender and age differences in mdx mice (Fig. 4). Specifically, there was a significant difference between males and females at 6 months of age and a significant difference between 6 and 20 months of age for both males and females.
FIGURE 4.
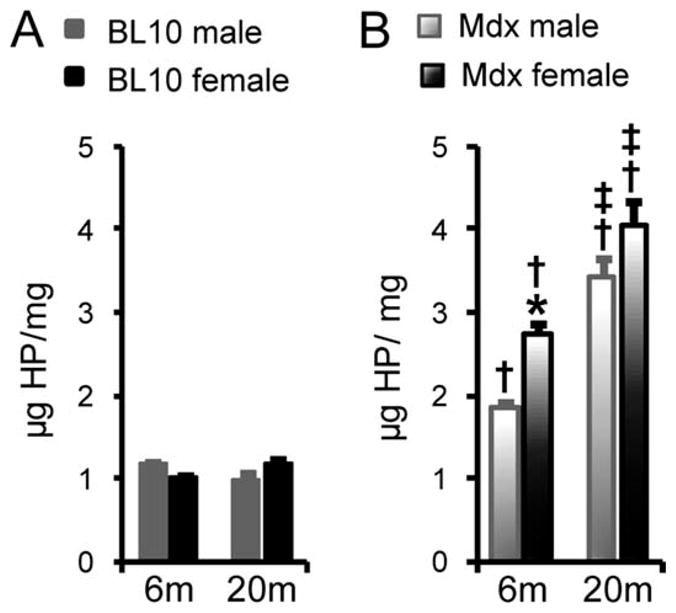
Quantification of the hydroxyproline content in the EDL muscle of BL10 (A) and mdx (B) mice. *Females significantly different from age- and strain-matched males; †mdx mice significantly different from BL10 mice within the same gender; ‡20-month-old groups significantly different from 6-month-old groups within the same gender and strain.
DISCUSSION
X-linked DMD mainly affects boys. Although female patients are rare,11 manifesting female carriers have been frequently reported.12,13 Both male and female dystrophin-null subjects have been included in preclinical studies. However, a gender difference has not been fully appreciated.6–8
Two studies have compared the serum creatine kinase (CK) level in male and female mdx mice. At the peak necrotic stage (3–4 weeks of age), both genders had similar levels of CK elevation.14,15 Interestingly, male mdx mice showed an approximately twofold higher CK concentration than that of female mdx mice at 3 months of age.14 However, this difference disappeared in 6-month-old mdx mice.14 One study addressed histopathological differences between male and female mdx mice.15 By morphometric quantification of Evans blue dye (EBD) uptake in the gastrocnemius muscle, the investigators concluded that there was significantly more myofiber damage in male mdx mice at the age of 6 weeks. However, at 24 weeks, there was more EBD-positive (damaged muscle) in females.15 Collectively, these studies suggest that male mdx mice are more severely affected at a younger age (1.5–3 months of age). However, as mdx mice get older (≥6 months), female mice may suffer more histopathological damage.14,15
We recently examined the gender difference in aged mdx heart, a model of Duchenne cardiomyopathy.16 Histological examination and hydroxyproline quantification revealed similar levels of myocardial fibrosis in both genders.16–18 However, physiological assays with electrocardiography and cardiac catheterization showed significant differences between male and female mdx mice.16 Aged female mdx mice displayed a dilated cardiomyopathy similar to that seen in human subjects.16 However, the heart chamber was reduced rather than enlarged in aged male mdx mice.16
To extend previous mdx gender comparison studies,14,15 in the present study we compared tetanic and passive forces in the EDL muscle of male and female mdx mice. We have focused our analysis on the EDL muscle, because it is one of the most commonly used muscles for study of mdx contractility.19–21 Young mdx mice are mildly affected. However, aged mdx mice exhibit severe muscle wasting and dystrophy.22–24 For this reason, we included two age groups (6 months and 20 months) in our study. Age- and gender-matched BL10 mice were also included as normal controls.
It is well established that male and female skeletal muscles display considerable differences in their metabolic properties and gene expression patterns.25,26 However, Glenmark et al. found that male and female EDL muscles generated similar levels of total tetanic force in wild-type mice.27 We compared the EDL muscle tetanic force in male and female BL10 mice. Consistent with the results of Glenmark et al., we observed comparable tetanic contractility between male and female BL10 mice (see Fig. 1A). Gender also did not affect the elastic and viscous properties in BL10 mice (see Figs. 2A and 3A). Hydroxyproline content quantification assay showed no significant difference in the collagen content in BL10 mice. Interestingly, there was a significant gender difference in the anatomical properties of the EDL muscle (20-month-old group only) and body weight (6-month-old group only) in the BL10 mice (Table 1). Collectively, our results suggest that gender may not affect the outcome of in vitro physiological assays in the EDL muscle of BL10 mice.
In contrast to BL10 mice, measurements performed in mdx EDL muscle showed significant gender disparity in several parameters. At the age of 6 months, female mdx EDL muscle displayed significantly higher specific tetanic force (Fig. 1B). However, the difference disappeared at 20 months. In fact, 20-month-old mdx females showed significantly lower total isometric tetanic force and a shorter half-relaxation time. It also took them longer to reach maximal force. It is interesting that adult female mdx mice performed better than males, but aged female mdx mice performed worse than males. The exact mechanisms behind this observation remain to be investigated. However, sex hormones may have contributed to this difference.28 Six-month-old female mice are at their reproductive peak, whereas at 20 months they should have reached menopause. Another point of interest is the body weight changes in 20-month-old mice. At this age, the body weight of mdx females was only ~50% of that of BL10 females. However, the difference was less in males (mdx males were ~20% of BL10 males). The gender-associated difference in body weight comparison (between mdx and BL10 mice) suggests that there may be global metabolic changes. These changes may have also contributed to our observations.
In addition to active muscle force, we also noticed significant gender differences in parameters of passive properties in mdx mice. Specifically, 20-month-old female mdx mice showed significantly higher peak stress than males (see Fig. 2B). In terms of viscosity, 6-month-old female mdx mice showed a significantly reduced SRR compared with male mdx mice from peak to 0.1 s. This difference extended through the entire assay time range in 20-month-old female mdx mice (see Fig. 3B). It remains unclear why these differences were only observed in mdx mice but not in BL10 mice. One likely reason is the level of fibrosis. Female mdx mice had a higher collagen content, and a significant difference was reached at 6 months of age (see Fig. 4). But why were female mdx mice more fibrotic than male mdx mice? Currently, there is no clear explanation for this observation, yet we suspect that it may relate to the renin–angiotensin system (RAS). The RAS is an important regulator of fibrosis.29 It has been shown that females seem more sensitive to perturbations of the RAS.30,31 It is worth noting that enhanced fibrosis was also seen in the hearts of female mdx mice. In fact, aged female mdx hearts had a significantly higher hydroxyproline content compared with male hearts.16 We recently demonstrated that the myotendinous junction (MTJ) also influences the outcome of passive property assays.10 Future studies are needed to clarify the role of the MTJ in gender-related stiffness changes (Fig. 3).
In conclusion, we have demonstrated that gender may profoundly influence the outcomes of physiological assays in mdx mice. Gender should be considered in study design and data interpretation when using mdx mice in DMD studies.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR-49419, to D.D.), Muscular Dystrophy Association (to D.D.), and an NIH training grant (T90DK70105 to C.H.). The authors thank Dr. Virginia Huxley for helpful discussions; Dr. Dick Madsen and Dr. Youngju Pak for help with statistical analysis; and Marianne Abdo, Juveria Nayeem, and Alexandra Kellogg for technical assistance.
Abbreviations
- CK
creatine kinase
- CSA
cross-sectional area
- DMD
Duchenne muscular dystrophy
- EBD
Evans blue dye
- EDL
extensor digitorum longus
- Lo
optimal length
- MTJ
myotendinous junction
- Po
absolute isometric force
- RAS
renin & angiotensin system
- SPo
specific isometric force
- SSR
stress–relaxation rate
References
- 1.Kunkel LM. 2004 William Allan Award address. Cloning of the DMD gene. Am J Hum Genet. 2005;76:205–214. doi: 10.1086/428143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks GB, Chamberlain JS. The value of mammalian models for duchenne muscular dystrophy in developing therapeutic strategies. Curr Top Dev Biol. 2008;84:431–453. doi: 10.1016/S0070-2153(08)00609-1. [DOI] [PubMed] [Google Scholar]
- 3.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 4.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol Dis. 2008;31:1–19. doi: 10.1016/j.nbd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wooddell CI, Zhang G, Griffin JB, Hegge JO, Huss T, Wolff JA. Use of Evans blue dye to compare limb muscles in exercised young and old mdx mice. Muscle Nerve. 2010;41:487–499. doi: 10.1002/mus.21527. [DOI] [PubMed] [Google Scholar]
- 7.Radley HG, Davies MJ, Grounds MD. Reduced muscle necrosis and long-term benefits in dystrophic mdx mice after cV1q (blockade of TNF) treatment. Neuromuscul Disord. 2008;18:227–238. doi: 10.1016/j.nmd.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Taniguti AP, Pertille A, Matsumura CY, Santo Neto H, Marques MJ. Prevention of muscle fibrosis and myonecrosis in mdx mice by suramin, a TGF-beta1 blocker. Muscle Nerve. 2011;43:82–87. doi: 10.1002/mus.21869. [DOI] [PubMed] [Google Scholar]
- 9.Hakim CH, Li D, Duan D. Monitoring murine skeletal muscle function for muscle gene therapy. Methods Mol Biol. 2011;709:75–89. doi: 10.1007/978-1-61737-982-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakim CH, Grange RW, Duan D. The passive mechanical properties of the extensor digitorum longus muscle are compromised in 2 to 20-month-old mdx mice. J Appl Physiol. 2011;110:1656–1663. doi: 10.1152/japplphysiol.01425.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verellen-Dumoulin C, Freund M, De Meyer R, Laterre C, Frederic J, Thompson MW, et al. Expression of an X-linked muscular dystrophy in a female due to translocation involving Xp21 and non-random inactivation of the normal X chromosome. Hum Genet. 1984;67:115–119. doi: 10.1007/BF00270570. [DOI] [PubMed] [Google Scholar]
- 12.Song TJ, Lee KA, Kang SW, Cho H, Choi YC. Three cases of manifesting female carriers in patients with Duchenne muscular dystrophy. Yonsei Med J. 2011;52:192–195. doi: 10.3349/ymj.2011.52.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soltanzadeh P, Friez MJ, Dunn D, von Niederhausern A, Gurvich OL, Swoboda KJ, et al. Clinical and genetic characterization of manifesting carriers of DMD mutations. Neuromuscul Disord. 2010;20:499–504. doi: 10.1016/j.nmd.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida M, Yonetani A, Shirasaki T, Wada K. Dietary NaCl supplementation prevents muscle necrosis in a mouse model of Duchenne muscular dystrophy. Am J Physiol Regul Integr Comp Physiol. 2006;290:R449–455. doi: 10.1152/ajpregu.00684.2004. [DOI] [PubMed] [Google Scholar]
- 15.Salimena MC, Lagrota-Candido J, Quirico-Santos T. Gender dimorphism influences extracellular matrix expression and regeneration of muscular tissue in mdx dystrophic mice. Histochem Cell Biol. 2004;122:435–444. doi: 10.1007/s00418-004-0707-8. [DOI] [PubMed] [Google Scholar]
- 16.Bostick B, Yue Y, Duan D. Gender influences cardiac function in the mdx model of Duchenne cardiomyopathy. Muscle Nerve. 2010;42:600–603. doi: 10.1002/mus.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bostick B, Yue Y, Long C, Duan D. Prevention of dystrophin-deficient cardiomyopathy in twenty-one-month-old carrier mice by mosaic dystrophin expression or complementary dystrophin/utrophin expression. Circ Res. 2008;102:121–130. doi: 10.1161/CIRCRESAHA.107.162982. [DOI] [PubMed] [Google Scholar]
- 18.Bostick B, Yue Y, Long C, Marschalk N, Fine DM, Chen J, Duan D. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged mdx mice. Mol Ther. 2009;17:253–261. doi: 10.1038/mt.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol. 2001;535:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS, Duan D. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther. 2005;11:245–256. doi: 10.1016/j.ymthe.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pastoret C, Sebille A. mdx mice show progressive weakness and muscle deterioration with age. J Neurol Sci. 1995;129:97–105. doi: 10.1016/0022-510x(94)00276-t. [DOI] [PubMed] [Google Scholar]
- 23.Lefaucheur JP, Pastoret C, Sebille A. Phenotype of dystrophinopathy in old mdx mice. Anat Rec. 1995;242:70–76. doi: 10.1002/ar.1092420109. [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 2007;21:2195–2204. doi: 10.1096/fj.06-7353com. [DOI] [PubMed] [Google Scholar]
- 25.Welle S, Tawil R, Thornton CA. Sex-related differences in gene expression in human skeletal muscle. PLoS One. 2008;3:e1385. doi: 10.1371/journal.pone.0001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green HJ, Fraser IG, Ranney DA. Male and female differences in enzyme activities of energy metabolism in vastus lateralis muscle. J Neurol Sci. 1984;65:323–331. doi: 10.1016/0022-510x(84)90095-9. [DOI] [PubMed] [Google Scholar]
- 27.Glenmark B, Nilsson M, Gao H, Gustafsson JA, Dahlman-Wright K, Westerblad H. Difference in skeletal muscle function in males vs. females: role of estrogen receptor-beta. Am J Physiol Endocrinol Metab. 2004;287:E1125–1131. doi: 10.1152/ajpendo.00098.2004. [DOI] [PubMed] [Google Scholar]
- 28.Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010;40:41–58. doi: 10.2165/11319760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Shahbaz AU, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC, McGee JE, et al. Fibrosis in hypertensive heart disease: molecular pathways and cardioprotective strategies. J Hypertens. 2010;28(suppl 1):S25–32. doi: 10.1097/01.hjh.0000388491.35836.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, et al. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension. 2004;43:957–962. doi: 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- 31.Duprez DA. Is the female heart more sensitive to aldosterone for early remodeling? Hypertension. 2004;43:936–937. doi: 10.1161/01.HYP.0000124253.98863.86. [DOI] [PubMed] [Google Scholar]